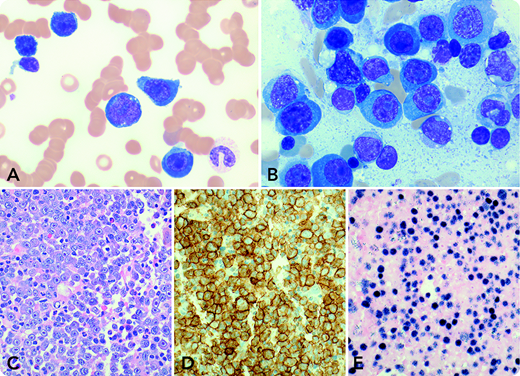
A 73-year-old man with a 2-year history of bilateral lung transplantation presented with progressive fatigue, nausea, and night sweats over the past 2 months. Laboratory tests showed abnormal serum components: creatinine, 460.0 µmol/L; lactate dehydrogenase, 3073 U/L; immunoglobulin A (IgA) lambda paraprotein, 43.7 g/L; free gamma light chain, 8.2 g/L; and Epstein-Barr virus (EBV) load, 1 555 723 IU/mL. Complete blood cell count demonstrated leukocytes 13.7 × 109/L, hemoglobin 74 g/L, and platelets 59 × 109/L. Blood smear examination showed 34% blastoid cells (panel A: Wright-Giemsa stain, original magnification ×1000) that were identified as plasma cells with aberrant CD56 and absent CD19 and CD20 by flow cytometry. Bone marrow biopsy revealed markedly increased large cells with plasmablastic morphology (panel B: aspirate smear, Wright-Giemsa stain, original magnification ×1000; panel C: core biopsy, hematoxylin and eosin stain, original magnification ×400) that were positive for CD138 (panel D: original magnification ×400), lambda light chain restriction, and EBV latency 1 infection (panel E: EBV-encoded RNA in situ hybridization, original magnification ×400). Cytogenetic studies detected a complex karyotype that included t(8;14)(q24;q32)/MYC-IGH. A radiologic scan demonstrated no lymphadenopathy or bone lytic lesions. Thereafter, diagnosis of a plasmablastic myeloma/leukemia variant of monomorphic posttransplant lymphoproliferative disorder (M-PTLD) was rendered. The patient’s condition deteriorated without therapy specifically designated for treating myeloma/lymphoma, and he expired 7 days after the diagnosis.
The myeloma variant of M-PTLD is rare. The variant with leukemic manifestation has not yet been reported in any detail. Our patient shows plasmablastic morphology and an MYC-IGH genotype that might have been the pathologic factors driving the lethal leukemic presentation and catastrophic clinical course.
A 73-year-old man with a 2-year history of bilateral lung transplantation presented with progressive fatigue, nausea, and night sweats over the past 2 months. Laboratory tests showed abnormal serum components: creatinine, 460.0 µmol/L; lactate dehydrogenase, 3073 U/L; immunoglobulin A (IgA) lambda paraprotein, 43.7 g/L; free gamma light chain, 8.2 g/L; and Epstein-Barr virus (EBV) load, 1 555 723 IU/mL. Complete blood cell count demonstrated leukocytes 13.7 × 109/L, hemoglobin 74 g/L, and platelets 59 × 109/L. Blood smear examination showed 34% blastoid cells (panel A: Wright-Giemsa stain, original magnification ×1000) that were identified as plasma cells with aberrant CD56 and absent CD19 and CD20 by flow cytometry. Bone marrow biopsy revealed markedly increased large cells with plasmablastic morphology (panel B: aspirate smear, Wright-Giemsa stain, original magnification ×1000; panel C: core biopsy, hematoxylin and eosin stain, original magnification ×400) that were positive for CD138 (panel D: original magnification ×400), lambda light chain restriction, and EBV latency 1 infection (panel E: EBV-encoded RNA in situ hybridization, original magnification ×400). Cytogenetic studies detected a complex karyotype that included t(8;14)(q24;q32)/MYC-IGH. A radiologic scan demonstrated no lymphadenopathy or bone lytic lesions. Thereafter, diagnosis of a plasmablastic myeloma/leukemia variant of monomorphic posttransplant lymphoproliferative disorder (M-PTLD) was rendered. The patient’s condition deteriorated without therapy specifically designated for treating myeloma/lymphoma, and he expired 7 days after the diagnosis.
The myeloma variant of M-PTLD is rare. The variant with leukemic manifestation has not yet been reported in any detail. Our patient shows plasmablastic morphology and an MYC-IGH genotype that might have been the pathologic factors driving the lethal leukemic presentation and catastrophic clinical course.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal