Key Points
Clinical data and in vivo xenograft models point out a key role for BMP4 in BCP-ALL development and CNS leukemic infiltration.
BMP4 levels in ALL cells could be a useful biomarker to identify children with poor outcomes in low-/intermediate-risk groups of BCP-ALL.
Abstract
Pediatric B-cell precursor acute lymphoblastic leukemia (BCP-ALL) outcome has improved in the last decades, but leukemic relapses are still one of the main problems of this disease. Bone morphogenetic protein 4 (BMP4) was investigated as a new candidate biomarker with potential prognostic relevance, and its pathogenic role was assessed in the development of disease. A retrospective study was performed with 115 pediatric patients with BCP-ALL, and BMP4 expression was analyzed by quantitative reverse transcription polymerase chain reaction in leukemic blasts at the time of diagnosis. BMP4 mRNA expression levels in the third (upper) quartile were associated with a higher cumulative incidence of relapse as well as a worse 5-year event-free survival and central nervous system (CNS) involvement. Importantly, this association was also evident among children classified as having a nonhigh risk of relapse. A validation cohort of 236 patients with BCP-ALL supported these data. Furthermore, high BMP4 expression promoted engraftment and rapid disease progression in an NSG mouse xenograft model with CNS involvement. Pharmacological blockade of the canonical BMP signaling pathway significantly decreased CNS infiltration and consistently resulted in amelioration of clinical parameters, including neurological score. Mechanistically, BMP4 favored chemoresistance, enhanced adhesion and migration through brain vascular endothelial cells, and promoted a proinflammatory microenvironment and CNS angiogenesis. These data provide evidence that BMP4 expression levels in leukemic cells could be a useful biomarker to identify children with poor outcomes in the low-/intermediate-risk groups of BCP-ALL and that BMP4 could be a new therapeutic target to blockade leukemic CNS disease.
Introduction
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is the most common form of pediatric cancer and constitutes the most frequent cause of death by disease in childhood.1-4 Although treatment advances have led to improvement of long-term survival rates, 10% to 15% of patients included in low/intermediate risk groups still suffer from medullary and extramedullary relapses, including the central nervous system (CNS).4-6 Although CNS infiltration is rarely detected at initial diagnosis of pediatric BCP-ALL, early studies from autopsies of children who died of ALL revealed CNS involvement in almost 60% of cases.7 Since most CNS relapses occur in children initially diagnosed as CNS-negative, intrathecal therapy is now mandatory in any protocol as part of the treatment of CNS leukemia. However, despite this, CNS relapse still represents one of the most important causes of morbidity and mortality, occurring in 3% to 6% of ALL patients, and remains one of the major challenges in pediatric oncology.8
Different cytokines, chemokines, and adhesion molecules have been implicated in CNS leukemic infiltration.9-14 Bone morphogenetic proteins (BMPs) are multifunctional secreted growth factors that belong to the TGF-β superfamily and are well known for their indispensable roles in vertebrate development, regulating cell proliferation, differentiation, apoptosis, and migration.15 Particularly, BMP4 has been described as a critical component of the hematopoietic microenvironment regulating both hematopoietic stem/progenitor cell number and function,16-18 having also been involved in homing and engraftment.19,20 Likewise, BMP4 has been shown to be a negative regulator of lymphocyte proliferation and differentiation.21-24 The contribution of BMP4 to the pathogenesis of cancer, including hematological tumors, has also been emphasized in recent years.18,25-28 In chronic myeloid leukemia, high levels of BMP4 maintain a quiescent fraction of leukemic stem cells and amplify leukemic progenitors,29-31 and in acute myeloid leukemia, increased levels of BMP4 have been described to regulate the expression of different survival and proliferation factors and induce stem-like features in leukemic cells.32,33 The role of BMP4 in B-cell leukemogenesis is scarcely known, but BMP4 pathway alterations in ALL and upregulated expression of BMP ligands, including BMP4 in ALL blasts infiltrating CNS, support a role for this factor in this hematological disorder as well.34-37 Therefore, the aim of the current study was to explore the involvement of BMP4 in BCP-ALL development and its relevance in CNS leukemic infiltration.
Patients and methods
Patient samples
Primary samples were obtained from 115 BCP-ALL patients at diagnosis. Routine diagnostic bone marrow (BM) aspirates were harvested following standard procedures as required for clinical diagnosis. Leukemia cells were isolated by density gradient centrifugation using Ficoll-Hypaque (all samples contained >85%). Samples were provided by the Onco-Hematology Unit at Niño Jesús University Children’s Hospital and Virgen de la Arrixaca University Hospital. Informed consent was provided according to the Declaration of Helsinki, and the study was approved by the Ethics Committee of Clinical Research at Niño Jesús Hospital (R-0063/18). All children were treated according to the SEHOP-PETHEMA 2013 clinical guideline, a Berlin-Frankfurt-Munich (BFM)-based protocol. The validation cohort included primary samples from 236 BCP-ALL patients at diagnosis (Kiel University Medical Center Schleswig-Holstein and Hannover Medical School) treated according to the ALL-BFM 2000 protocol.
Cell culture and reagents
Leukemia pre-B ALL cell line Nalm6 (ACC128) was purchased from DSMZ (German Collections of Microorganisms and Cell Cultures), BMP4 transduced-Nalm6 stable cell line (Nalm6-BMP4) was developed in our laboratory as previously described,37 and CNS microvascular endothelial cells (hCMEC/D3 cell line) were generously provided by J. Millán (CBMSO, Madrid, Spain). Lines were maintained according to the manufactureŕs recommendations.
The following treatments were used: rhBMP4 (Humanzyme, Chicago, IL), methotrexate, cytarabine, doxorubicin, and vincristine (Pfizer, New York, NY).
B-cell ALL xenograft model
NOD.Cg-Prkdc scid IL2rg tm1Wjl/SzJ (NSG) mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and housed under pathogen-free conditions. All animal experimentation was conducted in accordance with the Spanish guidelines for care and use of laboratory animals and protocols approved by the Complutense University and Community of Madrid (PROEX 015/19). Eight- to 12-week-old mice were IV-infused via the tail vein with human primary cells (characteristics of primary samples used for xenotransplantation studies are shown in supplemental Table 1 available on the Blood Web site) or ALL cell lines, and engraftment was weekly monitored by peripheral blood (PB) cell staining with antibodies against human CD19 (ImmunoStep, Salamanca, Spain) and murine CD45 (Biolegend, San Diego, CA). When disease symptoms, including rough hair, lethargy, hunched-back posture, loss of motor functions, and hind limb paralysis were evident, mice were anesthetized and euthanized. Blood, BM, spleen, and brain samples were collected to determine engraftment levels by flow cytometry using antibodies specific to human CD19 and mouse CD45.
For BMP inhibitor DMH1 (Tocris Bioscience, Bristol, United Kingdom) experiments, NSG mice were inoculated with BCP-ALL primary samples. On day 8, mice were randomly divided into 2 groups: 11 mice were treated with DMH1 by continuous subcutaneous infusion using Alzet osmotic pumps (Charles River Laboratories, L’arbresle Cedex, France) at a dose of 3 mg/kg per day, while the control group received the solvent DMSO, with the same pump system. Mice were monitored daily for survival and clinical appearance of nerve palsy and euthanized when control animals showed relevant clinical signs (week 5).
Additional methods, including methods for quantitative reverse transcription quantitative polymerase chain reaction (qRT_PCR), flow cytometry, viability and migration assays, cytokine measurements, histology, and olfactory habituation–dishabituation test, can be found in supplemental Methods.
Results
High BMP4 expression levels in leukemic blasts as a poor prognostic indicator in low/intermediate risk BCP-ALL patients
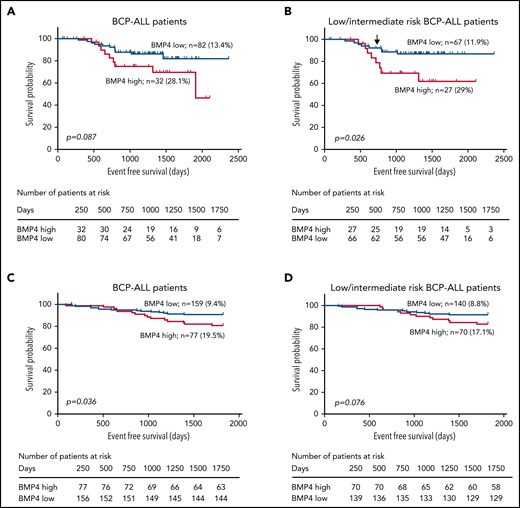
BMP4 mRNA expression levels were analyzed by qPCR in BM leukemic blasts in a cohort of 115 patients with BCP-ALL at diagnosis (supplemental Figure 1), and their clinical relevance was evaluated in terms of minimal residual disease (MRD) (+33 days) and event-free survival (EFS) (5 years follow-up). Kolmogorov–Smirnov test comparing probability distributions showed an association between high levels of BMP4 in leukemic blasts and MRD positivity (P = .054; patients analyzed, n = 60; MRD negative, n = 45; and MRD positive, n = 15). In addition, we observed an association between BMP4 expression (upper quartile) and risk of relapse. Quartiles were calculated based on the BMP4 expression values of the 115 patients (supplemental Figure 1). The cutoff corresponding to the value of BMP4 expression in the third quartile (18 arbitrary units [AU]) was associated with a significantly higher risk of relapse (BMP4high 28.1%, n = 32; BMP4low 13.4%, n = 82; P = .0317). Then, this value was chosen as the cutoff to define the BMP4 subgroups in the subsequent analyses, and patients with BMP4 expression levels higher than the third quartile value (18 AU) were considered as BMP4high and the remaining patients as BMP4low.
The mean time-to-relapse was 1138 (±488) for the whole group, with 1020 (±521) and 1145 (±478) for the BMP4high and BMP4low groups, respectively (P = .087 for log-rank test) (Figure 1A). These data suggest that increased levels of BMP4 at diagnosis could have a negative impact on the outcome of patients with BCP-ALL and the treatment effectiveness. Because no association was found between high BMP4 expression levels in leukemia cells at diagnosis and high-risk patients with ALL (P = .756), we next analyzed if high BMP4 levels could identify a subgroup of low/intermediate-risk ALL patients with poor prognosis who could benefit from more intensive therapy. Again, the relapse rate in low/intermediate-risk BCP-ALL patients was significantly higher (P = .019) in the BMP4high patient group (29%; n = 27) vs the BMP4low patient group (11.9%; n = 67). The logistic regression analysis showed that high BMP4 expression on blasts at diagnosis is a key factor affecting relapse (odds ratio, 3.1; 95% confidence interval [CI], 1.025-9.404; P = .045). In addition, EFS was significantly higher (P = .026) in the group of children with blasts expressing low BMP4 levels (Figure 1B). Then, the hazard ratio of relapse in the BMP4high group increased 2.85-fold (95% CI, 1.084-7.729) when compared with the group with BMP4low, as indicated by Cox’s proportional hazards regression analysis. Interestingly, as shown in Figure 1B, increased levels of BMP4 expression in blasts resulted in effects that were not graphically observable until near the end of chemotherapy treatment. However, an extended follow-up demonstrated that the survival curves gradually diverged, finally resulting in survival rates that were significantly lower (P = .026) in those patients who had blasts expressing high levels of BMP4 at diagnosis as above mentioned. Significant differences between both survival curves were observed from day 730 (P = .05), as indicated by a comparison test of proportions. The independent impact of high BMP4 mRNA levels in low/intermediate risk BCP-ALL patient relapse was corroborated with a multivariate analysis controlled for age and white blood cell count at diagnosis (supplemental Table 3).
Clinical data show that elevated BMP4 mRNA expression on leukemic blasts at diagnosis is associated with poor outcomes. (A) Using a cutpoint of 18 AU for the entire cohort (n = 114; EFS values from 1 BMP4low patient was not available), 5-year EFS was reduced in patients with high BMP4 expression levels on leukemic cells (P = .087; log-rank test). (B) Kaplan-Meier survival curve shows that, for low/intermediate risk patients with BCP-ALL (n = 94), the cutpoint of 18 AU identifies patients with significantly reduced EFS (P = .026; log-rank test). Significant differences between both survival curves were observed from day 730 (shown by an arrow; P = .05; test of proportions). (C) Kaplan-Meier survival curves for the entire validation cohort (n = 236) and (D) low/intermediate-risk patients with BCP-ALL (n = 210) show similar results.
Clinical data show that elevated BMP4 mRNA expression on leukemic blasts at diagnosis is associated with poor outcomes. (A) Using a cutpoint of 18 AU for the entire cohort (n = 114; EFS values from 1 BMP4low patient was not available), 5-year EFS was reduced in patients with high BMP4 expression levels on leukemic cells (P = .087; log-rank test). (B) Kaplan-Meier survival curve shows that, for low/intermediate risk patients with BCP-ALL (n = 94), the cutpoint of 18 AU identifies patients with significantly reduced EFS (P = .026; log-rank test). Significant differences between both survival curves were observed from day 730 (shown by an arrow; P = .05; test of proportions). (C) Kaplan-Meier survival curves for the entire validation cohort (n = 236) and (D) low/intermediate-risk patients with BCP-ALL (n = 210) show similar results.
Next, we investigated the association between BMP4 expression and CNS-relapse in patients with ALL. We found that BMP4 expression above the third quartile in ALL blasts at diagnosis was associated with a higher relapse rate (12.5%, n = 32; P = .036) compared with those in lower quartiles (3.6%, n = 83).
The negative impact in the outcome of patients with BCP-ALL of high BMP4 expression in leukemia cells at diagnosis was validated in an independent cohort of 236 patients with BCP-ALL (supplemental Figure 1). Again, high BMP4 expression (≥18 AU) was associated with a significantly higher risk of relapse both when complete cohort was considered (BMP4high 19.5%, n = 77; BMP4low 9.4%, n = 159; P = .014) and when low-/intermediat- risk patients were selected (BMP4high 17.1%, n = 70; BMP4low 8.8%, n = 140; P = .038). As described above, multivariant analysis in the low/intermediate risk ALL group showed that high BMP4 expression increased the risk of relapse (supplemental Table 3). In addition, EFS was reduced in the BMP4high group both when the complete cohort or only the low/intermediate risk BCP-ALL patient group were considered, although statistical significance was not reached in the latter (Figure 1C-D) (P = .036 complete cohort and P = .07 low/intermediate risk BCP-ALL patients). Finally, we could only observe no statistically significant association between BMP4 levels and CNS relapse in the validation cohort (BMP4high 3.9%, n = 77; BMP4low 2.5%, n = 159).
BMP4 expression levels in leukemic blasts are important in the establishment and development of disease
To investigate whether BMP4 mRNA levels influence the ability of primary patient cells to initiate leukemia and/or to infiltrate the CNS in vivo, 9 primary samples of pediatric BCP-ALL patients subgrouped according to BMP4 mRNA expression (4 BMP4high [>18 AU] and 5 BMP4low [<18 AU] primary samples) (supplemental Table 1) were xenografted into nonirradiated NSG mice (Figure 2A). Interestingly, whereas around 80% of mice (13 out of 16 mice) injected with BMP4high BCP-ALL blasts developed clinical leukemia symptoms in correlation with the presence of CD19+ cells in the BM, spleen, and CNS, only 15% of mice bearing BMP4low patient blasts engrafted successfully and showed the presence of leukemic cells in CNS (Figure 2B; supplemental Figure 2). The remaining 85% of the mice in this group neither died nor showed signs of disease until the time of euthanasia, 4 months after injection, which correlated with the absence of leukemic CD19+ cells. Moreover, both BMP4low and BMP4high leukemia-engrafted mice showed very similar BM and extramedullary infiltration.
BMP4high BCP-ALL blasts exhibit an increased leukemogenic activity. (A) Outline of the in vivo experimental design for panels B-E and H. BMP4high and BMP4low leukemic blasts were IV-transplanted into NSG mice at day 0. Recipient mice were monitored for disease symptoms and the presence of blasts in blood and were euthanized upon the appearance of clinical signs. (B) Nine primary samples of pediatric BCP-ALL patients were chosen according to BMP4 mRNA expression (4 BMP4high primary samples [patients #1, #2, #3, #8] and 5 BMP4low primary samples [patients #4, #5, #6, #7, #9] as shown in supplemental Table 1) and xenografted into 3 to 5 mice. Mice were followed for 4 months or were euthanized when they showed clinical signs. Kaplan-Meier curves show reduced survival in mice xenografted with BMP4high primary samples (P = .0001; log-rank test). (C) Nalm6 or Nalm6-BMP4 (0.5 x 105 cells per mouse) were xenografted into NSG mice. Reduced survival rate was observed in mice injected with Nalm6-BMP4 ALL blasts (P = .007; log-rank test). (D) IL-6 serum levels in NSG mice transplanted with Nalm6 or Nalm6-BMP4 cells and in control healthy mice (n = 5-6 mice per group). Each dot represents a single transplanted mouse, and lines represent the mean level of IL-6 in each group (**P ≤ .01 and #P ≤ .05 represent statistical significances relative to control healthy mice [*] or Nalm6 mice [#]). (E) Percentages (mean ± standard deviation [SD]) and absolute numbers of leukemic blasts recovered from the spleen of mice xenografted with either Nalm6 (n = 7) or Nalm6-BMP4 (n = 10) cells. Each dot represents a transplanted mouse, and bars and lines represent the mean percentages and absolute numbers (**P ≤ .01 and ***P ≤ .001). (F) Proliferation rate of Nalm6 and Nalm6-BMP4 leukemic cells recovered from the spleen of xenografted mice (n = 6), determined by 7-AAD staining and analyzed by flow cytometry. Results represent the mean ± SD of cells in G0/G1 phases (**P ≤ .01). Representative flow cytometry histograms are shown in the right panel. (G) Relative viability of Nalm6-BMP4 leukemic cells with respect to Nalm6 cells recovered from spleens of xenografted mice (n = 4) and cultured during 48 hours with different concentrations of methotrexate (MTX) and cytarabine (Ara-C) (*P ≤ .05). (H) Percentage of CD19+ leukemic blasts infiltrated in CNS in either Nalm6 (n = 7) or Nalm6-BMP4 (n = 10) mice. Lines represent average percentages of CNS-infiltrating CD19+ cells for each cohort (P = .058). Data in (D-H) panels were compared using 2-tailed Mann-Whitney U tests.
BMP4high BCP-ALL blasts exhibit an increased leukemogenic activity. (A) Outline of the in vivo experimental design for panels B-E and H. BMP4high and BMP4low leukemic blasts were IV-transplanted into NSG mice at day 0. Recipient mice were monitored for disease symptoms and the presence of blasts in blood and were euthanized upon the appearance of clinical signs. (B) Nine primary samples of pediatric BCP-ALL patients were chosen according to BMP4 mRNA expression (4 BMP4high primary samples [patients #1, #2, #3, #8] and 5 BMP4low primary samples [patients #4, #5, #6, #7, #9] as shown in supplemental Table 1) and xenografted into 3 to 5 mice. Mice were followed for 4 months or were euthanized when they showed clinical signs. Kaplan-Meier curves show reduced survival in mice xenografted with BMP4high primary samples (P = .0001; log-rank test). (C) Nalm6 or Nalm6-BMP4 (0.5 x 105 cells per mouse) were xenografted into NSG mice. Reduced survival rate was observed in mice injected with Nalm6-BMP4 ALL blasts (P = .007; log-rank test). (D) IL-6 serum levels in NSG mice transplanted with Nalm6 or Nalm6-BMP4 cells and in control healthy mice (n = 5-6 mice per group). Each dot represents a single transplanted mouse, and lines represent the mean level of IL-6 in each group (**P ≤ .01 and #P ≤ .05 represent statistical significances relative to control healthy mice [*] or Nalm6 mice [#]). (E) Percentages (mean ± standard deviation [SD]) and absolute numbers of leukemic blasts recovered from the spleen of mice xenografted with either Nalm6 (n = 7) or Nalm6-BMP4 (n = 10) cells. Each dot represents a transplanted mouse, and bars and lines represent the mean percentages and absolute numbers (**P ≤ .01 and ***P ≤ .001). (F) Proliferation rate of Nalm6 and Nalm6-BMP4 leukemic cells recovered from the spleen of xenografted mice (n = 6), determined by 7-AAD staining and analyzed by flow cytometry. Results represent the mean ± SD of cells in G0/G1 phases (**P ≤ .01). Representative flow cytometry histograms are shown in the right panel. (G) Relative viability of Nalm6-BMP4 leukemic cells with respect to Nalm6 cells recovered from spleens of xenografted mice (n = 4) and cultured during 48 hours with different concentrations of methotrexate (MTX) and cytarabine (Ara-C) (*P ≤ .05). (H) Percentage of CD19+ leukemic blasts infiltrated in CNS in either Nalm6 (n = 7) or Nalm6-BMP4 (n = 10) mice. Lines represent average percentages of CNS-infiltrating CD19+ cells for each cohort (P = .058). Data in (D-H) panels were compared using 2-tailed Mann-Whitney U tests.
To directly test whether high BMP4 expression confers a competitive advantage in the establishment and development of ALL disease, we used the Nalm6 human pre-B ALL cell line genetically modified to overexpress BMP4 (Nalm6-BMP4), as previously described by our group.37 Nalm6-BMP4 or Nalm6 control cells expressing different levels of BMP4 were IV-transplanted into NSG mice. The median survival of Nalm6-injected mice was 31 days after transplantation while, in contrast, all Nalm6–BMP4-injected mice developed very pronounced clinical symptoms significantly earlier (Figure 2C). This more aggressive phenotype correlated with enhanced levels of the proinflammatory cytokine IL-6 in the serum of Nalm6-BMP4 mice (Figure 2D). Surprisingly, and in comparison with the control Nalm6 mice group, mice transplanted with Nalm6-BMP4 cells demonstrated significantly reduced leukemic blast numbers in the BM, liver, and mainly spleen at the experimental endpoint (Figure 2E). Nalm6-BMP4 blasts recovered from the spleen of these animals showed a significantly decreased proliferation rate (Figure 2F) without affecting cell survival (data not shown). In correlation, a reduced sensitivity to death mediated by conventional chemotherapy drugs was observed in in vitro studies with blasts recovered from spleens of Nalm6-BMP4 mice, compared with the control Nalm6 group (Figure 2G; supplemental Figure 3). These results suggest that increased BMP4 levels in leukemia blasts induce a quiescent phenotype and promote chemoresistance in BCP-ALL.
On the other hand, and unlike what happened in the peripheral organs, the upregulated levels of BMP4 in leukemia cells led to an increase of blasts entering the CNS when compared with control Nalm6 cells (Figure 2H).
Pharmacological blockade of canonical BMP4 signaling pathway reduces CNS leukemia burden
To assess therapeutic relevance, we investigated whether blocking BMP4 signaling in vivo could have antileukemogenic activity when administered after disease establishment. For this purpose, the pharmacological intervention of BMP4 signaling in the xenograft model was performed using DMH1, an inhibitor of the BMP4 canonical pathway. Three primary samples (all showing engraftment, CNS involvement, and high BMP4 expression in previous experiments) were injected into NSG mice. Eight days after infusion, when blasts were detected in PB, mice were divided into 2 groups and treated with a control vehicle, DMSO, or inhibitor DMH1, using Alzet pumps until the clinical endpoint. On the onset of leukemia-related symptoms in control BCP-ALL mice, all mice were euthanized, and leukemia manifestation was quantified by flow cytometry in BM, spleen, PB, and CNS (Figure 3A). Although no difference in the frequency of CD19+ blasts recovered from blood, spleen, or BM was observed between the 2 experimental groups, a significant reduction in CNS leukemia was observed in DMH1-treated mice compared with control animals (Figure 3B-C). Also, IL-6 serum levels were significantly reduced in DMH1-treated animals (Figure 3D). In line with these observations, sensory and motor tests showed that DMH1-treated mice exhibited a less aggressive clinical phenotype related to a significantly less accumulation of leukemic blasts in the CNS compared with control DMSO-treated mice. Thus, the percentages of animals with the ability to discriminate divergent odors and hind limb paralysis were significantly different between DMH1- and vehicle-treated ALL mice (Figure 3E-F).
Inhibition of canonical BMP4 signaling reduces ALL CNS infiltration and neurological clinical symptoms. (A) Outline of the in vivo experimental design for panels (B-F). Mice were transplanted with BMP4high primary samples at day 0 (samples from patients #1, #2, and #3, which were shown to engraft in 100% animals in previous experiments, were used). Leukemia development was monitored, and 8 days after injection, mice transplanted with each sample (6-8 animals per sample) were randomly divided into 2 groups (n = 11 mice per group) and treated with DMH1 or control DMSO. All animals were euthanized upon detection of hind limb paralysis in DMSO control mice. (B) Both control DMSO- and DMH1-treated mice (n = 11 mice per group) showed similar engraftment in peripheral organs. Bars show mean percentages (±standard deviation [SD]) of CD19+ leukemic blasts in BM, spleen, and PB. (C) CNS leukemia infiltration was reduced in DMH1- vs control DMSO-treated animals (n = 11 mice per group). Each data point represents a single mouse transplanted with blasts from patients #1, #2, or #3 (shown as circles, squares, or triangles), and lines represent the mean percentage of CD19 leukemic blasts in each group (**P ≤ .01; 2-tailed Mann-Whitney U test). (D) Bars represent average serum IL-6 levels measured in DMSO- and DMH1-treated mice at euthanasia (n = 4 mice per group; *P ≤ .05; 2-tailed Mann-Whitney U test). (E) Olfactory habituation–dishabituation test of healthy (green triangles), DMSO- (blue circles), and DMH1-treated (red squares) mice (n = 4 mice per group). Data represent the exploration time (mean ± SD) of successive cotton swabs soaked in octanal, heptanal, or anisole. Two-way ANOVA followed by Bonferroni correction showed significant differences for heptanal and anisole odors: *P ≤ .05 and **P ≤ .01 show significant differences between vehicle-DMSO and healthy control mice; #P ≤ .05 shows significant differences between DMSO- and DMH1-treated mice. (F) The incidence of hind limb paralysis in DMH1-treated mice was notably reduced with respect to the DMSO control group.
Inhibition of canonical BMP4 signaling reduces ALL CNS infiltration and neurological clinical symptoms. (A) Outline of the in vivo experimental design for panels (B-F). Mice were transplanted with BMP4high primary samples at day 0 (samples from patients #1, #2, and #3, which were shown to engraft in 100% animals in previous experiments, were used). Leukemia development was monitored, and 8 days after injection, mice transplanted with each sample (6-8 animals per sample) were randomly divided into 2 groups (n = 11 mice per group) and treated with DMH1 or control DMSO. All animals were euthanized upon detection of hind limb paralysis in DMSO control mice. (B) Both control DMSO- and DMH1-treated mice (n = 11 mice per group) showed similar engraftment in peripheral organs. Bars show mean percentages (±standard deviation [SD]) of CD19+ leukemic blasts in BM, spleen, and PB. (C) CNS leukemia infiltration was reduced in DMH1- vs control DMSO-treated animals (n = 11 mice per group). Each data point represents a single mouse transplanted with blasts from patients #1, #2, or #3 (shown as circles, squares, or triangles), and lines represent the mean percentage of CD19 leukemic blasts in each group (**P ≤ .01; 2-tailed Mann-Whitney U test). (D) Bars represent average serum IL-6 levels measured in DMSO- and DMH1-treated mice at euthanasia (n = 4 mice per group; *P ≤ .05; 2-tailed Mann-Whitney U test). (E) Olfactory habituation–dishabituation test of healthy (green triangles), DMSO- (blue circles), and DMH1-treated (red squares) mice (n = 4 mice per group). Data represent the exploration time (mean ± SD) of successive cotton swabs soaked in octanal, heptanal, or anisole. Two-way ANOVA followed by Bonferroni correction showed significant differences for heptanal and anisole odors: *P ≤ .05 and **P ≤ .01 show significant differences between vehicle-DMSO and healthy control mice; #P ≤ .05 shows significant differences between DMSO- and DMH1-treated mice. (F) The incidence of hind limb paralysis in DMH1-treated mice was notably reduced with respect to the DMSO control group.
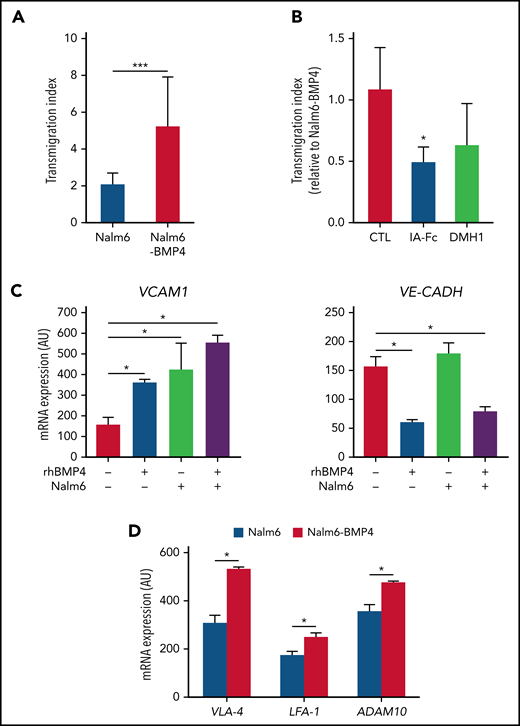
BMP4 promotes adhesion and transendothelial migration of ALL cells
Since our results suggested a migratory advantage to CNS for leukemic blasts expressing high BMP4 levels, we next analyzed the ability of Nalm6-BMP4 and Nalm6 control cells to undergo transendothelial migration across brain-derived microvascular endothelial cells that express functional BMP4 receptors (data not shown). As depicted in Figure 4A-B, after 4 hours of culture, a significantly higher proportion of Nalm6-BMP4 cells were able to cross through the brain endothelial monolayers, and this effect could be blocked by inhibiting the BMP signaling pathway. These results support the fact that high BMP4 expression in leukemic blasts could be a potential mechanism of greater invasiveness. In this sense, the analysis of endothelial cells cocultured with leukemic blasts in the presence or absence of rhBMP4 showed a significantly upregulated expression of VCAM-1, a molecule involved in leukocyte–endothelial cell adhesion, together with a reduced expression of VE-Cadherin, a transmembrane component of adherens junctions in endothelial cells, when leukemic cells and high levels of BMP4 were present in the cultures (Figure 4C). In addition, leukemic cells recovered from the spleens of NSG mice transplanted with Nalm6-BMP4 cells showed a significantly increased expression of VLA-4 and LFA-1 adhesion molecules (Figure 4D). Similarly, high levels of BMP4 were shown to induce a significant increase in the in vitro expression levels of the VCAM-1 ligand, VLA‐4, in Nalm6 cells (data not shown). In correlation, the expression of the metalloproteinase ADAM10, an important regulator of vascular permeability, was also found to be significantly increased in Nalm6-BMP4 cells recovered from xenografted mice (Figure 4D).
High levels of BMP4 induce changes in adhesion molecule expression favoring leukemia cell migration. (A) Comparison of the migration ability of Nalm6 and Nalm6-BMP4 leukemic cells across monolayers of human brain microvascular endothelial cells in a transwell culture system for 4 hours. Results represent the mean ± standard deviation (SD) of 9 independent experiments (***P ≤ .001; 2-tailed Student t test). (B) The migration ability of Nalm6-BMP4 cells was similarly assayed in the presence of BMP signaling pathway inhibitors, BMPRIA-Fc chimera protein (IA-Fc), and DMH1 (*P ≤ .05; 2-tailed Student t test). (C) qRT-PCR quantification of mRNA levels of VCAM-1 and VE-cadherin in human brain microvascular endothelial cells in the presence or absence of rhBMP4 with or without ALL cells. Results represent the mean ± SD of 4 independent experiments (*P ≤ .05; 2-tailed Mann-Whitney U test). (D) qRT-PCR quantification of mRNA levels of VLA-4 and LFA-1 integrins and ADAM10 in leukemic cells recovered from spleens of mice (n = 5) transplanted with Nalm6 or Nalm6-BMP4 cells (*P ≤ .05; 2-tailed Mann-Whitney U test).
High levels of BMP4 induce changes in adhesion molecule expression favoring leukemia cell migration. (A) Comparison of the migration ability of Nalm6 and Nalm6-BMP4 leukemic cells across monolayers of human brain microvascular endothelial cells in a transwell culture system for 4 hours. Results represent the mean ± standard deviation (SD) of 9 independent experiments (***P ≤ .001; 2-tailed Student t test). (B) The migration ability of Nalm6-BMP4 cells was similarly assayed in the presence of BMP signaling pathway inhibitors, BMPRIA-Fc chimera protein (IA-Fc), and DMH1 (*P ≤ .05; 2-tailed Student t test). (C) qRT-PCR quantification of mRNA levels of VCAM-1 and VE-cadherin in human brain microvascular endothelial cells in the presence or absence of rhBMP4 with or without ALL cells. Results represent the mean ± SD of 4 independent experiments (*P ≤ .05; 2-tailed Mann-Whitney U test). (D) qRT-PCR quantification of mRNA levels of VLA-4 and LFA-1 integrins and ADAM10 in leukemic cells recovered from spleens of mice (n = 5) transplanted with Nalm6 or Nalm6-BMP4 cells (*P ≤ .05; 2-tailed Mann-Whitney U test).
Infiltration of leukemia cells producing high BMP4 levels promotes angiogenesis in CNS
BMP4 has been demonstrated to be able to induce angiogenesis in physiological and pathological conditions.38,39 A marked angiogenesis has been described in the BM of both pediatric BCP-ALL patients and ALL xenografted mice,40-42 but to our knowledge, there is no information on a similar angiogenic situation in CNS after leukemic blast infiltration.
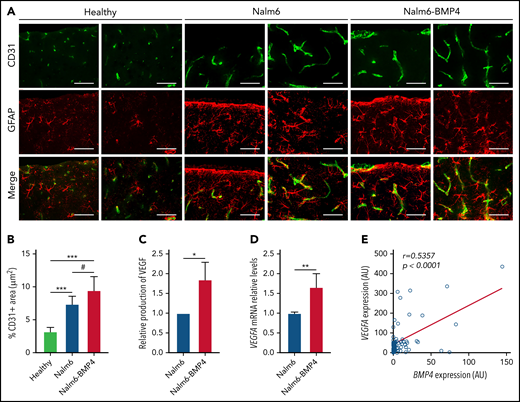
As indicated by the flow cytometry analysis (Figure 2H), histopathology studies of brains from mice injected with Nalm6-BMP4 or Nalm6 control cells revealed that both groups exhibited lymphoblastic infiltration of the subarachnoid space (data not shown). Of note, compared with healthy animals, brain sections of ALL mice showed the occurrence of an astroglial response as well as an active angiogenesis evidenced by the presence of neovessels and endothelial sprouts (Figure 5A). Immunofluorescence images and quantification of CD31 expression further indicated that BMP4-overexpressing leukemic blasts were able to induce a significantly greater GFAP+ astroglial reaction and neovascularization in the nervous parenchyma than control leukemic cells (Figure 5A-B). Also, Nalm6-BMP4 mice showed, in comparison with control Nalm6 mice, an increase in the number and size of blood vessels in other extramedullary tissues infiltrated by leukemic blasts (data not shown).
High levels of BMP4 increase VEGF expression in leukemic cells and exacerbate angiogenesis in CNS of xenografted mice. (A) Representative images of CD31+ endothelial cells (green) and GFAP+ astrocytes (red) in sagittal brain cryosections of healthy, Nalm6, and Nalm6-BMP4 mice (Scale bars, 50 μm). (B) Quantitation of microvessel density using CD31 immunofluorescence. Three random cortical areas per section were imaged at magnification ×40. Fiji software was used for computerized quantification of immunostained vascular structures, and positive pixels were quantified and expressed as a percentage (±standard deviation) of CD31+ area per tissue total area (4 cryosections/3 mice per group; #P ≤ .05 and ***P ≤ .001; 2-tailed Student t test). (C) VEGFα production in Nalm6 and Naml6-BMP4 cell cultures (n = 4 independent experiments). Results are shown as increments relative to Nalm6 control cultures (*P ≤ .05; 2-tailed Mann-Whitney U test). (D) VEGFA mRNA expression in leukemic cells recovered from spleens of Nalm6 (n = 4) and Nalm6-BMP4 (n = 6) mice. Results are presented as increments relative to Nalm6 control cells (**P ≤ .01; 2-tailed Mann-Whitney U test). (E) Pearson’s correlation coefficient (r) and P value between VEGFA and BMP4 mRNA expression in primary BCP-ALL samples at diagnosis are shown (n = 57).
High levels of BMP4 increase VEGF expression in leukemic cells and exacerbate angiogenesis in CNS of xenografted mice. (A) Representative images of CD31+ endothelial cells (green) and GFAP+ astrocytes (red) in sagittal brain cryosections of healthy, Nalm6, and Nalm6-BMP4 mice (Scale bars, 50 μm). (B) Quantitation of microvessel density using CD31 immunofluorescence. Three random cortical areas per section were imaged at magnification ×40. Fiji software was used for computerized quantification of immunostained vascular structures, and positive pixels were quantified and expressed as a percentage (±standard deviation) of CD31+ area per tissue total area (4 cryosections/3 mice per group; #P ≤ .05 and ***P ≤ .001; 2-tailed Student t test). (C) VEGFα production in Nalm6 and Naml6-BMP4 cell cultures (n = 4 independent experiments). Results are shown as increments relative to Nalm6 control cultures (*P ≤ .05; 2-tailed Mann-Whitney U test). (D) VEGFA mRNA expression in leukemic cells recovered from spleens of Nalm6 (n = 4) and Nalm6-BMP4 (n = 6) mice. Results are presented as increments relative to Nalm6 control cells (**P ≤ .01; 2-tailed Mann-Whitney U test). (E) Pearson’s correlation coefficient (r) and P value between VEGFA and BMP4 mRNA expression in primary BCP-ALL samples at diagnosis are shown (n = 57).
On the basis of these findings, we next analyzed whether high BMP4 levels on leukemic cells could autocrinally regulate the production of the proangiogenic factor VEGF, previously identified as a critical mediator of CNS disease.14Figure 5C shows that VEGF levels were significantly increased in the supernatants from Nalm6-BMP4 cultures, compared with Nalm6 control cultures. Likewise, a higher VEGF mRNA expression could be detected in leukemic blasts isolated from Nalm6–BMP4-injected mice (Figure 5D). Finally, we determined in leukemic blasts from BCP-ALL patients the possible correlation between BMP4 and VEGF expression, and we found a positive and significant correlation between these 2 factors (Figure 5E).
Discussion
The involvement of BMP4 in BCP-ALL has been pointed out by previous data from our group and others showing an upregulated BMP4 expression in CNS-infiltrating ALL blasts; the ability of ALL cell-derived BMP4 to impair the differentiation of dendritic cells and macrophages promoting an immunosuppressive and protumoural phenotype; and the occurrence of BMP4-dependent variations in the biology of ALL-derived BM mesenchymal stem cells.34,35,37 In the present work, we provide clinical and experimental evidence on the relevance of BMP4 in the establishment and development of BCP-ALL and CNS leukemia.
Our data show that high expression levels of BMP4 are associated with a poor prognosis in BCP-ALL, mainly of standard-risk patients, indicating that BMP4 could be considered as a new marker for the assessment of relapse in BCP-ALL. Together with the clinical data, the differential grafting capacity shown by leukemic blasts in the xenogeneic model depending on BMP4 levels supports the relevance of this BMP ligand in the establishment and maintenance of BCP-ALL. Previous work has demonstrated that BMP4 treatment significantly improves short and long-term repopulation of hematopoietic stem cells in lethally irradiated mice by increasing the adhesion of stem cell progenitors to the stroma through induction of VLA-4 expression.19,43 Pioneer work by Filshie et al44 demonstrated that a variant of the Nalm6 cell line lacking VLA-4 expression showed reduced BM infiltration in a xenograft mouse model. More recently, several authors, using different experimental approaches to block VLA-4/VCAM-1 signaling, have identified the α4 integrin as a central mediator of drug resistance in BCP-ALL.45,46 In this sense, high VLA-4 expression has been associated in BCP-ALL patients with adverse prognostic factors, poor molecular response to therapy, and significantly worse probabilities of event-free and overall survival.45,47 In our studies, the enforced expression of BMP4 in Nalm6 cells significantly increased the expression of VLA-4, and also LFA-1, mechanisms whereby leukemic blasts may enhance their adhesion capacity to stromal cells, which could favor leukemia establishment and maintenance in the BM and contribute to adhesion-mediated drug resistance. As described for normal hematopoiesis,19 this induction of VLA-4 expression by BMP4 would be dependent on noncanonical signaling, which would explain the fact that the administration of DMH1 inhibitor does not impact the establishment of leukemic blasts in the BM.
BMPs have been demonstrated to induce stem cell quiescence both in human normal tissues and in different cancers, including hematological tumors.31,33,48-50 BMP4 and the receptors BMPRIB or BMPRIA have been proposed to constitute a major signal driving stem cell persistence in chronic myeloid leukemia or acute myeloid leukemia, respectively.31,33 Furthermore, BMP4 produced by the BM microenvironment in myeloid leukemia directly controls the acquisition of stemness features, including quiescence and the induction of chemoresistance.31,51,52 In accordance, our results demonstrate that despite the greater severity of clinical symptoms, animals injected with BMP4-overexpressing blasts showed reduced numbers of leukemic cells, which exhibited chemoresistance in consonance with their low proliferative rate. This could favor residual leukemic survival in the BM of patients having achieved remission under treatment and be responsible for subsequent relapses, as indicated by the clinical data shown in the present study.
Inflammation and B-leukemogenesis have been previously reported to be interrelated and, indeed, increased levels of proinflammatory cytokines have been described in BM, plasma, and cerebrospinal fluid of BCP-ALL patients at diagnosis.53-55 Interestingly, BMP4 has been described as an inductor of inflammatory responses in different pathologies affecting distinct cell types,56-58 being able to induce the expression of proinflammatory cytokines such as IL-6 and TNF-α.59,60 We found that mice injected with blasts expressing high BMP4 levels had increased serum values of the proinflammatory IL-6 cytokine and developed more aggressive clinical symptoms, with complete paralysis of hind limbs, faster than ALL control mice. On the contrary, inhibition of BMP4 canonical signaling with DMH1, although it was not sufficient to ameliorate BM disease, had a significant effect reducing neural clinical symptoms in correlation with the lower IL-6 levels found in DMH1-treated mice. Therefore, the increased levels of BMP4 could promote the proinflammatory condition observed in xenografted mice and described in BCP-ALL patients.53,54 In this context, it could also be postulated that the local production of BMP4 by leukemic cells could contribute to the earlier appearance of neurological symptoms, such as hind limb paralysis, shown in our results from the mouse model. In support, the exogenous administration of BMPs for inducing bone formation in patients with fractures have been reported to provoke inflammatory complications, including radiculitis.61-63
Our results also point out a role for high levels of BMP4 in CNS leukemia involvement. Inhibition of BMP4 signaling in the in vivo model showed a compartment-specific effect with significantly decreased leukemia burden in CNS and associated clinical symptoms. Moreover, leukemic blasts expressing high BMP4 levels showed an increased CNS homing capacity, and mechanistic investigation in BMP4-treated brain microvascular endothelial cells revealed that BMP4 signaling increased the expression of adhesion molecules such as the VLA-4 receptor, VCAM-1, and reduced the levels of VE-cadherin, the major adhesion molecule in endothelial adherens junctions. Therefore, these BMP4-driven changes in brain endothelial cells along with an exacerbated central inflammation, suggested by an increased astroglial reaction, could favor the transendothelial migration of leukemic cells to CNS. It is necessary to note, however, that since the number of patients experiencing isolated CNS relapse in the cohorts analyzed is limited, it remains to be demonstrated whether BMP4 facilitates CNS infiltration independently of the risk of BM relapse.
A major contribution of this work is the finding that leukemic blasts expressing high levels of BMP4 induce an increased angiogenesis in the CNS of ALL xenografted mice. BMPs have been described to be involved in tumor angiogenesis by either directly regulating the proliferation and migration of vascular endothelial cells or indirectly through the upregulation of the expression of proangiogenic factors in tumor cells or the tumor microenvironment.39,64-67 More concretely, the canonical BMP4 signaling pathway, through the induction of VEGF expression, has been described as a requirement for the correct development of the embryonic vascular network, including brain capillaries.38,39,68,69 The expression of VEGF has been identified in ALL as a critical mediator of CNS disease favoring migration of leukemic cells and their hypoxic adaptation to the new microenvironment.14,70 Given that our results indicate that the overexpression of BMP4 in leukemic cells provokes similar effects to those described for VEGF and that BMP4 induces upregulation of VEGF in ALL cells, it is possible to suggest that BMP4 could be responsible, at least in part, for the previously described role of VEGF in CNS involvement. Supporting this, we found a positive correlation between BMP4 and VEGF mRNA expression levels in BCP-ALL samples. In addition, Gaynes et al,35 using the Nalm6 xenograft model, analyzed the gene expression profiling of approximately 700 cancer-associated genes in leukemic blasts located in the BM and CNS and found that BMP4 and VEGF were among the 30 genes upregulated in CNS leukemic cells.
Taken together, clinical and experimental results shown in this study indicate an important role for BMP4 in BCP-ALL development and CNS leukemic infiltration and point out that BMP4 could be a new therapeutic target to prevent leukemic CNS involvement and disease relapse.
Acknowledgments
This work was supported by grants RTI2018-093899-B-I00 (Spanish Ministry of Economy and Competitiveness), RD16/0011/0002, RD21/0017/0005, and RD21/0017/0010 (Institute of Health Carlos III, Madrid, Spain), B2017/BMD-3692 AvanCell-CM (Community of Madrid), and Beca I-UnoEntreCienMil (Uno Entre Cien Mil Foundation). L.M.F.d.S. was supported by a predoctoral fellowship (CT45/15‐CT46/15) from the Complutense University of Madrid. P.O.-S. is supported by a predoctoral fellowship (CT63/19-CT64/19) from the Complutense University. M.V.M.-S. is supported by Asociación Pablo Ugarte.
Authorship
Contribution: L.M.F.d.S. performed experiments, acquired data, analyzed results, interpreted data, and wrote the manuscript; J.V., P.O.-S., A.F.-R., E.J., and R.S. performed experiments and acquired data; P.Z. carried out the statistical analysis; A.M., M.V.M.-S., J.J., M.D., B.F., M.S., D.S., and G.C. provided patient samples and clinical information; M.R. obtained patient samples and clinical information and codesigned the study; and A. Varas and Á. Vicente conceived and designed the research, supervised the study, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: M.R. received a research grant from Orgenesis Inc. The remaining authors declare no competing financial interests.
Correspondence: Ángeles Vicente, Department of Cell Biology, Faculty of Medicine, Plaza Ramón y Cajal s/n, Complutense University, 28040 Madrid, Spain; e-mail: avicente@ucm.es; and Alberto Varas, Department of Cell Biology, Faculty of Medicine, Plaza Ramón y Cajal s/n, Complutense University, 28040 Madrid, Spain; e-mail: avaras@ucm.es.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
A. Varas and Á. Vicente contributed equally to this study.


![BMP4high BCP-ALL blasts exhibit an increased leukemogenic activity. (A) Outline of the in vivo experimental design for panels B-E and H. BMP4high and BMP4low leukemic blasts were IV-transplanted into NSG mice at day 0. Recipient mice were monitored for disease symptoms and the presence of blasts in blood and were euthanized upon the appearance of clinical signs. (B) Nine primary samples of pediatric BCP-ALL patients were chosen according to BMP4 mRNA expression (4 BMP4high primary samples [patients #1, #2, #3, #8] and 5 BMP4low primary samples [patients #4, #5, #6, #7, #9] as shown in supplemental Table 1) and xenografted into 3 to 5 mice. Mice were followed for 4 months or were euthanized when they showed clinical signs. Kaplan-Meier curves show reduced survival in mice xenografted with BMP4high primary samples (P = .0001; log-rank test). (C) Nalm6 or Nalm6-BMP4 (0.5 x 105 cells per mouse) were xenografted into NSG mice. Reduced survival rate was observed in mice injected with Nalm6-BMP4 ALL blasts (P = .007; log-rank test). (D) IL-6 serum levels in NSG mice transplanted with Nalm6 or Nalm6-BMP4 cells and in control healthy mice (n = 5-6 mice per group). Each dot represents a single transplanted mouse, and lines represent the mean level of IL-6 in each group (**P ≤ .01 and #P ≤ .05 represent statistical significances relative to control healthy mice [*] or Nalm6 mice [#]). (E) Percentages (mean ± standard deviation [SD]) and absolute numbers of leukemic blasts recovered from the spleen of mice xenografted with either Nalm6 (n = 7) or Nalm6-BMP4 (n = 10) cells. Each dot represents a transplanted mouse, and bars and lines represent the mean percentages and absolute numbers (**P ≤ .01 and ***P ≤ .001). (F) Proliferation rate of Nalm6 and Nalm6-BMP4 leukemic cells recovered from the spleen of xenografted mice (n = 6), determined by 7-AAD staining and analyzed by flow cytometry. Results represent the mean ± SD of cells in G0/G1 phases (**P ≤ .01). Representative flow cytometry histograms are shown in the right panel. (G) Relative viability of Nalm6-BMP4 leukemic cells with respect to Nalm6 cells recovered from spleens of xenografted mice (n = 4) and cultured during 48 hours with different concentrations of methotrexate (MTX) and cytarabine (Ara-C) (*P ≤ .05). (H) Percentage of CD19+ leukemic blasts infiltrated in CNS in either Nalm6 (n = 7) or Nalm6-BMP4 (n = 10) mice. Lines represent average percentages of CNS-infiltrating CD19+ cells for each cohort (P = .058). Data in (D-H) panels were compared using 2-tailed Mann-Whitney U tests.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/22/10.1182_blood.2021013506/3/m_bloodbld2021013506f2.png?Expires=1769080030&Signature=GdEpPspd9iXgOC-zCDrPCTbo52jVZ7I97RJUOe1Tf~GjILCeejRlHy3QJfNfGBJeuhiSNJ2TQKn0dvHX4dBxKq3Q-TsuuDT9QBx0mBU-WRiklSSI4-dn6v-hwjW3GZTaEoqWZvbFTUijFwXVjjjeYpb5AXnF88j8oiAj61Ve4fybiRh8CWLNqEM1-lZyBCzkuw6AQrRwBHMrmKEQX0zoUwFlqGuWCeLsuKeLIKzqDr60dP6a7iIp8pQ4LrpXkLaSvn9TUM~9Jx7X2et6L9vghQApRGF9~C7M8uZKzSROGSS9Vk2P7Yh8du6WSERyxnW6nQza~EWxgSVeq1OMi~-nEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Inhibition of canonical BMP4 signaling reduces ALL CNS infiltration and neurological clinical symptoms. (A) Outline of the in vivo experimental design for panels (B-F). Mice were transplanted with BMP4high primary samples at day 0 (samples from patients #1, #2, and #3, which were shown to engraft in 100% animals in previous experiments, were used). Leukemia development was monitored, and 8 days after injection, mice transplanted with each sample (6-8 animals per sample) were randomly divided into 2 groups (n = 11 mice per group) and treated with DMH1 or control DMSO. All animals were euthanized upon detection of hind limb paralysis in DMSO control mice. (B) Both control DMSO- and DMH1-treated mice (n = 11 mice per group) showed similar engraftment in peripheral organs. Bars show mean percentages (±standard deviation [SD]) of CD19+ leukemic blasts in BM, spleen, and PB. (C) CNS leukemia infiltration was reduced in DMH1- vs control DMSO-treated animals (n = 11 mice per group). Each data point represents a single mouse transplanted with blasts from patients #1, #2, or #3 (shown as circles, squares, or triangles), and lines represent the mean percentage of CD19 leukemic blasts in each group (**P ≤ .01; 2-tailed Mann-Whitney U test). (D) Bars represent average serum IL-6 levels measured in DMSO- and DMH1-treated mice at euthanasia (n = 4 mice per group; *P ≤ .05; 2-tailed Mann-Whitney U test). (E) Olfactory habituation–dishabituation test of healthy (green triangles), DMSO- (blue circles), and DMH1-treated (red squares) mice (n = 4 mice per group). Data represent the exploration time (mean ± SD) of successive cotton swabs soaked in octanal, heptanal, or anisole. Two-way ANOVA followed by Bonferroni correction showed significant differences for heptanal and anisole odors: *P ≤ .05 and **P ≤ .01 show significant differences between vehicle-DMSO and healthy control mice; #P ≤ .05 shows significant differences between DMSO- and DMH1-treated mice. (F) The incidence of hind limb paralysis in DMH1-treated mice was notably reduced with respect to the DMSO control group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/22/10.1182_blood.2021013506/3/m_bloodbld2021013506f3.png?Expires=1769080030&Signature=MH7Gp4Z1h75CGUITRXG~J~vrl-QwXZUdEljzzqDGmFmpHNg84o~wHQQdiqdGVu38gnELU0QxwKXkonq-T1V4~r1iebQJ6~o77IvGPoYovXYhNPhwTTaJE3FbDxw1LWViKTYzY9JwwONTXBwl7RtR6MCI0h60xvkoF7vMDDzzgW6Uqbhq2B0xcDXqv89rEKnloZ1UwE8fVtZuc38TGkP7z2wmxm56mPfhjlt54QbjAOJT89hRg~1lQJK8MW5EzZj2Kv4ITxgrBAUQpwz8fLW-oVLAy7E~Iupmv0U4pYjtyb9E~A6BoSzWMc4TVN5KNIJHm8ZM9NZTJzjX2M3ZKw1lCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal