Abstract
Although large-scale sequencing studies have elucidated key mutations in chronic lymphocytic leukemia (CLL), the molecular pathways underlying CLL transition to aggressive lymphoma (Richter's transformation, RT) are not completely understood. Co-expression of the two most common human CLL genomic alterations, 13q deletion, and SF3B1 mutation, in murine B cells results in indolent CLL. Seventy percent of RT cases involve MYC network aberrations, and MGA (Max-gene-associated), a functional MYC suppressor, is recurrently mutated/deleted in ~10% of RT cases. Given the function of MGA in MYC dysregulation, we sought to determine if loss-of-function MGA mutations accelerate CLL transformation.
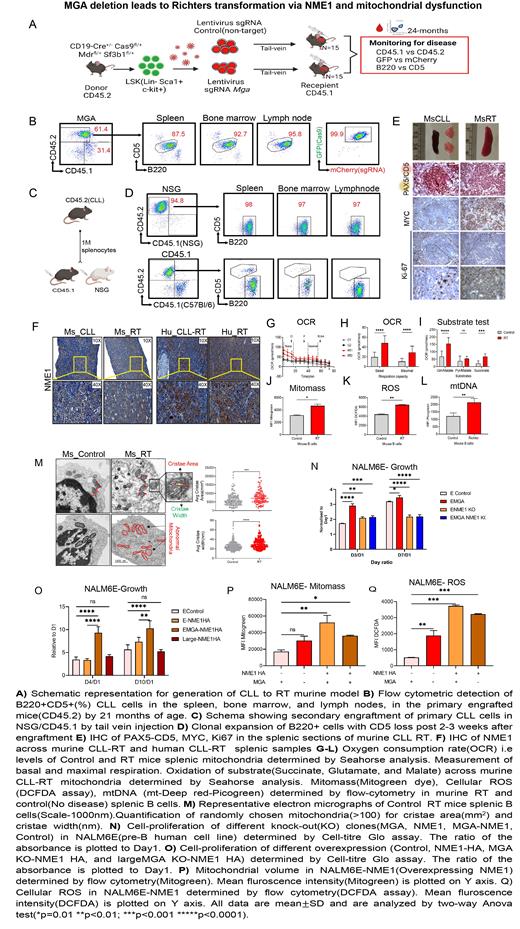
To determine the function of Mga deletion in CLL-RT, we in-vitro edited the genome of LSK (Lin - Sca-1 + Kit +) cells derived from the conditional CD19Cre/Cas9(GFP)/Mdr/Sf3b1-expressing mice (donor mice:CD45.2) to introduce Mga deletion in B cells with Mdr and the Sf3b1K700E mutations. Mga and control sgRNAs were lentivirally transduced in LSK cells and then transplanted into sub-lethally irradiated recipient mice (N=15, per group; C57BL/6: CD45.1). CLL onset (B220 +CD5 + CLL-like cells) was monitored bimonthly by flow-cytometry of peripheral blood, between ages 6 and 24 months. Three of 15 mice with Mga deletion had a clonal expansion of B220 +CD5 + cells with leukemic cells infiltration in the spleen and bone marrow by 21-months of age. However, no RT signs were observed in these mice. Transplantation of the CLL-like splenic cells into immunocompromised NSG or immunocompetent sub-lethally irradiated CD45.1 mice, led to rapid expansion of B220 + cells along with CD5 loss at 3 weeks post-engraftment and leukemic cells infiltrated various lymphoid tissues. H&E staining of splenic sections revealed the presence of CLL-like cells with a morphology resembling leukemia in the primary engrafted mice; however, these cells were larger and resembled aggressive lymphoma (RT) upon secondary engraftment. CLL and RT features were further confirmed by IHC staining of PAX5, CD5, Ki67, and MYC, suggesting the establishment of a murine CLL-to-RT transition murine model.
MYC was reported to contribute to cell growth by promoting mitochondrial dysfunction. To characterize mitochondrial changes in our RT model, oxidative phosphorylation (OXPHOS) was measured by Seahorse, and electron microscopy was performed on RT and no disease splenic B cells. Murine RT cells exhibited increased basal respiration and mitochondrial mass coupled with elevated mitochondria number and structural changes. To pinpoint the underlying transcriptional program, we performed RNA seq using splenic B cells derived from control, CLL, and RT mice. Through differential gene expression analysis, we identified >200 genes (mainly hallmark MYC targets and OXPHOS associated pathways) associated with CLL and RT. Of the 75 upregulated genes shared between CLL and RT cells, we focused on Nme1 (Nucleoside diphosphate kinase), an MGA transcriptional target reported in human lung adenocarcinoma. We confirmed Nme1 upregulation at both RNA and protein levels in murine and human RT samples by RT-PCR, immunoblot, and IHC, implicating a role of NME1 during CLL to RT transition.
To determine if the MGA/MYC/NME1 axis can be recapitulated in human cells, we generated combined genetic lesions in two human B-cell lines (harboring SF3B1K700E Nalm6E [E] or control Nalm6K [K]) by Crispr-Cas9 mediated deletion of MGA and MDR. In both MGA and MDR-MGA KO(Knockout) cells, NME1 RNA and protein were upregulated. MGA KO E cells displayed a mixture of large and small-sized cells with increased cell growth whereas K cells had no morphology change. Moreover, large E cells displayed increased mitochondria mass, basal and maximal OXPHOS, broken cristae, fully recapitulating the mitochondrial dysfunction observed in murine RT cells. Harnessing these cell lines, we discovered that NME1 KO diminished cell growth and OXPHOS in E/K cells, which can be rescued by NME1 overexpression, highlighting an essential but unrecognized role of NME1 in driving the CLL-RT transition.
With our established murine CLL-to-RT model, we demonstrated an RT-associated mitochondrial phenotype and revealed a novel role of the MGA/MYC/NME1 axis in driving the CLL-to-RT transition through mitochondrial regulation. This study opens a new therapeutic avenue for RT patients by targeting the MGA/MYC/NME1 axis.
Siddiqi: Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; Oncternal: Research Funding; TG Therapeutics: Research Funding. Danilov: Abbvie: Consultancy, Honoraria; Takeda Oncology: Research Funding; Genentech: Consultancy, Honoraria, Research Funding; TG Therapeutics: Consultancy, Research Funding; Beigene: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Gilead Sciences: Research Funding; Bristol-Meyers-Squibb: Honoraria, Research Funding; Rigel Pharm: Honoraria; Bayer Oncology: Consultancy, Honoraria, Research Funding; SecuraBio: Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal