Abstract
Introduction: SMM is an asymptomatic plasma cell disorder with heterogeneous clinical behavior. Both the Spanish Myeloma and ECOG Groups have demonstrated that patients (pts) at high risk of progression to active MM have prolonged time-to progression upon receiving early treatment with R-based regimens. Our next step was to perform a phase 2, single arm trial, focusing on the same population, but aiming at abrogating the risk of progression through the achievement of sustained minimal residual disease negativity (MRD-ve) at 3 and 5 years after HDT-ASCT.
Patients and methods: Ninety SMM pts at high-risk of progression (>50% at 2 yrs), younger than 70 years and transplant candidates were included. The high risk was defined by the presence of both ≥PC 10% and MC ≥3g/dL (Mayo criteria) or ifonly one criterion was present, pts should have >95%of aberrant PCs within the total PCsBM compartment by immunophenotyping plus immunoparesis (Spanish criteria). Induction therapy consisted of six 4-weeks cycles of KRd in which K was given at dose of 36 mg/m 2 twice per week plus R at dose of 25 mg on days 1-21 and dexamethasone at dose of 40 mg weekly. Melphalan at dose of 200 mg/m 2 followed by ASCT was given as intensification therapy followed by two KRd consolidation cycles and maintenance with R at dose of 10 mg plus dexamethasone at dose of 20 mg weekly for up to 2 yrs. The primary end-point was to evaluate the MRD-ve rate by next generation flow (NGF) after ASCT and MRD-ve rate maintained at 3 and 5 years after ASCT.
Results: Between June 2015 and June 2017, 90 high-risk SMM pts were recruited and 70 pts (78%) have completed the treatment protocol. The reasons for early discontinuations were: IC withdrawal (4 pts), adverse events (8 pts) or biological progression (BP), either biochemical or because of MRD conversion from negative to positive (1 pt during induction and 7 pts during maintenance). Thirty-one pts (34%) shared at least one of the biomarkers considered as myeloma defining events that currently reclassify SMM into active MM.
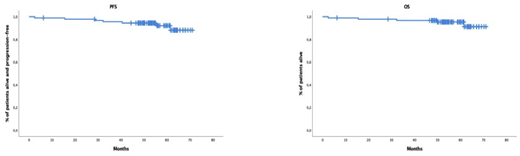
In the intent-to-treat (ITT) pts' population, after induction, the ≥CR rate was 41% and increased to 65% after HDT-ASCT and 72% after consolidation. During maintenance therapy, 7 pts experienced biological progression (2 pts conversion from MRD-ve into +ve and 5 pts biochemical progression) and the ≥CR rate at the end of treatment was 63.3%. In the ITT population, MRD-ve rates at 10 -5 were observed in 40% of pts after induction, 63% after HDT-ASCT, 68% after consolidation and 52% after maintenance therapy. Among MRD-ve patients after maintenance therapy that had MRD assessed one year after, 67% showed sustained MRD-ve. After a median f/u of 55 months (range: 6.2-71), only three patients progressed to symptomatic disease and the three had at baseline anyone of the biomarkers defining myeloma-defining events. At 5 years, 94% of pts remain alive and progression-free and 95% of pts alive (Figure 1). Overall, twenty-six pts (29%) have experienced biological progression (19 of them were conversion of MRD-ve into +ve), 8 of them during treatment phase (1 during induction and 7 during maintenance) and 16 pts during the follow-up period. The only factors predicting biological progression was failure to achieve MRD-ve at the end of treatment and unsustained MRD-ve at 1 year after finalizing maintenance.
Concerning toxicity, during induction, G3-4 neutropenia and thrombocytopenia were reported in 5 (6%) and 10 pts (11%), respectively. G3-4 infections were reported in 16 pts (18%), followed by skin rash in 8 pts (9%). One patient reported G1 atrial fibrillation and another cardiac failure secondary to respiratory infection. Three pts reported hypertension (G2 in two and G3 in one). In all but two of the pts, PBSC collection was successful with a median of 4.10 x 10 6/Kg CD34 cells collected. All pts engrafted but one patient developed late graft failure. During consolidation, 2 pts developed G3-4 neutropenia, 3 pts G3-4 infections and 1 pt skin rash. Seven pts had to discontinue maintenance therapy due to: G3-4 hematological toxicity (4 pts), SPM (2pts) and cardiac arrest (1pt). One additional patient withdrew the IC.
Conclusions: These results suggest that early treatment with intention to abrogate risk of progression in transplant candidate high risk SMM patients is associated with a 94% PFS at 55 months and a sustained MRD negative rate at 1 year post treatment of 67%.
Mateos: Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Honoraria; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria; Oncopeptides: Honoraria. Rodríguez-Otero: Celgene-BMS, Janssen, Amgen, Sanofi, GSK, Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria. Gonzalez-Calle: BMS, Janssen, Amgen: Honoraria. Oriol: Celgene: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Janssen: Consultancy; Amgen: Consultancy, Speakers Bureau. Rosinol: Janssen, Celgene, Amgen and Takeda: Honoraria. de la Rubia: Takeda: Consultancy; Amgen, Bristol Myers Squibb,: Honoraria, Speakers Bureau; GSK: Consultancy; Celgene, Takeda, Janssen, Sanofi: Honoraria; Ablynx/Sanofi: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Accommodations; Celgene: Consultancy; AbbVie: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Sanofi: Membership on an entity's Board of Directors or advisory committees. De Arriba: Amgen: Consultancy, Honoraria; Glaxo Smith Kline: Consultancy, Honoraria; BMS-Celgene: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau. Ocio: MSD: Honoraria; Sanofi: Consultancy, Honoraria; Karyopharm: Consultancy; Takeda: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers Squibb/Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Pfizer: Consultancy; Secura-Bio: Consultancy. Paiva: Bristol-Myers Squibb-Celgene, Janssen, and Sanofi: Consultancy; Adaptive, Amgen, Bristol-Myers Squibb-Celgene, Janssen, Kite Pharma, Sanofi and Takeda: Honoraria; Celgene, EngMab, Roche, Sanofi, Takeda: Research Funding. Puig: Celgene, Janssen, Amgen, Takeda: Research Funding; Celgene: Speakers Bureau; Amgen, Celgene, Janssen, Takeda: Consultancy; Amgen, Celgene, Janssen, Takeda and The Binding Site: Honoraria. Cedena: Janssen, Celgene and Abbvie: Honoraria. Lahuerta: Celgene: Other: Travel accomodations and expenses; Celgene, Takeda, Amgen, Janssen and Sanofi: Consultancy. San-Miguel: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Merck Sharpe & Dohme, Novartis, Regeneron, Roche, Sanofi, SecuraBio, Takeda: Consultancy, Other: Advisory board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal