Key Points
RIBE reduce the long-term hematopoietic reconstitution of human HSCs and the colony-forming ability of human HPCs.
RIBE impair human HSCs and HPCs via oxidative DNA damage, and antioxidants can lessen the effects.
Abstract
Total body irradiation (TBI) is commonly used in host conditioning regimens for human hematopoietic stem cell (HSC) transplantation to treat various hematological disorders. Exposure to TBI not only induces acute myelosuppression and immunosuppression, but also injures the various components of the HSC niche in recipients. Our previous study demonstrated that radiation-induced bystander effects (RIBE) of irradiated recipients decreased the long-term repopulating ability of transplanted mouse HSCs. However, RIBE on transplanted human HSCs have not been studied. Here, we report that RIBE impaired the long-term hematopoietic reconstitution of human HSCs as well as the colony-forming ability of human hematopoietic progenitor cells (HPCs). Our further analyses revealed that the RIBE-affected human hematopoietic cells showed enhanced DNA damage responses, cell-cycle arrest, and p53-dependent apoptosis, mainly because of oxidative stress. Moreover, multiple antioxidants could mitigate these bystander effects, though at different efficacies in vitro and in vivo. Taken together, these findings suggest that RIBE impair human HSCs and HPCs by oxidative DNA damage. This study provides definitive evidence for RIBE on transplanted human HSCs and further justifies the necessity of conducting clinical trials to evaluate different antioxidants to improve the efficacy of HSC transplantation for the patients with hematological or nonhematological disorders.
Introduction
Transplantation of hematopoietic stem cells (HSCs) is a critical therapy for various malignant and nonmalignant hematological disorders and immune dysfunction.1-5 The key to successful transplantation is that the transplanted HSCs home to the host’s bone marrow (BM) niche and differentiate into multilineage mature blood cells, thus providing the patient with a revitalized hematopoietic and immune system.6 Total body irradiation (TBI) is widely used for myeloablative conditioning regimens to eliminate malignant or autoimmune cells of the patients with acute lymphoblastic leukemia or acute myeloid leukemia before HSC transplantation.7 TBI has remained the first choice in many centers for acute lymphoblastic leukemia.8 However, nonirradiated engrafted donor cells are also subject to damage affecting their survival and function, known as radiation-induced bystander effects (RIBE), which are caused by harmful factors transmitted by irradiated cells.9-11 Moreover, we have previously demonstrated acute negative bystander effects of irradiated recipients on transplanted mouse HSCs12 and recently also shown rapid differentiation of transplanted HSCs in an irradiated host.13 Nevertheless, RIBE on human HSCs have not been established.
In this study, we used an in vivo RIBE model as well as an in vitro model to mimic RIBE in patients who undergo TBI, and investigated RIBE on human HSCs and comprehensive mechanisms. Our results indicated that RIBE also occurred in human HSCs both in vitro and in vivo: the long-term hematopoietic reconstitution of human HSCs was significantly decreased, and the clonogenic ability of HPCs was also reduced. RIBE induced the human hematopoietic cells to undergo enhanced DNA damage, which in turn led to cell-cycle arrest, cell apoptosis, or senescence. Moreover, the damage to bystander human hematopoietic cells was mainly from oxidative stress. Importantly, our results showed that multiple antioxidants could alleviate the damage of RIBE to bystander human hematopoietic stem and progenitor cells (HSPCs), although they differed in their degree of effectiveness. Taken together, these findings demonstrate that RIBE impair human HSCs through oxidative DNA damage. Our current study further justifies the plausible usefulness of specific antioxidants in improving the engraftment of transplanted HSCs for relevant patients.
Materials and methods
Exposure to irradiation in vivo and in vitro
For the in vivo RIBE model, CD34+ cells purified from human cord blood (CB) were intravenously injected into irradiated (10 Gy) or nonirradiated NOD/Shi-scid/IL-2Rγnull (NOG) mice separately. After 17 hours, homed human CD34+ cells (in vivo bystander cells) from recipients were individually detected or sorted for subsequent experiments. For the in vitro RIBE model, we cocultured CB-CD34+ cells with 10 Gy irradiated or nonirradiated human BM cells using a transwell system (see supplemental Methods, available on the Blood Web site). After 17 hours of coculture, the CD34+ cells in the insert (in vitro bystander cells) were collected for subsequent experiments.
BM transplantation assays
Female NOG mice were irradiated at a dose of 2.0 Gy 1 day before transplantation. The homed human CD34+ cells were transplanted into recipients at a dose of 5000 cells/mouse intravenously. For serial transplantations, 1 × 107 whole BM cells from each primary recipient were intravenously transplanted into secondary recipient mice that were exposed to sublethal irradiation (2.0 Gy). Details of processing and detection of mice are in the supplemental Methods.
Results
In vivo RIBE significantly dampen human long-term hematopoietic reconstitution and clonogenic ability
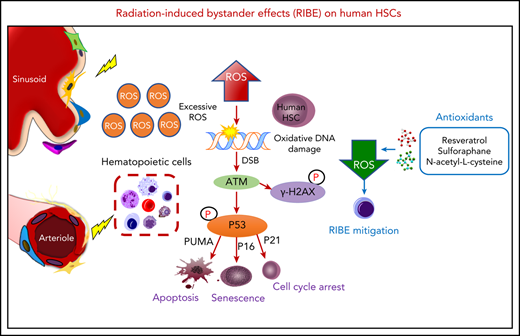
According to previous studies,12,14 almost all transplanted HSCs were observed to home to the BM and had not begun to divide 17 hours after transplantation. Therefore, we chose 17 hours after transplantation to assess the impact of RIBE on human hematopoietic cells (Figure 1A). First, we isolated human CD34+ cells and injected them into 10 Gy irradiated (RIBE group) or nonirradiated NOG mice (control group). Then, the homed cells from irradiated and nonirradiated recipients were flow-sorted 17 hours after transplantation individually, and transplanted at equivalent cell doses into sublethally (2 Gy) irradiated NOG mice intravenously. The percentage of homed CD34+ cells in the RIBE group was a little more than that in the control group (supplemental Figure 1A). However, no significant differences between groups were found in the absolute number of CD34+ cells because of the drastic decrease of the number of total bone marrow cells after irradiation (supplemental Figure 1B). Therefore, the homing ability of human CD34+ cells was similar between the control and RIBE groups (supplemental Figure 1A-B). The repopulation kinetics of human HSCs were measured at different time points after transplantation. We observed that the percentage of human CD45+ cells in the RIBE group was significantly lower than that of the control group as early as 4 weeks after transplantation (Figure 1B). Twenty weeks after transplantation, human CD45+ cell engraftment of the RIBE group was decreased by 2.7- and 2.3-fold in the BM and spleen, respectively, compared with the control group (Figure 1C-D; supplemental Figure 1C-D). Importantly, we found that the enrichment of HSC (CD34+CD38−) and HPC (CD34+CD38+) cells in the RIBE group were lower than in the control group (Figure 1E-F, respectively), suggesting a negative effect of irradiated recipients on donor HSCs/HPCs. The majority of human cells in the BM were CD19+ B cells in all the NOG mice (Figure 1G). The RIBE group had significantly decreased erythroid engraftment than the control group (Figure 1H).
In vivo RIBE significantly reduce human long-term hematopoietic reconstitution and clonogenic ability. (A) Experimental diagram of in vivo RIBE model. (B) Mean human cell engraftment level in peripheral blood (PB) at different time points after transplantation. (C) Representative flow plots of human hematopoietic cell engraftment in the bone marrow (BM) 20 weeks after transplantation (RIBE vs control [Ctrl]: 14.19% ± 3.809% vs 38.70% ± 7.168%, P = .009). (D) Percentage of human cell engraftment in the BM of recipients. (E-F) Frequency of human CD34+CD38− (E) and CD34+CD38+ (F) cells in the BM of recipients. (G-H) Lineage differentiation potential (G) and frequency (H) of human CD45−CD235a+ cells in the BM of recipients; n = 10-11 per group (B-H). (I-J) Percentage of human cell engraftment (I) and lineage differentiation potential (J) of the secondary mice (RIBE vs Ctrl: 0.19% ± 0.028% vs 6.29% ± 2.586%, P = .041; n = 9-11 per group). (K) Frequency of human hematopoietic cells in the recipients measured by LDA (n = 7-11 per group). (L) Number of hematopoietic colonies formed by hCD34+ cells in each group (n = 5 per group) (3 independent experiments; *P < .05; **P < .01; ***P < .001).
In vivo RIBE significantly reduce human long-term hematopoietic reconstitution and clonogenic ability. (A) Experimental diagram of in vivo RIBE model. (B) Mean human cell engraftment level in peripheral blood (PB) at different time points after transplantation. (C) Representative flow plots of human hematopoietic cell engraftment in the bone marrow (BM) 20 weeks after transplantation (RIBE vs control [Ctrl]: 14.19% ± 3.809% vs 38.70% ± 7.168%, P = .009). (D) Percentage of human cell engraftment in the BM of recipients. (E-F) Frequency of human CD34+CD38− (E) and CD34+CD38+ (F) cells in the BM of recipients. (G-H) Lineage differentiation potential (G) and frequency (H) of human CD45−CD235a+ cells in the BM of recipients; n = 10-11 per group (B-H). (I-J) Percentage of human cell engraftment (I) and lineage differentiation potential (J) of the secondary mice (RIBE vs Ctrl: 0.19% ± 0.028% vs 6.29% ± 2.586%, P = .041; n = 9-11 per group). (K) Frequency of human hematopoietic cells in the recipients measured by LDA (n = 7-11 per group). (L) Number of hematopoietic colonies formed by hCD34+ cells in each group (n = 5 per group) (3 independent experiments; *P < .05; **P < .01; ***P < .001).
Secondary transplantations remain the most commonly used strategy to assess the self-renewal ability of HSCs. Thus, we performed parallel secondary transplantations from both RIBE and control primary recipients. Interestingly, human cells from primary RIBE recipients generated a 33.1-fold decrease of the mean engraftment levels in the BM compared with those from the control group (Figure 1I-J). These data demonstrated that RIBE abrogated the long-term engraftment potential of HSPC.
The cells that initiated engraftment in xenotransplants were operationally defined as SCID-repopulating cells (SRCs). The SRC assay provided a direct quantitative in vivo assay to measure human HSC activity and engraftment. We next performed limiting dilution analysis (LDA)15,16 to measure the frequency of SRCs. One in 2979 cells in the RIBE group clonally initiated long-term hematopoiesis in NOG mice, whereas 1 in 1416 cells did so in the control group (Figure 1K; supplemental Table 2). In addition, colony-forming cell (CFC) assay showed that the number of CFCs in the RIBE group significantly decreased in comparison with that in the control group (Figure 1L). These data revealed that the clonogenic potential of human CD34+ cells was suppressed after exposure to irradiated recipients.
RIBE obstruct HSPC cell-cycle entry and increase apoptosis and senescence in vivo
The hematopoietic system is the most sensitive to irradiation, and irradiation leads to acute hematopoietic damage by inducing cell death of all the hematopoietic tissues.17-20 To explore the mechanisms underlying the impaired long-term engraftment of bystander hematopoietic cells (Figure 2A), we first examined the cell-cycle status of bystander human CD34+ cells by 5-bromo-2′-deoxyuridine (BrdU) incorporation. The proportion of BrdU+ cells in the CD34+ cells of the RIBE group was lower than that of the control group, suggesting that cell-cycle arrest occurred in bystander human HSPCs (Figure 2B). The fractions of apoptotic cells of CD34+ cells in the RIBE group were increased (Figure 2C). Furthermore, senescence-associated β-galactosidase (SA-β-gal) staining revealed a significant increase in the senescence of CD34+ cells in the RIBE group compared with the control group (Figure 2D). Altogether, the observed impaired long-term engraftment of bystander hematopoietic cells is partially attributed to the altered cell-cycle status, apoptosis, and senescence of HSPCs.
RIBE affect HSPC cell-cycle entry and increases apoptosis and senescence in vivo. (A) Experimental diagram of in vivo RIBE model for analysis. (B) The proportion of BrdU+ cells of human CD34+ cells from irradiated and nonirradiated mice. BrdU was injected simultaneously with human CD34+ cells. (C) Frequency of early apoptotic cells (Annexin V+7AAD−) and late apoptotic cells (Annexin V+7AAD+) in the homed human CD34+ cells from irradiated and nonirradiated mice. (D) Homed human CD34+ cells from irradiated and nonirradiated mice were sorted and cultured in vitro for 3 days to detect SA-β-gal activity. 5-dodecanoylaminofluorescein di-β-d-galactopyranoside (C12FDG) was used as a substrate. Bars represent fold change C12FDG MFI compared with that in the control (Ctrl) group. (E-F) Flow cytometric analysis of fold change of ROS levels by DCF-DA (E) and dihydroethidium staining (F) in homed human CD34+ cells. (G) Fold change of mitochondrial ROS levels in homed human CD34+ cells were detected by MitoSOX staining. (H-I) Fold change of mitochondrial membrane potential of homed human CD34+ cells from irradiated or nonirradiated NOG mice were determined with tetramethylrhodamine methyl ester (TMRE) (H) and DilC1(5) staining (I) (n = 4 mice per group, *P < .05; **P < .01; ***P < .001).
RIBE affect HSPC cell-cycle entry and increases apoptosis and senescence in vivo. (A) Experimental diagram of in vivo RIBE model for analysis. (B) The proportion of BrdU+ cells of human CD34+ cells from irradiated and nonirradiated mice. BrdU was injected simultaneously with human CD34+ cells. (C) Frequency of early apoptotic cells (Annexin V+7AAD−) and late apoptotic cells (Annexin V+7AAD+) in the homed human CD34+ cells from irradiated and nonirradiated mice. (D) Homed human CD34+ cells from irradiated and nonirradiated mice were sorted and cultured in vitro for 3 days to detect SA-β-gal activity. 5-dodecanoylaminofluorescein di-β-d-galactopyranoside (C12FDG) was used as a substrate. Bars represent fold change C12FDG MFI compared with that in the control (Ctrl) group. (E-F) Flow cytometric analysis of fold change of ROS levels by DCF-DA (E) and dihydroethidium staining (F) in homed human CD34+ cells. (G) Fold change of mitochondrial ROS levels in homed human CD34+ cells were detected by MitoSOX staining. (H-I) Fold change of mitochondrial membrane potential of homed human CD34+ cells from irradiated or nonirradiated NOG mice were determined with tetramethylrhodamine methyl ester (TMRE) (H) and DilC1(5) staining (I) (n = 4 mice per group, *P < .05; **P < .01; ***P < .001).
Previous studies have reported that exposure to radiation causes a persistent increase in reactive oxygen species (ROS) production, and high levels of ROS are toxic to HSPCs both in vitro and in vivo.21-26 Thus, we speculated that irradiation-induced oxidative stress may contribute to RIBE. To validate this hypothesis, we evaluated the ROS levels of bystander human HSPCs by analysis of oxidation of 2′,7′-dichlorofluorescein diacetate (DCF-DA) and dihydroethidium. A marked increase in ROS levels was observed in bystander human HSPCs (Figure 2E-F). In addition, mitochondrial ROS levels were significantly elevated in bystander human HSPCs (Figure 2G), resulting in impaired mitochondrial membrane potential (Figure 2H-I).
In vitro RIBE cause human HSPCs to undergo defects similar to the in vivo RIBE
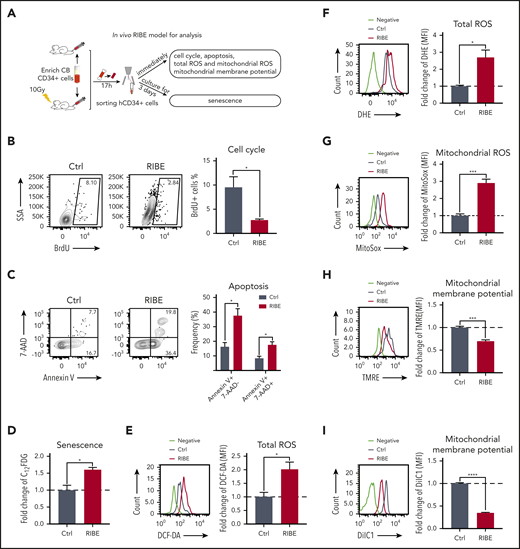
The number of bystander human HSPCs that can be collected from the in vivo RIBE model is limited. To better understand the mechanisms of RIBE, we designed in vitro experiments by coculturing human CD34+ cells with irradiated or nonirradiated human BM cells in a transwell system (Figure 3A). The reconstitution activity and clonogenic potential of CD34+ cells were evaluated in both the RIBE and control groups. There was a decreased level of human cell engraftment in the peripheral blood in the RIBE group (supplemental Figure 2A-B). Human CD45+ cell engraftment in the BM of the RIBE group was 1.9-fold lower than that in the control group (Figure 3B). Additionally, compared with the control group, with a lower level of HSC-enriched cells, the proportion of HPCs were slightly decreased in the RIBE group (supplemental Figure 2C-D). This was due to HSCs being more sensitive to radiation and oxidation than HPCs.27,28 Similar to the in vivo model, the majority of human cells were CD19+ B cells in the BM of all NOG mice (Figure 3C). However, unlike in vivo RIBE, the in vitro bystander human HSCs did not display erythroid differentiation defects, which may be caused by the absence of cell-to-cell contacts of the in vitro RIBE model (supplemental Figure 2E). As observed in the BM, the CD45+ cells in the SP of RIBE group were significantly fewer than those in the control group (supplemental Figure 2F) and the majority of cells in the SP were CD19+ B cells (supplemental Figure 2G). These experiments indicated that the long-term repopulating capacity of bystander human HSPCs was significantly reduced in the in vitro RIBE model.
In vitro RIBE lead to defects of human HSPCs. (A) Experimental design of in vitro RIBE model. CB-CD34+ cells were cocultured with irradiated or nonirradiated human bone marrow (BM) cells via transwell system. After 17 hours, CD34+ cells were collected for subsequent experiments. (B-C) Collected human CD34+ cells were transplanted into NOG mice. The frequency of human cell engraftment (B) and the lineage differentiation potential (C) were assessed in BM 20 weeks later (RIBE vs control [Ctrl]: 9.69% ± 1.903% vs 18.50% ± 3.301%, P = .023; n = 31 to 32 per group, 3 independent experiments). (D) Mean human cell engraftment in the BM of the secondary mice (RIBE vs Ctrl, 3.87 ± 1.494 vs 14.35 ± 4.430, P = .025; n = 15-17 per group). (E) Frequency of human cells in the recipient mice measured by LDA (n = 5-9 per group). (F) Number of hematopoietic colonies formed by human CD34+ cells in each group (n = 5 per group). (G) Proportion of BrdU+ cells of the human CD34+ cells from each group. BrdU was incubated with human CD34+ cells for 4 hours (n = 5 per group). (H) Frequency of early apoptotic cells (Annexin V+7AAD−) and late apoptotic cells (Annexin V+7AAD+) of human CD34+ cells from each group (n = 5 per group). (I) Fold change of SA-β-gal activity in human CD34+ cells was analyzed using C12FDG as a substrate from each group (n = 5 per group). (J) Difference of SA-β-gal activity of human CD34+ cells from each group was confirmed by the SA-β-gal enzyme activity assay as shown in the microscopic images, and bars represent the proportion of blue stained cells. (K) Flow cytometric analysis of fold change in mitochondrial ROS levels by MitoSOX staining in human hematopoietic cells (n = 5 per group). (L-M) Fold change of mitochondrial membrane potential of human CD34+ cells determined by flow cytometry with TMRE (L) and DilC1(5) staining (M) (n = 5 per group). (N) Transmission electron microscopy (TEM) analysis of the mitochondria damage in human CD34+ cells of in vitro RIBE model. (O-Q) The energy phenotype of CD34+ cells in the in vitro RIBE model (O), the oxygen consumption rate (OCR) (P), and extracellular acidification rate (ECAR) (Q) were assayed by Seahorse assay (n = 5 per group). (R) Relative ATP levels in human CD34+ cells of in vitro RIBE model (n = 5 per group) (*P < .05; **P < .01; ***P < .001).
In vitro RIBE lead to defects of human HSPCs. (A) Experimental design of in vitro RIBE model. CB-CD34+ cells were cocultured with irradiated or nonirradiated human bone marrow (BM) cells via transwell system. After 17 hours, CD34+ cells were collected for subsequent experiments. (B-C) Collected human CD34+ cells were transplanted into NOG mice. The frequency of human cell engraftment (B) and the lineage differentiation potential (C) were assessed in BM 20 weeks later (RIBE vs control [Ctrl]: 9.69% ± 1.903% vs 18.50% ± 3.301%, P = .023; n = 31 to 32 per group, 3 independent experiments). (D) Mean human cell engraftment in the BM of the secondary mice (RIBE vs Ctrl, 3.87 ± 1.494 vs 14.35 ± 4.430, P = .025; n = 15-17 per group). (E) Frequency of human cells in the recipient mice measured by LDA (n = 5-9 per group). (F) Number of hematopoietic colonies formed by human CD34+ cells in each group (n = 5 per group). (G) Proportion of BrdU+ cells of the human CD34+ cells from each group. BrdU was incubated with human CD34+ cells for 4 hours (n = 5 per group). (H) Frequency of early apoptotic cells (Annexin V+7AAD−) and late apoptotic cells (Annexin V+7AAD+) of human CD34+ cells from each group (n = 5 per group). (I) Fold change of SA-β-gal activity in human CD34+ cells was analyzed using C12FDG as a substrate from each group (n = 5 per group). (J) Difference of SA-β-gal activity of human CD34+ cells from each group was confirmed by the SA-β-gal enzyme activity assay as shown in the microscopic images, and bars represent the proportion of blue stained cells. (K) Flow cytometric analysis of fold change in mitochondrial ROS levels by MitoSOX staining in human hematopoietic cells (n = 5 per group). (L-M) Fold change of mitochondrial membrane potential of human CD34+ cells determined by flow cytometry with TMRE (L) and DilC1(5) staining (M) (n = 5 per group). (N) Transmission electron microscopy (TEM) analysis of the mitochondria damage in human CD34+ cells of in vitro RIBE model. (O-Q) The energy phenotype of CD34+ cells in the in vitro RIBE model (O), the oxygen consumption rate (OCR) (P), and extracellular acidification rate (ECAR) (Q) were assayed by Seahorse assay (n = 5 per group). (R) Relative ATP levels in human CD34+ cells of in vitro RIBE model (n = 5 per group) (*P < .05; **P < .01; ***P < .001).
To further assess the self-renewal potential of in vitro bystander human HSCs, we performed parallel secondary transplantation and SRC assays. Compared with that in the control group, the mean engraftment level of human cells from primary recipients in the RIBE group showed a 3.7-fold decrease (Figure 3D). The majority of human cells were CD19+ B cells in the BM of all secondary NOG mice (supplemental Figure 2F). Next, LDA showed that 1 in 977 cells in the RIBE group clonally initiated long-term hematopoiesis compared with 1 in 638 cells in the control group (Figure 3E; supplemental Table 2). Moreover, the number of CFCs in the RIBE group significantly decreased compared with that in the control group (Figure 3F). Taken together, these data indicated that, although not as strong as the in vivo RIBE, the long-term repopulating capacity and clonogenic potential of human HSPCs were also dampened after exposure to irradiated BM cells, further confirming the negative bystander effects of irradiated BM cells on human HSPCs.
We next detected the cell-cycle status and apoptosis status of in vitro bystander human CD34+ cells and demonstrated cell-cycle arrest (Figure 3G; supplemental Figure 3A) and increased apoptosis in the in vitro bystander human HSPCs (Figure 3H; supplemental Figure 3B). Furthermore, cellular senescence detected by SA-β-gal staining showed that senescent cells accumulated in the RIBE group compared with that in control group (Figure 3I; supplemental Figure 3C). These results were confirmed by SA-β-gal enzymatic activity assay as shown in microscopic images (Figure 3J). Taken together, these data indicated that RIBE caused cell-cycle arrest, apoptosis, and senescence in human HSPCs.
Previous studies have confirmed that mitochondria are the main sources of ROS.29,30 Therefore, we further examined mitochondria and energy metabolism in bystander human HSPCs. Our data showed an elevated mitochondrial ROS level of in vitro bystander human CD34+ cells (Figure 3K; supplemental Figure 3D), resulting in impaired mitochondrial membrane potential (Figure 3L-M; supplemental Figure 3E-F). Furthermore, transmission electron microscopy analysis showed that the mitochondria in bystander human CD34+ cells were swollen and round, and the number of elongated mitochondria was significantly decreased, accompanied by prevalent mitophagosome formation (Figure 3N). Mitochondrial dysfunction leads to a reduction in adenosine triphosphate (ATP) synthesis because of the disruption of energy metabolism. We thus investigated whether the energy phenotype of bystander human CD34+ cells was altered. Their energy metabolism was generally reduced (Figure 3O), including aerobic respiration (indicated by extracellular oxygen consumption rate) (Figure 3P) and glycolysis (indicated by extracellular acidification rate) (Figure 3Q), which led to a reduction in intracellular ATP (Figure 3R). These results suggest that the mitochondria in bystander human HSPCs display severe dysfunction.
Excessive ROS in bystander human hematopoietic cells leads to DNA damage in HSPCs
Our data in Figures 1 and 3 showed that the damage to human HSPCs caused by the in vitro RIBE was consistent with the in vivo RIBE. Therefore, the in vitro model was appropriate for the study of detailed mechanisms. Our further studies showed that the ROS level was also elevated in the in vitro bystander HSPCs (Figures 4A; supplemental Figure 4A-B). To elucidate the molecular mechanism of RIBE, we performed RNA-sequencing of the in vitro human CD34+ cells in both the RIBE and control groups. Gene set enrichment analysis revealed that there was activation of the p53 signaling pathway, negative regulation of the cell cycle, and positive regulation of the apoptotic signaling pathway in HSPCs in the RIBE group (Figure 4B). Real-time quantitative polymerase chain reaction results showed the upregulation of DNA damage response (DDR) markers including ATM, CHK1, and CHK2, P53; the cell-cycle inhibitors P16INK4a and P21CIP1; and apoptosis-related caspases in bystander HSPCs (Figure 4C). Further analyses by western blot and immunofluorescence assays revealed cytological evidence of activated DDR in human HSPCs cocultured with irradiated BM. The activated forms of ATM, p53 binding protein, forkhead box O3a, γ-H2AX, among others, were detected by western blot or immunofluorescence (Figure 4D-E; supplemental Figure 4C). Furthermore, the protein expression of the cell-cycle and apoptotic signaling pathway confirmed that the activation of the p53 signaling pathway by DDR led to negative regulation of the cell-cycle and positive regulation of the apoptotic signaling pathway (Figure 4D-E; supplemental Figure 4C). ROS levels of HSPCs were elevated as soon as 30 minutes after coculture with irradiated BM (Figure 4F). However, the level of γ-H2AX was enhanced at about 2 hours after coculture (Figure 4G). Thus, our data suggested that the activation of a series of DDR pathways was initiated by increased ROS levels in bystander human HSPCs. Abundant evidence indicates that HSPCs are highly sensitive to ROS, which can cause oxidative DNA damage and lead to cell damage.21,23,31,32 Our data confirmed that RIBE induced excessive increases in ROS and oxidative DNA damage in bystander human HSPCs, which may lead to their exhaustion.
Excessive ROS in bystander human hematopoietic cells results in DNA damage in HSPCs. (A) Flow cytometric analysis of fold change of ROS levels by DCF-DA staining in human CD34+ cells of in vitro RIBE model. (B) Gene set enrichment analysis of the activation of the p53 signaling pathway, negative regulation of the cell cycle, and positive regulation of the apoptotic signaling pathway in human CD34+ cells of the in vitro RIBE model. (C) Real-time quantitative polymerase chain reaction analysis of the related genes in human CD34+ cells of in vitro RIBE model. (D) Western blot verified the expression of related signaling pathway at the protein levels. Each bar represents the mean ± standard deviation for biological triplicate experiments. (E) Human CD34+ cells of in vitro RIBE model were immunostained for p-p53, γ-H2AX, p-ATM, p-53BP1, and FOXO3a (p-p53, γ-H2AX, p-ATM, p-53BP1, and FOXO3a, green; 4′,6-diamidino-2-phenylindole, blue). Scatter plots represent foci per cell from each group (scale bars, 7 μm). (F) Flow cytometric analysis of fold change of ROS levels by DCF-DA staining of human CD34+ cells after different processing times. (G) γ-H2AX expression of CD34+ cells at the protein level after different processing times. (H) Relative RNA expression of cytokines of nonirradiated or irradiated human bone marrow cells (n = 3 per group). (I) Human cytokine array showed the relative expression of IL-1ra in nonirradiated or irradiated bone marrow supernatant; the right panel shows the quantification results. (J) γ-H2AX expression of CD34+ cells at the protein level in different cytokine-treated groups (*P < .05; **P < .01; ***P < .001).
Excessive ROS in bystander human hematopoietic cells results in DNA damage in HSPCs. (A) Flow cytometric analysis of fold change of ROS levels by DCF-DA staining in human CD34+ cells of in vitro RIBE model. (B) Gene set enrichment analysis of the activation of the p53 signaling pathway, negative regulation of the cell cycle, and positive regulation of the apoptotic signaling pathway in human CD34+ cells of the in vitro RIBE model. (C) Real-time quantitative polymerase chain reaction analysis of the related genes in human CD34+ cells of in vitro RIBE model. (D) Western blot verified the expression of related signaling pathway at the protein levels. Each bar represents the mean ± standard deviation for biological triplicate experiments. (E) Human CD34+ cells of in vitro RIBE model were immunostained for p-p53, γ-H2AX, p-ATM, p-53BP1, and FOXO3a (p-p53, γ-H2AX, p-ATM, p-53BP1, and FOXO3a, green; 4′,6-diamidino-2-phenylindole, blue). Scatter plots represent foci per cell from each group (scale bars, 7 μm). (F) Flow cytometric analysis of fold change of ROS levels by DCF-DA staining of human CD34+ cells after different processing times. (G) γ-H2AX expression of CD34+ cells at the protein level after different processing times. (H) Relative RNA expression of cytokines of nonirradiated or irradiated human bone marrow cells (n = 3 per group). (I) Human cytokine array showed the relative expression of IL-1ra in nonirradiated or irradiated bone marrow supernatant; the right panel shows the quantification results. (J) γ-H2AX expression of CD34+ cells at the protein level in different cytokine-treated groups (*P < .05; **P < .01; ***P < .001).
Cytokines may exert multiple functions involving stress and inflammatory responses, which are also associated with RIBE.33 We observed several cytokines including interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor-α were increased after irradiation, as reported33 (Figure 4H). Interestingly, the messenger RNA and protein levels of IL-1ra were significantly elevated after irradiation (Figure 4I). Moreover, we added IL-1α or IL-1ra to the in vitro culture system and then measured the γ-H2AX protein by western blot. Based on the protein levels, we found that IL-1α could lessen DNA damage of HSPCs both in the control and RIBE groups, and IL-1ra can slightly increase DNA damage of bystander HSPCs (Figure 4J). However, ROS levels of HSPCs did not change in either IL-1α– or IL-1ra–treated groups, suggesting that the alterations of DNA damage induced by these cytokines may not be attributable to ROS levels (supplemental Figure 4D).
Inhibition of ROS elevation rescues human HSCs from functional deterioration
Next, we asked whether the pharmacological inhibition of ROS elevation could protect human HSPCs from functional degradation. We used 3 antioxidants: N-acetyl-l-cysteine (NAC), sulforaphane (SF), and resveratrol (Res), to eliminate excessive ROS from bystander human HSPCs in vitro (Figure 5A-B). In addition, treatment with these antioxidants prevented DNA damage (Figure 5C-D) and reduced apoptosis (Figure 5E) of in vitro bystander human HSPCs. However, combination of these antioxidants did not exhibit further improvement (Figure 5B-E).
Inhibition of ROS elevation by antioxidant treatment in an in vitro RIBE model lessens the functional deterioration of human HSPCs. (A) Experimental diagram of in vitro antioxidant treatment RIBE model; human BM cells were treated with single antioxidant alone or in combination for 30 minutes before irradiation, and the antioxidants continued to exist in the coculture system for 40 hours. (B) Flow cytometric analysis of fold change of ROS levels by DCF-DA staining of human CD34+ cells from each group. (C-D) Human CD34+ cells from each group were immunostained for p-p53 and γ-H2AX (p-p53 and γ-H2AX, green; 4′,6-diamidino-2-phenylindole, blue). Scatter plots represent foci per cell from each group (scale bars, 7 μm). (E) Frequency of Annexin V+ cells of human CD34+ cells from each group. (F) Collected human CD34+ cells from panel A were transplanted into NOG mice. Mean human cell engraftment (left) was detected and bars represent the fold difference of engraftment levels from each group (right, n = 6-8 per group). Frequency of human (G) CD34+CD38− cells and (H) CD34+CD38+ cells in the BM of recipient. (I) Mean engraftment level of human cells in the spleen of recipients. (J) Number of hematopoietic clones formed by human CD34+ cells of each group (*P < .05; **P < .01; ***P < .001).
Inhibition of ROS elevation by antioxidant treatment in an in vitro RIBE model lessens the functional deterioration of human HSPCs. (A) Experimental diagram of in vitro antioxidant treatment RIBE model; human BM cells were treated with single antioxidant alone or in combination for 30 minutes before irradiation, and the antioxidants continued to exist in the coculture system for 40 hours. (B) Flow cytometric analysis of fold change of ROS levels by DCF-DA staining of human CD34+ cells from each group. (C-D) Human CD34+ cells from each group were immunostained for p-p53 and γ-H2AX (p-p53 and γ-H2AX, green; 4′,6-diamidino-2-phenylindole, blue). Scatter plots represent foci per cell from each group (scale bars, 7 μm). (E) Frequency of Annexin V+ cells of human CD34+ cells from each group. (F) Collected human CD34+ cells from panel A were transplanted into NOG mice. Mean human cell engraftment (left) was detected and bars represent the fold difference of engraftment levels from each group (right, n = 6-8 per group). Frequency of human (G) CD34+CD38− cells and (H) CD34+CD38+ cells in the BM of recipient. (I) Mean engraftment level of human cells in the spleen of recipients. (J) Number of hematopoietic clones formed by human CD34+ cells of each group (*P < .05; **P < .01; ***P < .001).
Then, we attempted to rescue bystander human HSPCs from functional exhaustion in long-term repopulation and clonogenic potential through treatment with antioxidants alone both in vitro and in vivo. For the in vitro RIBE, the BM cells were treated with NAC, SF, or Res for 30 minutes before irradiation, and the antioxidants continued to exist in the coculture system until the CB CD34+ cells were collected and transplanted into sublethally irradiated NOG mice intravenously (Figure 5A). Twenty weeks after transplantation, the engraftment of bystander human CD45+ cells was improved by antioxidants treatment (Figure 5F; supplemental Figure 5A). Furthermore, we found that antioxidants could also improve HSC-enriched cell engraftment, but only SF and Res could improve HPC engraftment (Figure 5G-H). The majority of human cells were CD19+ B cells in the BM of all NOG mice, whereas the Res group had significantly higher myeloid and erythroid (supplemental Figure 5B-C) engraftment than the RIBE group, suggesting that Res administration in vitro had positive effects on myeloid and erythroid differentiation, which was consistent with the observations of a previous study.34 As was observed in the BM, the antioxidants could also improve the in vitro bystander human cell engraftment in the SP (Figure 5I). Secondary transplantation demonstrated that antioxidants could partially improve human HSPC long-term repopulation compared with that in the RIBE group (supplemental Figure 5D). The LDA assay showed that HSPCs in antioxidant-treated groups contained more long-term repopulating cells than the RIBE group (supplemental Figure 5E; supplemental Table 5). Additionally, we used a CFC assay to evaluate the clonogenic potential of in vitro antioxidant-treated bystander CB-CD34+ cells. The number of CFCs significantly increased after antioxidant treatment (Figure 5J).
For the in vivo RIBE, the mice were treated with NAC, SF, or Res for 7 days. CB CD34+ cells were injected into nonirradiated or 10 Gy irradiated NOG mice in either the control, RIBE, or RIBE with antioxidant treatment groups. After 17 hours, the homed human CD34+ cells were sorted individually and transplanted into sublethally irradiated NOG mice (Figure 6A). At 20 weeks, the transplantation results demonstrated that these antioxidants could also improve in vivo bystander human cell engraftment (Figure 6B; supplemental Figure 6A). The engraftment of HSC-enriched cells was improved (Figure 6C), and unlike treatment in vitro, only Res could improve HPC engraftment in vivo (Figure 6D). Furthermore, antioxidants used in vivo showed that the majority of human cells in the BM were CD19+ B cells in NOG mice, and antioxidants could not improve the erythroid potential of human HSPCs within the in vivo RIBE model (supplemental Figure 6B-C). The antioxidants could also improve in vivo bystander human HSPC engraftment in the SP, as observed in the BM (Figure 6E). Moreover, we assessed the clonogenic potential of in vivo antioxidant-treated bystander CB CD34+ cells and found that the number of CFCs significantly increased after antioxidant treatment (Figure 6F). Secondary transplantation and LDA assay showed that antioxidants could partially improve human HSPC long-term repopulation activity damaged by RIBE (supplemental Figure 6D-E; supplemental Table 5). Taken together, our data show that the impaired long-term engraftment and clonogenic potential of bystander human HSPCs can be restored by antioxidants. These results show that the excessive ROS level is the primary cause of DNA damage in bystander human HSPCs, and the pharmacological inhibition of ROS elevation effectively prevents deterioration of human HSPC function both in vitro and in vivo.
Inhibition of ROS elevation protects the human HSPCs from functional deterioration in an in vivo RIBE model. (A) Experimental diagram of in vivo antioxidant treatment RIBE model. NOG mice were given antioxidants for 7 days before irradiation. All of the following procedures were consistent with those shown in Figure 1A (n = 4-7 per group). (B) Mean human cell engraftment (top) and bars represent the fold difference of engraftment levels between each group (bottom). (C-D) Frequency of human CD34+CD38− (C) and CD34+CD38+ (D) cells in the BM of recipient. (E) Mean engraftment level of human cells in the spleen of recipients. (F) Number of hematopoietic clones formed by human CD34+ cells of each group (*P < .05; **P < .01; ***P < .001).
Inhibition of ROS elevation protects the human HSPCs from functional deterioration in an in vivo RIBE model. (A) Experimental diagram of in vivo antioxidant treatment RIBE model. NOG mice were given antioxidants for 7 days before irradiation. All of the following procedures were consistent with those shown in Figure 1A (n = 4-7 per group). (B) Mean human cell engraftment (top) and bars represent the fold difference of engraftment levels between each group (bottom). (C-D) Frequency of human CD34+CD38− (C) and CD34+CD38+ (D) cells in the BM of recipient. (E) Mean engraftment level of human cells in the spleen of recipients. (F) Number of hematopoietic clones formed by human CD34+ cells of each group (*P < .05; **P < .01; ***P < .001).
Discussion
Human HSCs are uniquely able to be implanted into the BM of a recipient and to expand and provide long-term multilineage hematopoiesis. However, sustained and qualitative implantation of donor HSCs requires preconditioning, traditionally with radiation or chemotherapeutics, which not only ablates most of the hematopoietic and immune system of the host, but also exposes the implanted donor hematopoietic cells to bystander effects. Previously, we reported that RIBE significantly injured the long-term repopulating ability of transplanted mouse HSCs.12 However, RIBE on human HSCs have not been well studied. In this study, we found that RIBE also impaired human HSPCs, which showed that the long-term repopulating ability of bystander HSCs and the clonogenic capacity of bystander HPCs were significantly reduced. Our data showed that erythroid engraftment of bystander HSPCs was impaired in the NOG mice model. Although CD45−CD235a+ erythroid cells could be detected in the NOG mouse model, they were at very low levels.15 Human red blood cells were rejected by macrophages in immunodeficient mice,35 although an improved mouse model for red blood cell reconstitution has been reported.36,37 Further investigations of improving erythroid engraftment are needed. However, to our knowledge, the current study is the first to investigate RIBE on transplanted human hematopoietic cells.
Multiple mechanisms underlying RIBE have been reported, among which excessive ROS generation is one of the main culprits.33,38,39 For example, Lyng et al found that ROS was responsible for in vitro bystander cell death.40 Moreover, our previous study demonstrated that elevated ROS was at least partially responsible for the in vivo RIBE on mouse HSCs.12 Importantly, our current results showed that oxidative stress in bystander human hematopoietic cells leads to DDR, cell-cycle arrest, senescence, and p53-dependent apoptosis through the in vitro RIBE model. Excessive ROS produced by irradiation can lead to mitochondrial dysfunction, which in turn reduces ATP production, alters cellular metabolism, and induces apoptosis.41 Therefore, we investigated the mitochondria in bystander human HSPCs and found that they were severely impaired and greatly diminished, leading to reduced ATP production and altered cellular metabolism. Moreover, we found that IL-1α could partially weaken but IL-1ra tends to increase DNA damage of bystander human HSPCs. However, the opposite effects of these 2 cytokines appeared to be independent of ROS levels. Pietras et al42 previously showed that chronic IL-1 exposure negatively affects HSC function and promotes differentiation, and reverses the interruption of IL-1 exposure. It will be interesting to investigate the long-term effect and further mechanisms of acute increase of IL-1ra following RIBE.
Based on the mediating role of ROS in RIBE, we explored the effects of NAC, SF, and Res on ROS reduction and bystander human HSC engraftment. As reported previously, treatment with NAC significantly reduced the level of intracellular ROS and increased cell viability.43-45 Kong et al showed the effectiveness of preventive NAC therapy in the recovery of megakaryocytopoiesis and hematopoietic reconstitution after haploidentical transplantation (registered at www.clinicaltrials.gov as #NCT03236220 and #NCT02978274).46,47 SF can upregulate the expression of endogenous antioxidants through Nrf2 activation to protect against oxidative stress and damage.48 It has been suggested that Res not only promotes the expansion of CD34+ cells in vitro but also ameliorates irradiation-induced long-term BM injury by inhibiting oxidative stress in HSCs.49,50 In this study, we found that all the antioxidants above could reduce the level of intracellular ROS and rescue the impaired long-term engraftment of bystander human HSCs, although at different efficacies. However, combinations of these treatments showed similar effects to using a single antioxidant alone (Figure 5B-E). We suppose that these 3 antioxidants may reduce ROS levels via signaling pathways in a redundant manner. These results may suggest that simultaneously using 2 or more antioxidants in clinical bone marrow transplantations would not provide a further improvement of therapeutic effects. Taken together, these findings indicate the plausible usefulness of specific antioxidants in improving the engraftment of transplanted HSCs for relevant patients, although potential risks of promoting malignant cell growth must also be weighed.
In summary, our study presents definitive evidence for the adverse effects of RIBE on transplanted human HSCs and also has implications for improving the prognosis of the patients after radiotherapy. Because of the limitations of the xenograft mouse model, we investigated the mechanism of RIBE-induced human HSC injury also involving an in vitro model. However, the exact cell-to-cell contacts may differ between the in vivo and in vitro models, thereby accounting for the different functional readouts observed. In addition, we focused on RIBE on human HSCs in a very short time frame after exposure to radiation; thus, the long-term impact of RIBE on human HSCs has not been addressed. More systematic and comprehensive analyses for the short-term and long-term impacts of RIBE on engrafted human HSCs are needed in future studies. Nonetheless, given the nontoxic nature of many antioxidants, our current study justifies a strong rationale to conduct clinical trials on multiple antioxidants in preconditioning regimens for stem cell transplant patients in a broader scope.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Our present study encompasses RNA-seq data that have been uploaded to Gene Expression Omnibus (GEO) database and are accessible through GEO Series accession number GSE149649.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2016YFA0100600, 2015CB964400), the National Natural Science Foundation of China (81970104, 81890990, 81730006, 81421002, 81900113, 82070192, 81872195), Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (2019-I2M-1-006, 2016-I2M-1-017, 2017-I2M-1-015, 2017-I2M-B&R-04), Key Project of Tianjin Natural Science Foundation (20JCZDJC00410), and the Graduate Innovation Fund of PUMC (2017-1002-1-24).
Authorship
Contribution: T.C. conceptualized the study, supervised experiments, and reviewed the manuscript; L.H., X.Y., and Y. Zhang designed the research, performed most of the experiments, analyzed data, and wrote the manuscript; Y. Zhang performed RNA-sequencing library preparation; X.X. and C.Z. performed bioinformatics analysis; S.Y., Y.L., B.Z., Y.H., and Y.T. provided discussion of the data and reviewed the manuscript; M.W., W.C., S.C., Y. Zheng, and S.F. provided human bone marrow samples; Y.R. performed transmission electron microscopy assay; S.M., F.D., S.H., and H.C. advised parts of the study and reviewed the manuscript; A.P., S.F., and E.J. supervised experiments and reviewed the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tao Cheng, State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Collaborative Innovation Center for Cancer Medicine, 288 Nanjing Rd, Tianjin 300020, China; e-mail: chengtao@ihcams.ac.cn; and Erlie Jiang, State Key Laboratory of Experimental Hematology, Hematopoietic Stem Cell Transplantation Center, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences–Peking Union Medical College, 288 Nanjing Rd, Tianjin 300020, China; e-mail: jiangerlie@ihcams.ac.cn.
REFERENCES
Author notes
L.H., X.Y., Y. Zhang, and A.P. contributed equally to this work and are considered co-first authors.

![In vivo RIBE significantly reduce human long-term hematopoietic reconstitution and clonogenic ability. (A) Experimental diagram of in vivo RIBE model. (B) Mean human cell engraftment level in peripheral blood (PB) at different time points after transplantation. (C) Representative flow plots of human hematopoietic cell engraftment in the bone marrow (BM) 20 weeks after transplantation (RIBE vs control [Ctrl]: 14.19% ± 3.809% vs 38.70% ± 7.168%, P = .009). (D) Percentage of human cell engraftment in the BM of recipients. (E-F) Frequency of human CD34+CD38− (E) and CD34+CD38+ (F) cells in the BM of recipients. (G-H) Lineage differentiation potential (G) and frequency (H) of human CD45−CD235a+ cells in the BM of recipients; n = 10-11 per group (B-H). (I-J) Percentage of human cell engraftment (I) and lineage differentiation potential (J) of the secondary mice (RIBE vs Ctrl: 0.19% ± 0.028% vs 6.29% ± 2.586%, P = .041; n = 9-11 per group). (K) Frequency of human hematopoietic cells in the recipients measured by LDA (n = 7-11 per group). (L) Number of hematopoietic colonies formed by hCD34+ cells in each group (n = 5 per group) (3 independent experiments; *P < .05; **P < .01; ***P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/137/24/10.1182_blood.2020007362/1/m_bloodbld2020007362f1.png?Expires=1765883730&Signature=2XgSnjqTapSIL2IK1jik76Ymi6zxzTsndZdbFx4Q2L5BSb6OiiwmchzlyMz5P3sB3LyQUpoCnPNmuMTtHc71NGi26nkQVoG5NjubOHTgt17D5hw6SoOzyUfmGtRloSTaRa3nCCdGNMvkW7g5mZNjc-eDcqg9lhh2QUleOVuqVB4dSybXDjTGanUTQFEpgYxfEFT9pIcv6DApL9m3rIWm-jx7GNnv4uSIyuWt-Nr64-Of8LNT6uz2EUm9cKEGiUPIpoQXgRSAVYeyAIs5GoTET4QoCOo7UsI1Avx8eBUkqhgXVOn~qYIxQOxt-hTEDJlPALGa9NZitvbU5FG6-UE6hA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![In vitro RIBE lead to defects of human HSPCs. (A) Experimental design of in vitro RIBE model. CB-CD34+ cells were cocultured with irradiated or nonirradiated human bone marrow (BM) cells via transwell system. After 17 hours, CD34+ cells were collected for subsequent experiments. (B-C) Collected human CD34+ cells were transplanted into NOG mice. The frequency of human cell engraftment (B) and the lineage differentiation potential (C) were assessed in BM 20 weeks later (RIBE vs control [Ctrl]: 9.69% ± 1.903% vs 18.50% ± 3.301%, P = .023; n = 31 to 32 per group, 3 independent experiments). (D) Mean human cell engraftment in the BM of the secondary mice (RIBE vs Ctrl, 3.87 ± 1.494 vs 14.35 ± 4.430, P = .025; n = 15-17 per group). (E) Frequency of human cells in the recipient mice measured by LDA (n = 5-9 per group). (F) Number of hematopoietic colonies formed by human CD34+ cells in each group (n = 5 per group). (G) Proportion of BrdU+ cells of the human CD34+ cells from each group. BrdU was incubated with human CD34+ cells for 4 hours (n = 5 per group). (H) Frequency of early apoptotic cells (Annexin V+7AAD−) and late apoptotic cells (Annexin V+7AAD+) of human CD34+ cells from each group (n = 5 per group). (I) Fold change of SA-β-gal activity in human CD34+ cells was analyzed using C12FDG as a substrate from each group (n = 5 per group). (J) Difference of SA-β-gal activity of human CD34+ cells from each group was confirmed by the SA-β-gal enzyme activity assay as shown in the microscopic images, and bars represent the proportion of blue stained cells. (K) Flow cytometric analysis of fold change in mitochondrial ROS levels by MitoSOX staining in human hematopoietic cells (n = 5 per group). (L-M) Fold change of mitochondrial membrane potential of human CD34+ cells determined by flow cytometry with TMRE (L) and DilC1(5) staining (M) (n = 5 per group). (N) Transmission electron microscopy (TEM) analysis of the mitochondria damage in human CD34+ cells of in vitro RIBE model. (O-Q) The energy phenotype of CD34+ cells in the in vitro RIBE model (O), the oxygen consumption rate (OCR) (P), and extracellular acidification rate (ECAR) (Q) were assayed by Seahorse assay (n = 5 per group). (R) Relative ATP levels in human CD34+ cells of in vitro RIBE model (n = 5 per group) (*P < .05; **P < .01; ***P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/137/24/10.1182_blood.2020007362/1/m_bloodbld2020007362f3.png?Expires=1765883730&Signature=4PZJ-EFDoHc83jAVq-1B5wMbHZKoL2XJZp6eretNQvUXLCM6e7NhisCAf6sFZzch3jKRX12iN4lccErjIU4cI-UQj1zzualKkxL~5ir0dZSRE~K0bwRDCTPL4l-XHsEDLaVtxKckPT~-YYWnh4x70ccJugkjAKkp6~PkwZawmQPd6ZWmpL14ZcgWONPvSCkLhKYvQlP9YiEor~CWuxqECSAMNbd5mvE6hhh~~oRWqiHIr13LrKDKy3QE8FAF46cJZFBDaVq1U4xnFOf9mZD9e~KlswMBqI1twlCf~1uKBL80RFPqmgPxrKBX-Zlx-ZrwbxAqkYH9Oh6e-gVGhovyMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal