Introduction
Primary cerebral lymphoma (PCNSL) is an uncommon subtype of diffused large B-cell lymphoma (DLBCL) with a particular poor outcome as compared to systemic DLBCL, especially in elderly. For patients older than 60 years, standard treatment consists of high-dose methotrexate (HD-MTX) chemotherapy without consolidation brain radiotherapy to reduce the risk of leukoencephalopathy. Rituximab in combination with HD-MTX, procarbazine, vincristine followed by HD-cytarabine consolidation is one of standard of treatments for PCNSL patients with a 2-year PFS rate of 47% for patients aged of 60 or older in prospective trial (Morris JCO 2013). Etoposide and Ifosfamide are two drugs that can diffuse across blood-brain barrier and commonly used for relapsed/refractory PCNSL. To improve efficacy of R-MPVA protocol, we developed a new regimen which consisted in adding etoposide and ifosfamide for patients with a newly diagnosed PCNSL aged between 60 and 75 years old.
Patients & Methods
The protocol consisted of 3 cycles every 28 days of rituximab (375mg/m2, J1 and J15), MTX (3.5 g/m2, J1 and J15), vincristine (1.4 mg/m2, J1 and J15), vepeside (100mg/m2, J2) and procarbazine (100mg/m2, J1-7). Consolidation therapy consisted of 2 cycles every 21 days rituximab (375mg/m2, J1) in combination with cytarabine (3g/m2, J1-2) with ifosfamide (1.5 g/m2, J1-3). Response evaluations were planned after the 3 cycles of induction (R-MPV-VP16) and after consolidation (R-AraC-Ifo). We retrospectively reviewed treatment modalities, toxicities, response and outcome with this protocol and compared results with a matched group of patients with the same range age (60 - 75 years) treated with R-MPVA.
Results
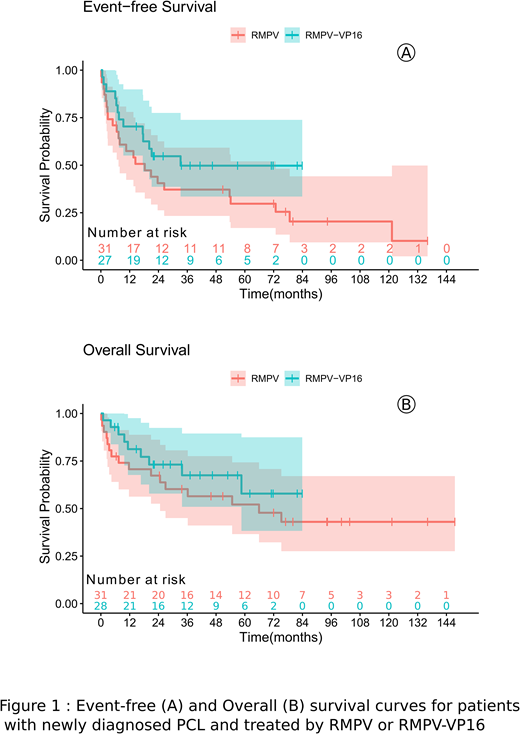
Between 2013 and 2018, 28 PCNSL patients were treated with this protocol. The median age was 67.5 years old (range, 61-74). Poor performance status (PS 3-4) was presented in 9 patients (32%). As compared to 31 patients treated between 2007 and 2018 with R-MPVA, patients treated with intensive protocol were younger (66 vs. 69 years, P=0.01) and had less frequently a poor PS 3-4 (32% vs. 61%, P=0.04). In intent-to-treat analysis, 27 patients received 3 cycles of R-MPV-VP16 but one received only 2. Among them, five patients achieved PR and then received 1 to 2 additional cycles of R-MPV-VP16. Following this induction, 25 patients underwent 2 cycles R-AraC-ifo consolidation, 3 of them did not received ifosfamide for the second cycle because of hematological toxicity and poor PS. One patient in complete response (CR) after whole treatment received high-dose therapy followed by autologous stem cell transplantation. After R-MPV-VP16, 10 patients (36%) achieved CR and 14 partial responses (50%) (PR) as compared to 12 CR (39%) and 12 PR (39%) for patients treated with R-MPVA. After consolidation phase, 23 patients (82%) achieved CR after R-AraC-Ifo as compared to 21 CR (68%) after R-AraC in the historical arm. Differences were not statistically significant. R-MPV-VP16 regimen was associated with favorable toxicity profile with 13 (46%) grade 4 hematological toxicity, 8 (28%) grade 3 and one grade 4 (3%) renal toxicity, 3 (10%) grade 3 and one grade 4 (3%) hepatic toxicity, 6 (21%) grade 3 and 4 grade 4 (14%) and infectious toxicity. With a median follow-up of 46.5 months, patients treated with R-MPV-VP16 followed by R-AraC-ifo had a median event-free survival (EFS) of 33.2 months (95%CI, 17.6 - not reached [NR]) with a 2-year EFS rate of 52%; the median overall survival (OS) was not reached (95%CI, 58.6-NR) with a 2-year OS rate of 70%. With a median follow-up of 94.2 months, patients treated with R-MPVA had a median EFS of 18.3 months with a 2-year EFS rate of 39% (P=0.14, Fig 1); the median OS was 65.9 months with 2-year OS rate of 64% (P=0.33, Fig 1).
Conclusions
In this retrospective analysis of two HD-MTX and HD-AraC based regimens for PCNSL patients aged between 60 and 75 years performed in real-life setting, R-MPVA was more frequently proposed for older patients with a poorer PS. Combination of vepeside to R-MPV and ifosfamide to R-AraC was feasible with a favorable toxicity profile. Despite not statistically different, we observed a trend for an improvement of response rate at the end of treatment (82% vs. 68% of CR) and reduced rate of relapses (2-year EFS rates: 52% vs. 39%) with the intensified protocol. These first results deserve a confirmative larger prospective study of R-MPV-VP16 followed by R-AraC-ifosfamide for elderly PCNSL patients.
Ferrant:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Karlin:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, personal fees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, personal fees; Celgene: Other: Personal fees; Sanofi: Honoraria; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, personal fees; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Bachy:Beigene: Membership on an entity's Board of Directors or advisory committees; Roche, Celgene, Amgen, Janssen, Gilead, Novartis, Sanofi: Honoraria; Amgen: Research Funding; Roche, Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal