Abstract
Treatment outcomes for pediatric patients with acute myeloid leukemia (AML) have continued to lag behind outcomes reported for children with acute lymphoblastic leukemia (ALL), in part because of the heterogeneity of the disease, a paucity of targeted therapies, and the relatively slow development of immunotherapy compared with ALL. In addition, we have reached the limits of treatment intensity, and, even with outstanding supportive care, it is highly unlikely that further intensification of conventional chemotherapy alone will impact relapse rates. However, comprehensive genomic analyses and a more thorough characterization of the leukemic stem cell have provided insights that should lead to tailored and more effective therapies in the near future. In addition, new therapies are finally emerging, including the BCL-2 inhibitor venetoclax, CD33- and CD123-directed chimeric antigen receptor T-cell therapy, CD123-directed antibody therapy, and menin inhibitors. Here, we present 4 cases to illustrate some of the controversies regarding the optimal treatment of children with newly diagnosed or relapsed AML.
Introduction
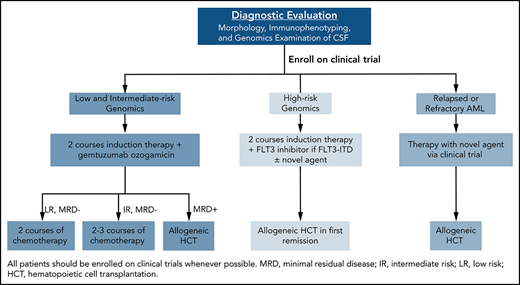
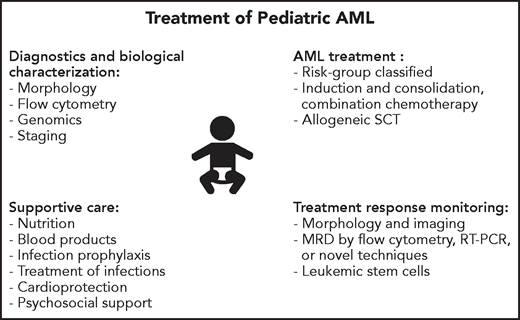
Survival rates for children with acute myeloid leukemia (AML) treated on clinical trials in the late 1990s and early 2000s reached 70% in high-income countries, primarily through improvements in supportive care and the adaptation of therapy based on genomics and response to therapy. However, despite a better understanding of the biology of AML,1-6 modest progress in treatment outcome has been made in the past 10 years.7,8 Nevertheless, the development of a broad spectrum of new agents, as well as further advances in supportive care and allogeneic hematopoietic cell transplantation (allo-HCT), provide great hope. Important questions faced by pediatric oncologists today include how to rapidly identify targetable lesions and incorporate targeted agents, by what method should response to therapy be assessed, how should the activity of certain agents be balanced against their potential late effects, and for which patients should allo-HCT be used. In this review, we present 4 patients to demonstrate our approach to the treatment of childhood AML (Figure 1). We refer the reader to a previous review for a more thorough description of the results and implications of clinical trials conducted by pediatric AML consortia.8
Overview of key components of the evaluation and treatment of children with AML.
Overview of key components of the evaluation and treatment of children with AML.
Patient 1: intermediate (standard)-risk AML with no targetable lesions
A 10-year-old boy presented with a 1-week history of cough, congestion, and excessive bruising. A complete blood count obtained in the local emergency department revealed a leukocyte count of 48 × 103/μL, hemoglobin concentration of 10.6 g/dL, and a platelet count of 51 × 103 /μL, prompting a referral to our institution. A review of the bone marrow aspirate revealed a diagnosis of AML with maturation (French-American-British [FAB] classification M2 without Auer rods), with 43% blasts that were positive for CD33, CD11c, CD34, CD117, CD133, CD13, and myeloperoxidase. Cytogenetic analysis revealed 47,XY,+6, transcriptome (RNA-sequencing) analysis identified no recurrent fusion transcripts, and next-generation sequencing was positive for alterations in NRAS and BCORL1.
Issues to consider when deciding treatment of this patient include risk classification, the incorporation of gemtuzumab ozogamicin (GO), the number of courses of therapy, and the optimal use of conventional agents.8 Risk classification of this patient, whose leukemic blasts contain trisomy 6 and mutations of NRAS and BCORL1, lack favorable or unfavorable genetic features, is best determined by careful measurements of his response to therapy. Whereas conventional morphologic examination of the bone marrow is insensitive and can be imprecise, minimal residual disease (MRD) assays that distinguish residual leukemia cells from normal hematopoietic precursors provide specific and sensitive assessments of response. These assays include flow cytometric detection of leukemia-specific immunophenotypes9 or abnormal phenotypes,10 RT-PCR detection of fusion transcripts, and next-generation sequencing to measure clearance of leukemia-associated variants.11-13
Flow cytometric assessment of MRD has been used prospectively to adapt therapy14 and has been analyzed retrospectively in several clinical trials for childhood AML.10,15,16 In the St Jude AML02 trial, in which flow cytometric detection of leukemia-associated immunophenotypes was used to assess response to therapy, MRD levels >1% at the end of induction 1 (EOI1) were associated with a poor outcome, whereas the outcome of patients with MRD levels 0.1% to 1% was similar to that of patients with negative MRD.14 However, any detectable MRD at end of induction 2 (EOI2) was associated with a high risk of relapse. Similarly, in the Nordic Society for Pediatric Haematology and Oncology (NOPHO) AML 200415 study, detectable MRD at EOI2 was the strongest predictor of outcome, and results of the AML-BFM 98 trial17 showed that MRD was a significant prognostic factor even in analyses limited to patients without morphologically detectable blasts. Investigators from the Children’s Oncology Group (COG) recently reported that among patients treated on the AAML0531 trial, in which MRD was assessed by the “different from normal” flow cytometric method, detection of any MRD (>0.02%) at EOI1 was associated with an increased risk of relapse and inferior overall survival.10 In this study, there were no differences in outcome between patients with low (0.1% to 1%) or high levels (1% to 5%) of MRD at EOI1 or between EOI1 MRD- positive patients who cleared their MRD after induction 2 compared with those with persistent MRD.10 A meta-analysis confirmed the predictive value of MRD in AML across age groups and methods.18 There is now uniform agreement that assessment of response by MRD, if available, should be used to tailor therapy according to the clinical trial on which the patient is treated.19 It should be noted, however, that few studies actually demonstrate that intensification of therapy based on MRD results in improvement in outcome. We suggest that this failure represents limited treatment options for patients with persistent MRD rather than a failure of the techniques. In addition, the optimal cutoff values and time points of assessment have not been established. We suggest that study groups agree on an approach that allows comparisons of MRD data among clinical trials. In addition, there is a need to further improve the sensitivity of MRD measurements, since many patients who achieve MRD negativity still relapse. Monitoring with techniques other than flow cytometry may be helpful in this respect and so might be the identification of leukemic stem cells in bone marrow during treatment. In adult AML, the latter was shown to add prognostic impact to conventional MRD detection.20
Based on the results of the COG AAML0531 trial,21 the Food and Drug Administration approved GO for the treatment of pediatric patients with newly diagnosed CD33-positive AML in June 2020. In this trial, GO was associated with an improvement in event-free survival from 47% to 53% but did not impact overall survival, except in specific subgroups.21 GO was particularly beneficial for the majority of patients with high (second through fourth quartiles) CD33 expression22 or those with the CC CD33 splicing polymorphism.23 In adults with AML, GO is associated with improved outcome among patients with core-binding-factor leukemia, intermediate-risk (designated standard-risk by some investigators) AML with activating signaling mutations, or NPM1 mutations, together comprising approximately two-thirds of adult patients with AML.24-26 Importantly, in pediatric and adult trials, patients with high-risk disease did not benefit from GO. Together, these results indicate that GO should be given only to patient subgroups who are most likely to benefit, ideally in the context of a clinical trial. Any benefit of GO may also depend on the backbone of treatment. In the context of a more effective backbone, it is less likely that GO will impart a clinical benefit.
The number of courses of chemotherapy needed to minimize the risk of relapse without increasing toxicity remains controversial. Based on the results of the Medical Research Counicl (MRC) AML trials,27,28 in which relapse and overall survival rates were the same for children who received 4 or 5 courses of chemotherapy, 4 courses of therapy were given to patients enrolled on the St Jude AML0829 and COG AAML103130 trials, except for those who underwent allo-HCT. However, current US-based trials (AML16 and AAML1831) give 4 courses only to patients with favorable genetics who are MRD negative at EOI1, whereas all other patients receive 5 courses because of poor outcome with only 4 courses in these previous, nonrandomized trials. In Europe and Asia, many cooperative groups have continued to recommend 5 courses of chemotherapy, although the MyeChild 01 trial prescribes 4 courses. Clearly, the optimal number of courses for each subgroup remains controversial.7,8,31,32 Moreover, the optimal number of courses needed to minimize relapse without increasing toxicity likely depends on the intensity and efficacy of induction therapy, the components of consolidation therapy, and the proportion of patients allocated to allo-HCT in first remission.
Anthracyclines are a key component of AML therapy, but are associated with dose-dependent late cardiotoxicity, leading to significant morbidity and mortality in AML survivors.33-38 In addition, early treatment-related cardiotoxicity has been reported to be associated with a worse outcome.38 On most cooperative trials, children with AML receive >350 mg/m2 cumulative doses of doxorubicin equivalents, which may actually be an underestimate for patients who receive mitoxantrone, as recent data suggest that the equivalence ratio for mitoxantrone to doxorubicin might be as high as 10.5.36 Two approaches can be considered to reduce the risk of cardiotoxicity. In the AML08 trial, we demonstrated that we could replace 150 mg/m2 of daunorubicin with clofarabine during induction therapy without negatively impacting event-free or overall survival.29 Nevertheless, we do not recommend the routine use of clofarabine to reduce anthracycline exposure. An alternative approach is to use the cardioprotectant dexrazoxane, which was associated with preserved cardiac function in a small subset of patients treated on the AAML1031 trial.37 However, there is not sufficient data specific to pediatric AML to recommend the routine use of dexrazoxane in this disease. Thus, the total anthracycline dose, the choice of anthracycline, and the use of dexrazoxane remain controversial.8
The use of etoposide also varies among study groups. Based on the results of the MRC AML15 trial, in which adult patients with AML who were randomly assigned to receive daunorubicin and cytarabine or daunorubicin, cytarabine, and etoposide during induction had similar complete remission, overall survival, and relapse rates, the current MRC (MyeChild 01) and COG (AAML1831) trials do not include etoposide. However, the activity of etoposide is clearly demonstrated in the ongoing NOPHO Dutch, Belgium, Hong Kong (DBH) AML-2012 study, in which hundreds of children have received single-agent etoposide at 150 mg/m2 once daily for 5 consecutive days with activity in all patients (Gertjan J. L. Kaspers, Princess Máxima Center For Pediatric Oncology, written communication). The Japanese group applied this regimen very successfully, with a 96% complete response (CR) rate.39 Moreover, children may be more likely to have AML cells that are sensitive to etoposide,40 perhaps because of having a different distribution of biological subtypes. Indeed, children with t(8;21) seem to had benefited from higher cumulative doses of etoposide at both induction and consolidation.41 Some investigators are therefore reluctant to remove an active agent from treatment regimens for a disease in which relapse remains the major cause of failure. Although we acknowledge that there may be a small risk of treatment-related AML, we currently include etoposide in our clinical trials and recommend it as part of standard of care for childhood AML.
Because central nervous system (CNS) relapse occurs in <5% of cases, it has not been possible to conduct randomize trials to define the best strategy to prevent this event. Nevertheless, all cooperative group trials routinely administer intrathecal chemotherapy, generally consisting of cytarabine or the combination of cytarabine, methotrexate, and hydrocortisone.42 However, some groups, including the NOPHO-DBH consortium, use single-agent intrathecal methotrexate, with apparently similar efficacy.43 The results of a retrospective analysis of patients with CNS involvement at the time of diagnosis suggests that intensified intrathecal is warranted, but the addition of cranial irradiation was not beneficial.44 We recommend one dose of intrathecal therapy prior to each course of systemic therapy for patients without CNS leukemia at the time of diagnosis and at least 8 doses (4 weekly doses followed by 4 monthly doses) for patients with CNS disease.
Treatment summary
The patient was enrolled on the St Jude AML16 clinical trial and was MRD negative by flow cytometry at EOI1. He was therefore considered to be at intermediate (standard) risk of relapse, completed 5 courses of chemotherapy, and remains in complete remission 2 years after completing therapy.
Patient 2: KMT2A-rearranged AML
This 12-year-old boy presented to the local clinic with a 2-week history of intermittent fevers, fatigue, and malaise. Initial complete blood count was significant for a leukocyte count of 17 × 103/μL, hemoglobin concentration of 3.4 g/dL, and a platelet count of 99 × 103/μL. His bone marrow contained 90% blasts that were positive for CD33, CD11c, CD34, CD117, CD133, CD13, CD11b, and CD7 and negative for cCD3, CD19, and myeloperoxidase, confirming a diagnosis of AML with minimal differentiation (FAB classification M0). His leukemia was characterized by the t(6;11)(q27;q23) and was positive for KMT2A-MLLT4.
Rearrangements of KMT2A, which involve over 100 partner genes, are seen in both AML and acute lymphoblastic leukemia (ALL), with particularly high prevalence in treatment-related AML and infant ALL.45 Among children with AML, the prognostic significance of most KMT2A fusions is not certain, although most investigators agree that t(4;11)(q21;q23.3)/KMT2A-MLLT2, t(6;11)(q27;q23)/KMT2A-MLLT4, t(10;11)(p12;q23)/KMT2A-AF10 and t(10;11)(p11.2;q23)/KMT2A-ABI1 are associated with a poor prognosis.46,47 The results of a recent study suggest that patients with reciprocal translocations have a favorable outcome, but this finding will need to be confirmed before it can be used clinically.48 Currently, many study groups consider patients whose blasts harbor any of the 4 fusions mentioned above to be at high risk of relapse and candidates for allo-HCT in first remission, whereas patients with other KMT2A fusions are stratified based on MRD. However, there is a remarkable lack of consensus on this topic. For example, some groups transplant patients with KMT2A-MLLT2 regardless of response, while other groups take MRD assessments into account. Similarly, this particular patient would not be eligible for allo-HCT based on the KMT2A-MLLT4 alone according to the NOPHO-DBH consortium approach, but is considered high-risk by other study groups.
The role of allo-HCT in first remission has been recently reviewed and remains a topic of intense debate.49 As the list of genetic abnormalities that are associated with a poor outcome grows, many cooperative groups have expanded the indications for allo-HCT to include many of the lesions in Table 1, the RAM phenotype,50 and low levels of MRD. As a result, nearly 50% of patients enrolled on the current US trials (AML16 and AAML1831) are classified as high risk and are candidates for allo-HCT in first remission. In contrast, on the DB-AML-01 trial, allo-HCT was not recommended for any patient who achieved remission after 2 courses of therapy.31 Although the relapse rate was nearly 40%, the 3-year overall survival of 74% was equivalent to or better than other contemporary trials.29,30 It must be noted that having a biological subtype associated with an unfavorable outcome with current chemotherapy does not imply that this will remain the case with improved chemotherapy or that allo-HCT will improve outcome. The approach taken in the DB-AML01 trial will likely lead to a lower number of patients ultimately being transplanted, which might translate into fewer late effects of treatment and a higher quality of life.44 Long-term follow-up of all patients, including those who need salvage treatment, and taking these factors into account are required to address this issue of indications for allo-HCT in first remission.
Genetic abnormalities with prognostic significance
| Favorable genetic lesions |
| t(8;21 )(q22;q22)/RUNX1-RUNX1T1 |
| inv(16)(p13.1;q22)/t(16;16)(p13.1;q22)/CBFB-MYH11 |
| NPM1 mutation with or without FLT3-ITD |
| CEBPA mutation with or without FLT3-ITD |
| Unfavorable genetic lesions |
| inv(16)(p13.3q24.3)/CBFA2T3-GLIS2 |
| t(10;11)(p12;q23)/KMT2A-AF10 |
| t(10;11)(p11.2;q23)/KMT2A-ABI1 |
| t(6;11)(q27;q23)/KMT2A-MLLT4 |
| t(4;11)(q21;q23.3)/KMT2A-MLLT2 |
| t(11;12)(p15;p13)/NUP98-KDM5A |
| t(7;11)(p15.4;p15)/NUP98-HOXA9 |
| t(5;11)(q35;p15)/NUP98-NSD1 |
| t(6;9)(p23;q34)/DEK-NUP214 |
| t(8;16)(p11;p13)/KAT6A-CREBBP |
| t(16;21)(q24;q22)/RUNX1-CBFA2T3 |
| t(7;12)(q36;p13)/ETV6-HLXB |
| t(3;21)(26.2;q22)/RUNX1-MECOM |
| t(16;21)(p11.2;q22.2)/ FUS-ERG |
| FLT3-ITD without CEPBA or NPM1 mutation |
| inv(3)(q21.3q26.2)/t(3;3)(q21.3q26.2)/RPN1-MECOM |
| t(3;5)(q25;q34)/NPM1-MLF1 |
| t(10;11)(p12.3;q14.2)/PICALM-MLLT10 |
| −7, −5, 5q− |
| Favorable genetic lesions |
| t(8;21 )(q22;q22)/RUNX1-RUNX1T1 |
| inv(16)(p13.1;q22)/t(16;16)(p13.1;q22)/CBFB-MYH11 |
| NPM1 mutation with or without FLT3-ITD |
| CEBPA mutation with or without FLT3-ITD |
| Unfavorable genetic lesions |
| inv(16)(p13.3q24.3)/CBFA2T3-GLIS2 |
| t(10;11)(p12;q23)/KMT2A-AF10 |
| t(10;11)(p11.2;q23)/KMT2A-ABI1 |
| t(6;11)(q27;q23)/KMT2A-MLLT4 |
| t(4;11)(q21;q23.3)/KMT2A-MLLT2 |
| t(11;12)(p15;p13)/NUP98-KDM5A |
| t(7;11)(p15.4;p15)/NUP98-HOXA9 |
| t(5;11)(q35;p15)/NUP98-NSD1 |
| t(6;9)(p23;q34)/DEK-NUP214 |
| t(8;16)(p11;p13)/KAT6A-CREBBP |
| t(16;21)(q24;q22)/RUNX1-CBFA2T3 |
| t(7;12)(q36;p13)/ETV6-HLXB |
| t(3;21)(26.2;q22)/RUNX1-MECOM |
| t(16;21)(p11.2;q22.2)/ FUS-ERG |
| FLT3-ITD without CEPBA or NPM1 mutation |
| inv(3)(q21.3q26.2)/t(3;3)(q21.3q26.2)/RPN1-MECOM |
| t(3;5)(q25;q34)/NPM1-MLF1 |
| t(10;11)(p12.3;q14.2)/PICALM-MLLT10 |
| −7, −5, 5q− |
ITD, internal tandem duplication.
Treatment summary
This patient was enrolled on AML16 and initially received azacitidine 75 mg/m2 per day for 5 days followed by cytarabine (100 mg/m2 every 12 hours on days 1-10), daunorubicin (50 mg/m2 on days 1, 3, and 5), and etoposide (100 mg/m2 on days 1-5) but had a very poor response, with 81% residual disease at EOI1. His MRD decreased to 0.1% after treatment with azacitidine, idarubicin (8 mg/m2 on days 3-5), fludarabine (30 mg/m2 on days 1-5), and cytarabine (2 g/m2 on days 1-5) and became undetectable after receiving azacitidine, mitoxantrone (12 mg/m2 on days 3-5), and cytarabine (1 g/m2 every 12 hours on days 1-4 ). He then underwent matched unrelated donor allo-HCT but relapsed ∼300 days after transplant, demonstrating that allo-HCT may not always overcome the underlying biology associated with high-risk leukemia.
Patient 3: refractory/relapsed AML
A 7-year-old girl was diagnosed with AML with normal karyotype, no recurrent gene fusions, and mutations in WT1 and NPM1; she was treated on the COG AAML1031 protocol, relapsed 6 months after completing therapy, achieved second remission, and underwent matched-sibling donor allo-HCT. However, she suffered a second relapse and was then refractory to treatment with decitabine, vorinostat, fludarabine, and cytarabine on trial NCT02412475, with 40% residual disease, and was referred to St Jude.
Until recently, minimal progress had been made in the treatment of relapsed pediatric AML, in part because of a lack of enrollment of such patients on clinical trials. We estimated that in the Untied States and Canada, <25% of children with relapsed AML were treated on clinical trials between 2006 and 2016.51 In addition, a literature review of this topic identified only 12 publications, of which a single one reported the results of a randomized trial.52,53 Fortunately, the landscape is changing as new agents become available. For example, we demonstrated that selinexor, a selective inhibitor of nuclear export, is safe and active when combined with fludarabine and cytarabine in patients with relapsed AML.54 The remarkable single-agent activity of selinexor in a subset of these patients indicates that there is a subgroup of patients who may benefit from this agent, even in the absence of cytotoxic chemotherapy. However, the lack of biomarkers to identify which patients will respond to selinexor has prevented further development of this agent in pediatric AML. Investigators from the COG reported that Vyxeos (CPX-351), a liposomal formulation of daunorubicin and cytarabine, was safe and active in children with relapsed AML.55 However, because treatment was limited to patients in first, untreated relapse, the activity of Vyxeos in refractory patients or those in later relapses is unknown. Recently, we recently reported the results of VENAML, a phase 1 study of the BCL2 inhibitor, venetoclax, in combination with chemotherapy in pediatric patients with relapsed or refractory AML.56 We demonstrated that venetoclax could be safely combined with low-dose or high-dose chemotherapy and identified venetoclax 360 mg/m2 per day combined with cytarabine (1000 mg/m2 per dose for 8 doses) with or without 1 dose of idarubicin (12 mg/m2) as the recommended phase 2 dose. Although many of the patients were in second or greater relapse and others were refractory to salvage therapy, 14 (70%) of the 20 evaluable patients who were treated at the recommended phase 2 dose achieved CR or CR with incomplete count recovery after 1 cycle of therapy, including 10 who became MRD negative.56 The promising results of our study, as well as extensive safety and activity data from clinical trials in adults,57-60 suggest that venetoclax should be studied in carefully designed clinical trials in children with newly diagnosed and relapsed AML.
Targeted therapies that are in early clinical development include menin inhibitors, which disrupt interactions between menin and KMT2A and have potent activity in mouse xenograft models derived from human KMT2A-rearranged leukemias.61 Two menin inhibitors are in early-phase trials in adults (NCT04067336 and NCT04065399) and are expected to move into pediatric trials in 2021. Like venetoclax, menin inhibitors should only be considered in the context of clinical trials. For patients with IDH mutations, ivosidenib62 and enasidenib63 have proven to be safe and active in adults, but, because of the rarity of these mutations in children with AML, safety has not yet been established. However, investigators from the COG are currently testing enasidenib in patients with relapsed or refractory IDH2-mutated AML (NCT04203316). Many FLT3 inhibitors are commercially available and are considered standard of care for newly diagnosed and relapsed patients with FLT3 activating mutations.64 Although there are no active trials of FLT3 inhibitors in pediatric patients with relapsed AML in the United States, quizartinib is being evaluated in children with relapsed or refractory FLT3-ITD–positive AML in Europe, while gilteritinib is being investigated in the COG AAML1831 trial in newly diagnosed children with this AML subtype.
The lack of known leukemia-specific antigens that are not expressed on normal hematopoietic precursors has hampered the development of immunotherapy for AML, which lags significantly behind the advances in immunotherapy for ALL.65 The targets against which most current efforts are directed are CD33 and CD123, both of which are expressed in ∼90% of AML cases. CD123 is also expressed in precursor B-cell ALL, some cases of T-cell ALL, and, most importantly, on AML leukemic stem cells. Although CD123 is expressed on normal hematopoietic precursors, its lower expression on normal stem cells compared with leukemic stem cells may offer a therapeutic window. The safety and activity of GO against CD33-positive AML has led to the development of CD33-directed chimeric antigen receptor (CAR) T-cell therapy, including a pediatric trial (NCT03971799) that is recruiting patients at the Children’s Hospital of Philadelphia and the National Cancer Institute. CD123-directed therapies include flotetuzumab, a CD123 × CD3 bispecific designed to target CD123-positive blasts for recognition by CD3-positive T cells66; CD123-targeted CAR T-cell therapy; IMGN632, in which a humanized anti-CD123 antibody is conjugated to indolino-benzodiazepine dimers67; and tagraxofusp (SL-401), a CD123-directed protein consisting of human interleukin-3 fused to truncated diphtheria toxin.68 Flotetuzumab is currently being investigated in the PEPN1812 trial (NCT04158739), which is open for enrollment at many sites in the United States, while the CATCHAML trial (NCT04318678) is exploring CD123-directed CAR therapy at St Jude. Clinical trials of IMGN632 and tagraxofusp are expected to open in 2021.
Treatment summary
Evaluation here confirmed relapse of NPM1-mutated AML with complex karyotype, and she was enrolled on the VENAML clinical trial. After receiving 7 days of single-agent venetoclax, her MRD decreased to 0.3%. After completing 28 days of venetoclax and 8 doses of cytarabine, she became MRD negative with complete blood count recovery, proceeded to haploidentical allo-HCT, and remains in MRD-negative remission 18 months after transplant.
Patient 4: AML case with invasive fungal infection
A 6-year-old girl was diagnosed with AML with maturation (FAB classification M2) with alterations in BRAF, CIC, POLD1, SD3B1, and TET2; she was enrolled on AML16 and achieved MRD negativity after 1 course of induction chemotherapy. After receiving her second course of therapy, she fell and suffered an abrasion of her right knee. She developed fever and local erythema and was therefore treated with cefepime and vancomycin while continuing to receive prophylactic voriconazole. However, because her systemic and local symptoms persisted, she underwent a skin biopsy that showed epidermal hyperplasia, mild perivascular inflammation, and fungal hyphae without evidence of septation. Because Mucor was suspected, voriconazole was discontinued, and the patient was started on Ambisome and posaconazole. Importantly, she underwent a wide excision of the region that revealed invasive aseptate fungal infection involving the dermis; cultures confirmed the presence of Mucor species. A repeat biopsy 1 week later was negative for fungus, and the patient subsequently underwent a skin graft.
Despite improvements in supportive care, early death and treatment-related death in remission remain important causes of treatment failure in AML, occurring in 5% to 10% of patients.69 In addition, the cumulative incidence of any grade 3 or 4 infection may be >50%.29,70
Although 1 report from the COG indicated that the incidence of viridans group streptococcal infections, hypoxia and hypotension were higher among patients who were discharged after completion of chemotherapy,71 and another study showed that mandatory hospitalization reduced median hospital stay but did not reduce mortality.72 Of note, prophylactic antimicrobials, given prior to the onset of fever or suspected infection, may reduce the risk of bacterial infections. We previously demonstrated that systemic antibacterial prophylaxis with vancomycin (given IV as oral absorption is very poor) plus ciprofloxacin, or levofloxacin alone, is effective at preventing bacterial infections and severe sepsis in pediatric patients with AML.73,74 Similarly, Boztug et al reported that teicoplanin on alternate days significantly reduced the incidence of febrile neutropenia and viridans sepsis.75 However, it should be noted that these studies were not conducted in a randomized fashion. In contrast, a large, open-label, randomized trial conducted by the COG demonstrated that levofloxacin prophylaxis administered during the neutropenic period after each of 2 cycles of chemotherapy in patients with acute leukemia reduced the risk of bacteremia from 43% to 22% and also reduced other suspected or proven bacterial infections, although there were no deaths from bacterial sepsis in either group.76 Importantly, there were no differences in Clostridioides difficile–related diarrhea or invasive fungal infection between groups. Although adoption of routine antibacterial prophylaxis has been limited by concerns about the long-term impact of prolonged exposure to these agents on antibiotic resistance, the impact of prophylaxis on antibiotic resistance is unclear. The most consistent finding across various studies is that resistance to the antibiotic used for prophylaxis is increased, but there is no conclusive evidence of cross-resistance to other antibiotics. Currently, antibiotic prophylaxis with levofloxacin is strongly recommended for all patients treated on the COG AAML1831 trial. However, because many episodes of viridans group streptococcal infections were reported among patients treated with levofloxacin,76 prophylaxis with vancomycin and ciprofloxacin is recommended for patients treated on the St Jude AML16 trial. In Europe, the use of prophylactic antibiotics varies by institution and may include gram-negative prophylaxis, gram-positive prophylaxis, or both.77 The NOPHO-DB-SHIP consortium will soon investigate the usefulness of teicoplanin (a glycopeptide) 3 times per week for prophylaxis of viridans group streptococcal blood stream infections.
Patients with AML are at especially high risk of fungal infections, including candidiasis, aspergillosis, and mucormycosis. Recommendations for antifungal prophylaxis are based on primarily on randomized trials conducted in adults and have been summarized elsewhere.77-79 Effective regimens include echinocandins (caspofungin and micafungin) or azoles. Some investigators prefer voriconazole or posaconazole rather than fluconazole or itraconazole because of better absorption or broader activity against Aspergillus species and molds, but these preferences are not based on randomized trials and practices vary across institutions.77 When voriconazole or posaconazole are used, monitoring of drug levels to ensure that therapeutic levels are reached and maintained should be performed regularly. In addition, voriconazole and posaconazole are strong CYP3A inhibitors and must be avoided or used cautiously when given concomitantly with chemotherapy agents that are metabolized by CYP3A4.
Treatment summary
Whenever possible, treatment of invasive fungal infections should include emergent local control and systemic treatment. As in this patient, local control may include extensive excision if possible, depending on the location of the infection as well as the organism and the likelihood of control with antifungal treatment only. Although there is no evidence that coverage with 2 antifungal agents is beneficial, we recommend starting liposomal amphotericin (Ambisome) and posaconazole, only discontinuing Ambisome after therapeutic levels of posaconazole are documented. This patient remained on posaconazole throughout her 3 remaining courses of therapy, completed therapy in July 2020, and is doing well. With more effective antifungal treatment being available nowadays, it is often possible to control fungal infection and simultaneously continue AML treatment.
Conclusion
Although we have presented our approach to the treatment of 4 pediatric patients with AML, we strongly believe that all children with newly diagnosed or relapsed AML should be enrolled and treated on clinical trials (Tables 2 and 3). Globally, there is consensus that treatment consists of induction of complete remission followed by consolidation of remission. In most trials, induction therapy consists of 2 courses of combination chemotherapy that include cytarabine and an anthracycline or the alternative mitoxantrone. Consolidation therapy, also termed intensification, includes 2 or 3 additional chemotherapy courses, except for higher-risk patients, who undergo allo-HCT as soon as they are in an MRD-negative remission and a suitable donor is identified. While all study groups now apply extensive biological characterization of the AML cells and treatment response based on MRD assessments for risk-group adapted treatment, several controversies remain. Only through carefully conducted trials that incorporate biological studies to identify biomarkers of response and characterize mechanisms of resistance will we be able to make progress in the treatment of pediatric AML. However, one limitation of most current trials is that, despite the tremendous heterogeneity of AML, all patients except those with FLT3-mutated AML receive the same chemotherapy, with or without allo-HCT. Thus, it is possible that the benefit of a new treatment strategy for a subgroup of patients is not detected because that intervention is not beneficial to the overall study population. Future trials must therefore test new agents only in those patients who are most likely to benefit. Such trials will require not only the availability of new drugs and the identification of biomarkers but also the implementation of novel clinical trial designs80-83 that will allow us to more efficiently study molecularly defined patient subgroups.
Current and planned trials for children with newly diagnosed AML
| Trial . | Primary objectives . |
|---|---|
| St Jude AML16 (NCT03164057) | Safety and activity of epigenetic priming with DNA methyltransferase inhibitors |
| COG AAML1831 (NCT04293562) | Comparison of Vyxeos and GO vs daunorubicin, cytarabine, and GO as induction therapy |
| NOPHO-DBH AML-2012 (NCT01828489) | Comparison of daunorubicin/cytarabine/etoposide vs daunorubicin/cytarabine/fludarabine during induction II |
| Myechild01 (NCT02724163) | Induction: comparison of mitoxantrone/cytarabine with 1 vs 3 doses of gemtuzumab ozogamicin Consolidation: comparison of high-dose cytarabine vs fludarabine/cytarabine Conditioning at allo-HCT: comparison of busulfan/cyclophosphamide vs busulfan/fludarabine |
| NOPHO-DB-SHIP (planned) | Induction: comparison of mitoxantrone/etoposide/cytarabine with or without gemtuzumab ozogamicin Consolidation: comparison of 3 vs 2 courses in non-high-risk patients Conditioning at allo-HCT: comparison of busulfan/cyclophosphamide/melphalan vs busulfan/clofarabine/fludarabine |
| JPLSG-AML-20 (planned) | Phase 3 trial evaluating MRD-based risk stratification and a randomized study of GO in combination with postinduction consolidation chemotherapy for non–low-risk patients |
| AIEOP-BFM21 (planned) | Induction: comparison of 2 courses of Vyxeos vs idarubicin/cytarabine/etoposide followed by high-dose cytarabine/mitoxantrone Conditioning at allo-HCT: comparison of busulfan/cyclophosphamide/melphalan vs treosulfan/fludarabine/thiotepa |
| Trial . | Primary objectives . |
|---|---|
| St Jude AML16 (NCT03164057) | Safety and activity of epigenetic priming with DNA methyltransferase inhibitors |
| COG AAML1831 (NCT04293562) | Comparison of Vyxeos and GO vs daunorubicin, cytarabine, and GO as induction therapy |
| NOPHO-DBH AML-2012 (NCT01828489) | Comparison of daunorubicin/cytarabine/etoposide vs daunorubicin/cytarabine/fludarabine during induction II |
| Myechild01 (NCT02724163) | Induction: comparison of mitoxantrone/cytarabine with 1 vs 3 doses of gemtuzumab ozogamicin Consolidation: comparison of high-dose cytarabine vs fludarabine/cytarabine Conditioning at allo-HCT: comparison of busulfan/cyclophosphamide vs busulfan/fludarabine |
| NOPHO-DB-SHIP (planned) | Induction: comparison of mitoxantrone/etoposide/cytarabine with or without gemtuzumab ozogamicin Consolidation: comparison of 3 vs 2 courses in non-high-risk patients Conditioning at allo-HCT: comparison of busulfan/cyclophosphamide/melphalan vs busulfan/clofarabine/fludarabine |
| JPLSG-AML-20 (planned) | Phase 3 trial evaluating MRD-based risk stratification and a randomized study of GO in combination with postinduction consolidation chemotherapy for non–low-risk patients |
| AIEOP-BFM21 (planned) | Induction: comparison of 2 courses of Vyxeos vs idarubicin/cytarabine/etoposide followed by high-dose cytarabine/mitoxantrone Conditioning at allo-HCT: comparison of busulfan/cyclophosphamide/melphalan vs treosulfan/fludarabine/thiotepa |
AIEOP, Associazione Italiana di Ematologia e Oncologia Pediatrica; JPLSG, Japanese Pediatric Leukemia/Lymphoma Study Group; SHIP, Spain, Hong Kong, Israel, Portugal.
Current and planned trials for children with relapsed AML
| Target . | Agent(s) . | ID (www.clinicaltrials.gov) . | Study group . | Status . |
|---|---|---|---|---|
| BCL2 | Venetoclax | NCT03194932 | St Jude | Recruiting |
| BCL2 | Venetoclax | Pending | PedAL | Planned |
| BCL2 and XPO1 | Venetoclax and selinexor | Pending | St Jude | Planned |
| PD-1 | Nivolumab | NCT03825367 | TACL | Recruiting |
| IDH2 | Enasidenib | NCT04203316 | COG | Recruiting |
| NEDD8 | Pevonedistat | NCT03813147 | COG | Closed to accrual |
| MDM2 | Idasanutlin | NCT04029688 | Hoffmann-La Roche | Recruiting |
| Menin | SNDX-5613 | NCT04065399 | Syndax Pharmaceuticals | Recruiting |
| Menin | KO-539 | Pending | Kura Oncology | Planned |
| Mesothelin | Anetumab ravtansine | Pending | PedAL | Planned |
| E-selectin | Uproleselan | Pending | PedAL | Planned |
| CD123 | CART | NCT04318678 | St Jude | Recruiting |
| CD123 | Flotetuzumab | NCT04158739 | COG | Recruiting |
| CD123 | IMGN632 | Pending | PedAL | Planned |
| CD123 | Tagraxofusp | Pending | TACL | Planned |
| CD33 | CART | NCT03971799 | CIBMTR | Recruiting |
| Nonspecific | NK cells | NCT03068819 | Washington University | Recruiting |
| Target . | Agent(s) . | ID (www.clinicaltrials.gov) . | Study group . | Status . |
|---|---|---|---|---|
| BCL2 | Venetoclax | NCT03194932 | St Jude | Recruiting |
| BCL2 | Venetoclax | Pending | PedAL | Planned |
| BCL2 and XPO1 | Venetoclax and selinexor | Pending | St Jude | Planned |
| PD-1 | Nivolumab | NCT03825367 | TACL | Recruiting |
| IDH2 | Enasidenib | NCT04203316 | COG | Recruiting |
| NEDD8 | Pevonedistat | NCT03813147 | COG | Closed to accrual |
| MDM2 | Idasanutlin | NCT04029688 | Hoffmann-La Roche | Recruiting |
| Menin | SNDX-5613 | NCT04065399 | Syndax Pharmaceuticals | Recruiting |
| Menin | KO-539 | Pending | Kura Oncology | Planned |
| Mesothelin | Anetumab ravtansine | Pending | PedAL | Planned |
| E-selectin | Uproleselan | Pending | PedAL | Planned |
| CD123 | CART | NCT04318678 | St Jude | Recruiting |
| CD123 | Flotetuzumab | NCT04158739 | COG | Recruiting |
| CD123 | IMGN632 | Pending | PedAL | Planned |
| CD123 | Tagraxofusp | Pending | TACL | Planned |
| CD33 | CART | NCT03971799 | CIBMTR | Recruiting |
| Nonspecific | NK cells | NCT03068819 | Washington University | Recruiting |
CIBMTR, Center for International Blood and Marrow Transplant Research; PedAL, Pediatric Acute Leukemia; TACL, Therapeutic Advances in Childhood Leukemia Consortium.
Acknowledgments
The authors thank all cooperative groups for designing and conducting well-designed, informative clinical trials and the patients who participate in the trials. They also thank their colleagues from around the world for collaborating on retrospective studies and for their willingness to openly share data and ideas with the entire pediatric oncology community.
Authorship
Contribution: J.E.R. and G.J.L.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey E. Rubnitz, St Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN, 38105-9005; e-mail: jeffrey.rubnitz@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal