
Background. Proteasome inhibitor (PI)-based induction/consolidation proved to be effective in newly diagnosed multiple myeloma (NDMM) patients (pts) eligible for melphalan 200 mg/m2 plus autologous stem-cell transplantation (MEL200-ASCT). High response rates have been reported with carfilzomib (K) plus lenalidomide-dexamethasone (KRd) or cyclophosphamide-dexamethasone (KCd). Lenalidomide (R) alone is a standard of care for post-ASCT maintenance; K maintenance showed promising results in phase I/II studies, but no data on KR maintenance vs R are available.
Aims. The aims of this analysis were to evaluate the progression-free survival (PFS) of KRd induction-ASCT-KRd consolidation (KRd_ASCT) vs 12 cycles of KRd (KRd12) vs KCd induction-ASCT-KCd consolidation (KCd_ASCT) and the PFS of KR vs R maintenance. Secondary aims were efficacy in different subgroups of pts and safety of the maintenance phase.
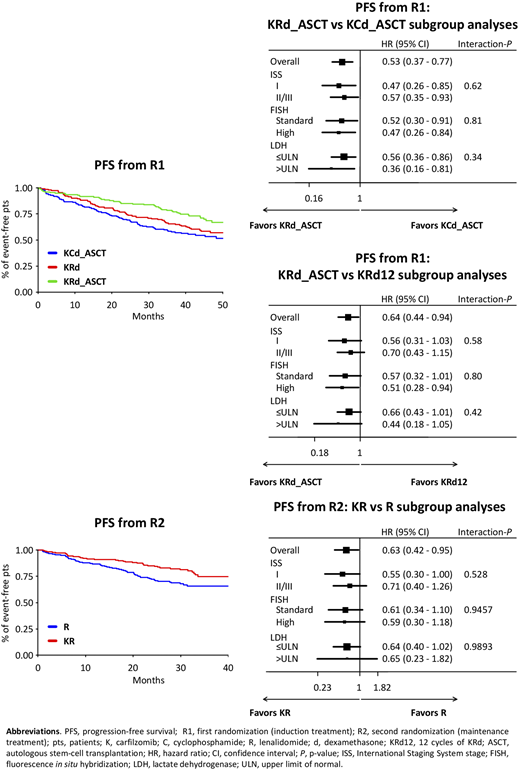
Methods. NDMM pts ≤65 years were randomized [R1: 1:1:1, stratification International Staging System (ISS) and age] to: KRd_ASCT: 4 28-day cycles with KRd induction (K 20/36 mg/m2 IV days 1,2,8,9,15,16; R 25 mg days 1-21; dexamethasone [d] 20 mg days 1,2,8,9,15,16) followed by MEL200-ASCT and 4 KRd consolidation cycles; KRd12: 12 KRd cycles; KCd_ASCT: 4 28-day induction cycles with KCd (K 20/36 mg/m2 IV days 1,2,8,9,15,16; cyclophosphamide 300 mg/m2 days 1,8,15; d 20 mg days 1,2,8,9,15,16) followed by MEL200-ASCT and 4 KCd consolidation cycles. Thereafter, pts were randomized (R2) to maintenance with KR (K 36 mg/m2 days 1,2,15,16, subsequently amended to 70 mg/m2 days 1,15 for up to 2 years; plus R 10 mg days 1-21 every 28 days until progression) or R alone (10 mg days 1-21 every 28 days until progression). Centralized minimal residual disease (MRD) evaluation (8-color second-generation flow cytometry, sensitivity 10-5) was performed in pts achieving ≥very good partial response before maintenance and every 6 months (m) during maintenance. Data cut-off was June 30, 2020.
Results. 474 NDMM pts were randomized (KRd_ASCT, n=158; KRd12, n=157; KCd_ASCT, n=159) and analyzed. Pt characteristics were well balanced. Intention-to-treat (ITT) data of pre-maintenance MRD (KRd_ASCT, 62%; KRd12 56%, KCd_ASCT 43%) and safety of the induction/consolidation phases in the 3 arms were already reported (F. Gay et al. ASH 2018; S. Oliva et al. ASH 2019). After a median follow-up from R1 of 45 m, median PFS was not reached with KRd_ASCT, 57 m with KRd12 and 53 m with KCd_ASCT (KRd_ASCT vs KCd_ASCT: HR 0.53, P<0.001; KRd_ASCT vs KRd12: HR 0.64, P=0.023; KRd12 vs KCd_ASCT: HR 0.82, P=0.262). The benefit of KRd_ASCT vs both KCd_ASCT and KRd12 was observed in most subgroups (Figure). 3-year overall survival (OS) was 90% with KRd_ASCT and KRd12 vs 83% with KCd. 356 pts (KR, n=178; R, n=178) were randomized to maintenance; pt characteristics, pre-maintenance response (≥complete response [CR]: KR 62% vs R 59%; stringent CR: KR 50% vs R 48%) including MRD negativity (KR 65% vs R 66%) in the 2 groups were well balanced. After a median follow-up from R2 of 31 m and a median duration of maintenance of 27 m in both arms, 46% of MRD-positive pts at randomization turned negative in KR vs 32% in R (P=0.04). By ITT analysis, 3-year PFS from R2 was 75% with KR vs 66% with R (HR 0.63; P=0.026). The benefit of KR vs R was observed in most subgroups (Figure). 3-year OS was 90% in both arms.
During maintenance, a similar proportion of pts experienced ≥1 grade (G)3-4 hematologic adverse events (AEs)/serious AEs (SAEs) in the 2 arms (KR 22% vs R 23%); the most frequent were neutropenia (KR 18% vs R 21%) and thrombocytopenia (KR 3% vs R 3%). Rate of ≥1 G3-4 non-hematologic AEs/SAEs was higher with KR (27%) compared with R (15%), P=0.012; the most frequent were infections (KR 4% vs R 7%); all other events were reported in ≤5% of pts and included: gastrointestinal (KR 5% vs R 2%), cardiac (KR 4% vs R 1%), hypertension (KR 3% vs R 0%), and thrombotic microangiopathy (3% vs 0%). 4 pts developed a second primary malignancy in KR (breast 1 pt; thyroid 1 pt; myelodysplastic syndrome 1 pt; non-melanoma skin cancer 1pt) vs 1 pt in R (acute lymphoblastic leukemia). Dose reductions of R were reported in 23% of KR and 29% of R pts; dose reductions of K were reported in 20% of pts. The rate of discontinuation due to AEs was similar in the 2 arms (KR 10% vs R 9%).
Conclusions. Treatment with KRd_ASCT significantly improved PFS compared with both KRd12 and KCd_ASCT. Maintenance with KR also improved PFS vs R.
Gay:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Musto:Celgene: Honoraria; Amgen: Honoraria. Galli:BMS: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Takeda: Honoraria. Belotti:Jannsen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Zamagni:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau. Zambello:Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. De Sabbata:Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. D'Agostino:GSK: Membership on an entity's Board of Directors or advisory committees. Liberati:VERASTEM: Honoraria, Research Funding; ROCHE: Honoraria, Research Funding; PFIZER: Honoraria, Research Funding; ONCOPEPTIDES AB: Honoraria, Research Funding; TAKEDA: Honoraria, Research Funding; MORPHOSYS: Honoraria, Research Funding; ONCONOVA: Honoraria, Research Funding; ABBVIE: Honoraria, Research Funding; NOVARTIS: Honoraria, Research Funding; KARYOPHARM: Honoraria, Research Funding; INCYTE: Honoraria; JANSSEN: Honoraria; CELGENE: Honoraria; AMGEN: Honoraria; BMS: Honoraria; BEIGENE: Honoraria; ARCHIGEN: Honoraria; BIOPHARMA: Honoraria; FIBROGEN: Honoraria. Offidani:Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Cavo:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau. Boccadoro:AbbVie: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Research Funding; Amgen: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding.
The presentation includes discussion of off-label use of a drug or drugs for the treatment of multiple myeloma (including carfilzomib, cyclophosphamide, lenalidomide and dexamethasone).
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal