Key Points
Inhibition of Sphk2 sustains long-term self-renewal, increases regenerative potential, and functionally attenuates the aging of HSCs.
Loss of Sphk2 improves anaerobic metabolism and stem cell fitness through the Sphk2-HIF1α-PDK3 axis.
Abstract
Hematopoietic stem cells (HSCs) have reduced capacities to properly maintain and replenish the hematopoietic system during myelosuppressive injury or aging. Expanding and rejuvenating HSCs for therapeutic purposes has been a long-sought goal with limited progress. Here, we show that the enzyme Sphk2 (sphingosine kinase 2), which generates the lipid metabolite sphingosine-1-phosphate, is highly expressed in HSCs. The deletion of Sphk2 markedly promotes self-renewal and increases the regenerative potential of HSCs. More importantly, Sphk2 deletion globally preserves the young HSC gene expression pattern, improves the function, and sustains the multilineage potential of HSCs during aging. Mechanistically, Sphk2 interacts with prolyl hydroxylase 2 and the Von Hippel-Lindau protein to facilitate HIF1α ubiquitination in the nucleus independent of the Sphk2 catalytic activity. Deletion of Sphk2 increases hypoxic responses by stabilizing the HIF1α protein to upregulate PDK3, a glycolysis checkpoint protein for HSC quiescence, which subsequently enhances the function of HSCs by improving their metabolic fitness; specifically, it enhances anaerobic glycolysis but suppresses mitochondrial oxidative phosphorylation and generation of reactive oxygen species. Overall, targeting Sphk2 to enhance the metabolic fitness of HSCs is a promising strategy to expand and rejuvenate functional HSCs.
Introduction
Maintaining hematopoietic stem cell (HSC) quiescence vs cell proliferation and differentiation requires a dynamically regulated metabolic program during homeostasis, after injury regeneration, and aging.1-4 HSCs generate ATP(adenosine triphosphate) via anaerobic glycolysis in hypoxic microenvironments.5,6 Restricted mitochondrial respiration keeps HSCs in a low oxidative phosphorylation (OXPHOS) and low reactive oxygen species (ROS) state; this metabolic fitness maintains the quiescence and genomic integrity to protect HSCs from exhaustion.7,8 Perturbation of this metabolic state impairs function in young and old HSCs.3,5,6,9-15 The critical role of metabolic program in HSC maintenance is well known; however, altering the metabolic machinery to improve the function of HSCs is an unmet and challenging task.
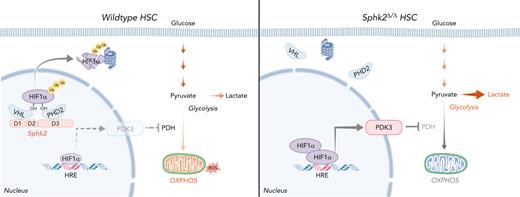
S1P (sphingosine-1-phosphate) is a pleiotropic sphingolipid metabolite that regulates numerous biologically significant functions, including cancer, vascular maturation, blood cell circulation, and erythrocyte differentiation.16-18 S1P gradient in the bloodstream, which is generated by erythrocytes, platelets, and vessel endothelial cells,19,20 regulates lymphocyte trafficking, T-lymphoblastic lymphoma intravasation, and AMD3100 or granulocyte-colony stimulating factor–induced hematopoietic stem and progenitor cell (HSPC) mobilization via S1P receptor 1.21-26 Although S1P signaling regulates hematopoiesis, the role of S1P generating sphingosine kinase isoenzymes, Sphk1 and Sphk2, in HSCs remains unknown.27,28 In this study, we found that Sphk2 regulates hypoxic responses and metabolic fitness in HSCs independent of its catalytic activity. Deletion of Sphk2 expands the pool, maintains the quiescence, promotes the regenerative potential, and functionally attenuates the aging of HSCs by improving their metabolic fitness through the Sphk2-HIF1α-PDK3 axis.
Methods
Animals
B6.129S6-Sphk1tm1Rlp/J (Sphk1Δ/Δ), B6.129S6-Sphk2tm1Rlp/J (Sphk2Δ/Δ), and Vav-Cre mice were obtained from the Jackson Laboratory. Pdk3Δ/Δ mice were generated by deleting exons 1-2 (Shang Hai Model Organisms). Sphk2flox/flox mice have loxP sites flanking exon 3 of the Sphk2 gene (Cyagen Biosciences Inc). Hif1αflox/flox mice have loxP sites flanking exon 2 of the HIF1α gene (Shang Hai Model Organisms).
Transplantation
A total of 2 × 105 CD45.2+ bone marrow (BM) cells or 100 HSCs (CD45.2+) and 2 × 105 CD45.1+ rescue BM cells were transplanted into lethally irradiated (9 Gy) CD45.1+ recipient mice. For secondary or tertiary transplantation, BM cells from each recipient were injected into 1 or 2 new irradiated mice (1 × 106 per recipient, depending on the number of recipients) in each round of transplantation. None of the mice were excluded unless they died during the 16-week observation period.
Repopulation assay
Every 4 weeks after transplantation, peripheral blood was collected from the tail vein. Hematopoietic repopulation was measured from donor-derived blood cells (CD45.2+).
Results
Sphk2 deletion increases HSC function during homeostasis and injury
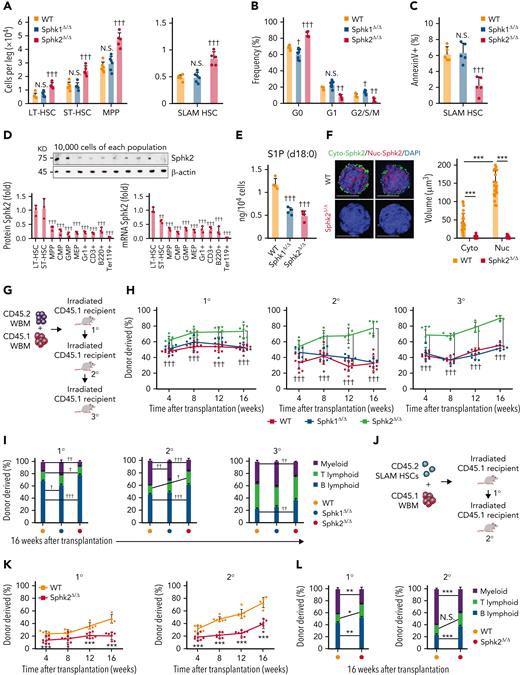
To investigate the role of sphingosine kinase isoenzymes in HSCs, we used Sphk1 and Sphk2 knockout mice. We observed an increase in the number of long-term HSCs (LT-HSCs), short-term HSCs (ST-HSCs), multipotent progenitor cells (MPPs), and SLAM HSCs (hereinafter referred to as HSCs) in Sphk2-deficient mice but not in Sphk1-deficient mice (Figure 1A). The hematopoietic cell pool was expanded in HSPCs and early lymphoid progenitors but not in other progenitor populations, mature blood cells, or platelets in Sphk2-knockout mice (supplemental Figure 1A-E in the data supplement). Sphk2 deletion did not affect the T-cell development in the thymus or B-cell development in the BM and spleen (supplemental Figure 1F-G). The increased HSC numbers in Sphk2-deficient mice were not because of HSC activation because HSC quiescence was increased in Sphk2-deficient mice but not in the Sphk1-deficient or control mice (Figure 1B). Increased quiescence was also observed in LT-HSCs and other HSC subpopulations in Sphk2-deficient mice (supplemental Figure 1H). We repeatedly observed increased HSC numbers and quiescence in Vav-Cre+; Sphk2f/f mice compared with Vav-Cre–; Sphk2f/f or Vav-Cre+; Sphk2+/+ control mice, which ruled out the potential influence of nonhematopoietic tissues (supplemental Figure 1I-J). Sphk2-deficient HSCs showed a decreased apoptosis rate (Figure 1C), which partially explained how Sphk2 deletion increased the HSC pool size without decreasing quiescence.
Sphk2 deletion increases HSC function during homeostasis. (A-C) (A) The absolute number of HSPCs (LT-HSC, ST-HSC, MPP, and SLAM HSC) (WT n = 6 mice, Sphk1Δ/Δ n = 6 mice, and Sphk2Δ/Δ n = 5 mice) in the BM, (B) cell cycle analysis (WT n = 4 mice, Sphk1Δ/Δ n = 8 mice, and Sphk2Δ/Δ n = 4 mice), and (C) apoptosis analysis (n = 5 mice per group) of HSCs in BM from Sphk1Δ/Δ, Sphk2Δ/Δ, or control mice. (D) Western blots (top) and quantification (bottom left) of relative Sphk2 protein expression and Sphk2 mRNA expression (bottom right) in each hematopoietic population as indicated from wild-type C57BL/6J mice (n = 3 mice). (E) Targeted metabolomics detection of S1P via LC/MS in HSCs (n = 3 mice). (F) Representative images of sorted HSCs immunostained with Sphk2 (left) and quantification of Sphk2 expression (right) in the cytoplasm (Cyto-Sphk2) and nuclear (Nuc-Sphk2) in HSCs from Sphk2Δ/Δ or control mice (n = 25 cells from 3 mice). (G) Scheme for quantification of functional HSCs by transplantation assay. A total of 2 × 105 BM cells from Sphk1Δ/Δ, Sphk2Δ/Δ, or control mice were transplanted into irradiated mice along with 2 × 105 recipient BM cells. A total of 1 × 106 BM cells from primary or secondary recipient mice were transplanted into irradiated mice in secondary transplantation or tertiary transplantation, respectively. (H) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (I) percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (1° WT n = 9-10 mice, Sphk1Δ/Δ n = 8-10 mice, and Sphk2Δ/Δ n = 7-8 mice; 2° WT n = 9 mice, Sphk1Δ/Δ n = 6-7 mice, and Sphk2Δ/Δ n = 6-7 mice; 3° WT n = 4-7 mice, Sphk1Δ/Δ n = 5-8 mice, and Sphk2Δ/Δ n = 4-6 mice). (J) Scheme for quantification of HSC self-renewal potential by transplantation assay. A total of 100 purified HSCs from Sphk2Δ/Δ or control mice were transplanted into irradiated mice along with 2 × 105 recipient BM cells. A total of 1 × 106 BM cells from primary recipient mice were transplanted into irradiated mice in secondary transplantation. (K) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (L) percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (1° WT n = 7-8 mice, Sphk2Δ/Δ n = 4-6 mice; 2° WT n = 6-8 mice, and Sphk2Δ/Δ n = 5-8 mice). 1°, primary transplantation; 2°, secondary transplantation; 3°, tertiary transplantation. (F) Scale bar 5 μm. Data represented as mean ± standard deviation. Two-tailed Student t test assessed statistical significance, ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. One-way ANOVA with Tukey‘s multiple comparison post hoc test, †P < .05, ††P < .01, and †††P < .001. N.S., not significant.
Sphk2 deletion increases HSC function during homeostasis. (A-C) (A) The absolute number of HSPCs (LT-HSC, ST-HSC, MPP, and SLAM HSC) (WT n = 6 mice, Sphk1Δ/Δ n = 6 mice, and Sphk2Δ/Δ n = 5 mice) in the BM, (B) cell cycle analysis (WT n = 4 mice, Sphk1Δ/Δ n = 8 mice, and Sphk2Δ/Δ n = 4 mice), and (C) apoptosis analysis (n = 5 mice per group) of HSCs in BM from Sphk1Δ/Δ, Sphk2Δ/Δ, or control mice. (D) Western blots (top) and quantification (bottom left) of relative Sphk2 protein expression and Sphk2 mRNA expression (bottom right) in each hematopoietic population as indicated from wild-type C57BL/6J mice (n = 3 mice). (E) Targeted metabolomics detection of S1P via LC/MS in HSCs (n = 3 mice). (F) Representative images of sorted HSCs immunostained with Sphk2 (left) and quantification of Sphk2 expression (right) in the cytoplasm (Cyto-Sphk2) and nuclear (Nuc-Sphk2) in HSCs from Sphk2Δ/Δ or control mice (n = 25 cells from 3 mice). (G) Scheme for quantification of functional HSCs by transplantation assay. A total of 2 × 105 BM cells from Sphk1Δ/Δ, Sphk2Δ/Δ, or control mice were transplanted into irradiated mice along with 2 × 105 recipient BM cells. A total of 1 × 106 BM cells from primary or secondary recipient mice were transplanted into irradiated mice in secondary transplantation or tertiary transplantation, respectively. (H) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (I) percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (1° WT n = 9-10 mice, Sphk1Δ/Δ n = 8-10 mice, and Sphk2Δ/Δ n = 7-8 mice; 2° WT n = 9 mice, Sphk1Δ/Δ n = 6-7 mice, and Sphk2Δ/Δ n = 6-7 mice; 3° WT n = 4-7 mice, Sphk1Δ/Δ n = 5-8 mice, and Sphk2Δ/Δ n = 4-6 mice). (J) Scheme for quantification of HSC self-renewal potential by transplantation assay. A total of 100 purified HSCs from Sphk2Δ/Δ or control mice were transplanted into irradiated mice along with 2 × 105 recipient BM cells. A total of 1 × 106 BM cells from primary recipient mice were transplanted into irradiated mice in secondary transplantation. (K) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (L) percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (1° WT n = 7-8 mice, Sphk2Δ/Δ n = 4-6 mice; 2° WT n = 6-8 mice, and Sphk2Δ/Δ n = 5-8 mice). 1°, primary transplantation; 2°, secondary transplantation; 3°, tertiary transplantation. (F) Scale bar 5 μm. Data represented as mean ± standard deviation. Two-tailed Student t test assessed statistical significance, ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. One-way ANOVA with Tukey‘s multiple comparison post hoc test, †P < .05, ††P < .01, and †††P < .001. N.S., not significant.
HSCs had the highest expression level of Sphk2 compared with committed progenitors and differentiated cells (Figure 1D). Sphk2 deletion did not influence the expression of Sphk1 in HSCs (supplemental Figure 1K-L). Our targeted metabolomics assay showed that S1P was enriched in HSCs compared with differentiated cells (supplemental Figure 1M) and remarkably reduced in HSCs when Sphk1 or Sphk2 was deleted (Figure 1E), with a compensatory increase of sphingomyelin and ethanolamine-1-phosphate (supplemental Figure 1N). In contrast to Sphk1, Sphk2 regulated HSC function, which suggested that Sphk2 might regulate HSCs independent of its catalytic activity. Furthermore, Sphk2 was more abundant in the nucleus than in the cytoplasm of HSCs (Figure 1F), suggesting that Sphk2 might have a nuclear function.
We performed serial transplantation assays to test the impact of Sphk deficiency on the long-term function of HSCs (Figure 1G). Sphk2-deficient but not Sphk1-deficient BM cells showed significantly higher reconstitution levels than control cells throughout the 16-week observation period (Figure 1H). Sphk2-deficient donor cells also showed higher reconstitution levels in the secondary and tertiary recipient mice (Figure 1H). Increased reconstitution was also observed in Vav-Cre+; Sphk2f/f mice compared with that in Vav-Cre–; Sphk2f/f littermates, which demonstrated that Sphk2 cell autonomously regulates HSC function (supplemental Figure 1O). Sphk2 deletion robustly enhanced lymphopoiesis, which was compromised during serial transplantation in Sphk1-deficient or control recipients (Figure 1I; supplemental Figure 1P), which suggested that Sphk2 deletion preserved the lineage-output balance of HSCs. Sphk2-deficient BM cells also generated more donor-derived BM cells and HSPCs in recipients during serial transplantations (supplemental Figure 1Q-Y). In addition, Sphk2 deletion did not affect HSC homing (supplemental Figure 1Z). To analyze whether Sphk2 deletion increased the self-renewal activity of HSCs, we transplanted Sphk2-deleted or control HSCs into irradiated recipients (Figure 1J). Sphk2-deficient HSCs showed significantly higher reconstitution levels than control HSCs through 2 rounds of 16-week observations (Figure 1K). In addition, Sphk2-deficient HSCs preserved the lineage-output balance (Figure 1L) and generated more donor-derived BM cells and HSPCs in recipients (supplemental Figure 1AA-AD).
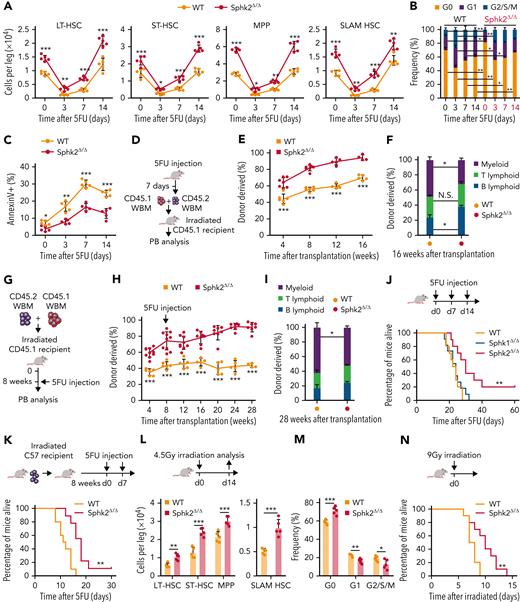
To evaluate whether Sphk2 deficiency regulates HSC regenerative potential, we tested the response of mice to 5-fluorouracil (5FU), which ablates cycling cells and induces HSC regeneration to recover hematopoiesis. Sphk2-deficient mice showed increased pool size (Figure 2A; supplemental Figure 2A), accelerated quiescence restoration (Figure 2B; supplemental Figure 2B), and reduced apoptosis (Figure 2C) in HSCs during 5FU-induced regeneration. We performed a competitive repopulation analysis using BM cells on day 7 after 5FU treatment (Figure 2D). Sphk2-deficient BM donors showed higher reconstitution that was multilineage (Figure 2E-F) and generated more donor-derived HSPCs (supplemental Figure 2C) than control donors. To further assess whether Sphk2-deficient HSCs improved the regenerative capacity, we challenged recipient mice with 5FU 8 weeks after transplantation with Sphk2-deficient or control BM donor cells (Figure 2G). Sphk2-deficient BM donors showed higher reconstitution that was multilineage (Figure 2H-I) and more donor-derived HSCs (supplemental Figure 2D) than the control donors. Sphk2-deficient mice showed significantly greater survival than Sphk1-deficient or control mice after multiple 5FU challenges (P = .004) (Figure 2J). To further test whether the increased survival was because of improved hematopoietic recovery, we transplanted lethally irradiated wild-type recipients with BM cells from Sphk2-deficient mice or littermate controls and further challenged the recipients with multiple 5FU injections 8 weeks after transplantation. Transplantation of Sphk2-deficient BM cells significantly increased survival in wild-type recipients compared with control BM cells (P = .001) (Figure 2K). Sphk2-deficient mice also showed improved survival under lethal-dose irradiation challenge owing to the increased HSC pool size and protected quiescence, which were observed on day 14 after half-lethal irradiation (Figure 2L-N).
Sphk2 deletion promotes HSC regeneration after chemotherapy. (A) HSPC (LT-HSC, ST-HSC, MPP, and SLAM HSC) numbers from Sphk2Δ/Δ or control mice at indicated time after 5FU treatment (n = 5 mice per group). (B) Cell cycle and (C) apoptosis analysis of SLAM HSCs from Sphk2Δ/Δ or control mice after 5FU treatment (n = 5 mice per group). (D) Scheme for quantification of functional HSCs via transplantation assay. A total of 2 × 105 BM cells from Sphk2Δ/Δ or control mice on day 7 after 5FU treatment were transplanted into irradiated mice along with 2 × 105 recipient BM cells. (E) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (F) percentage of donor-derived B, T, and myeloid lineage cells 16 weeks after transplantation (WT n = 5-6 mice and Sphk2Δ/Δ n = 4-7 mice). (G) Scheme for quantification of HSC function in response to chemotherapy by transplantation assay. A total of 2 × 105 BM cells from Sphk2Δ/Δ or control mice were transplanted into irradiated mice along with 2 × 105 recipient BM cells. Recipient mice were treated with 5FU at 8 weeks after transplantation. (H) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (I) percentage of donor-derived B, T, and myeloid lineage cells at 28 weeks after transplantation (WT n = 4 to 6 mice and Sphk2Δ/Δ n = 7-10 mice). (J) Survival of Sphk2Δ/Δ, Sphk1Δ/Δ, or control mice after serial 5FU treatment every 7 days (WT n = 10 mice, Sphk1Δ/Δ n = 11 mice, and Sphk2Δ/Δ n = 10 mice). (K) Survival of irradiated recipient mice that received 1 × 106 BM cells from Sphk2Δ/Δ or control mice. Injections (5FU) were performed 8 weeks after transplantation every 7 days (WT n = 10 mice and Sphk2Δ/Δ n = 9 mice). (L) HSPC numbers and (M) cell cycle of HSC from Sphk2Δ/Δ or control mice at day 14 after half-lethal irradiation (n = 5 mice per group). (N) Survival of Sphk2Δ/Δ or control mice after lethal irradiation (n = 10 mice per group). Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Sphk2 deletion promotes HSC regeneration after chemotherapy. (A) HSPC (LT-HSC, ST-HSC, MPP, and SLAM HSC) numbers from Sphk2Δ/Δ or control mice at indicated time after 5FU treatment (n = 5 mice per group). (B) Cell cycle and (C) apoptosis analysis of SLAM HSCs from Sphk2Δ/Δ or control mice after 5FU treatment (n = 5 mice per group). (D) Scheme for quantification of functional HSCs via transplantation assay. A total of 2 × 105 BM cells from Sphk2Δ/Δ or control mice on day 7 after 5FU treatment were transplanted into irradiated mice along with 2 × 105 recipient BM cells. (E) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (F) percentage of donor-derived B, T, and myeloid lineage cells 16 weeks after transplantation (WT n = 5-6 mice and Sphk2Δ/Δ n = 4-7 mice). (G) Scheme for quantification of HSC function in response to chemotherapy by transplantation assay. A total of 2 × 105 BM cells from Sphk2Δ/Δ or control mice were transplanted into irradiated mice along with 2 × 105 recipient BM cells. Recipient mice were treated with 5FU at 8 weeks after transplantation. (H) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (I) percentage of donor-derived B, T, and myeloid lineage cells at 28 weeks after transplantation (WT n = 4 to 6 mice and Sphk2Δ/Δ n = 7-10 mice). (J) Survival of Sphk2Δ/Δ, Sphk1Δ/Δ, or control mice after serial 5FU treatment every 7 days (WT n = 10 mice, Sphk1Δ/Δ n = 11 mice, and Sphk2Δ/Δ n = 10 mice). (K) Survival of irradiated recipient mice that received 1 × 106 BM cells from Sphk2Δ/Δ or control mice. Injections (5FU) were performed 8 weeks after transplantation every 7 days (WT n = 10 mice and Sphk2Δ/Δ n = 9 mice). (L) HSPC numbers and (M) cell cycle of HSC from Sphk2Δ/Δ or control mice at day 14 after half-lethal irradiation (n = 5 mice per group). (N) Survival of Sphk2Δ/Δ or control mice after lethal irradiation (n = 10 mice per group). Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
These data demonstrate that the loss of Sphk2 markedly promotes expansion, protects quiescence, and improves stem cell function of HSCs during homeostasis and under injury.
Sphk2 deletion prevents the accumulation of HSC aging phenotypes
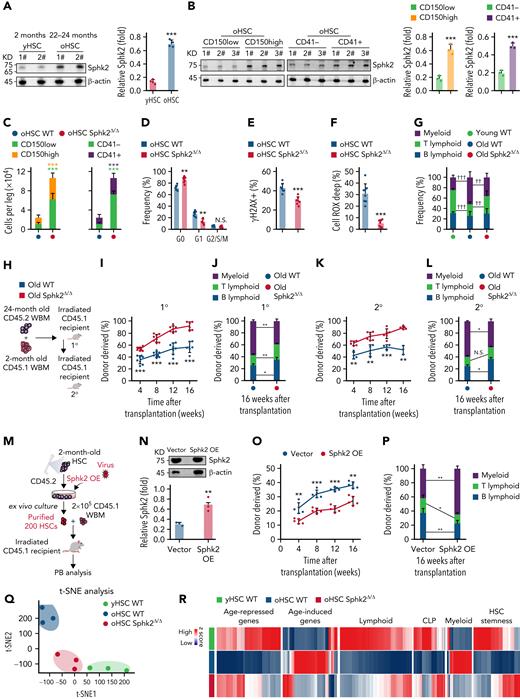
Old HSCs (oHSCs) had higher Sphk2 protein concentrations than young HSCs (yHSCs) (Figure 3A), and aging-associated CD150high oHSCs and CD41+ oHSCs29-31 exhibited high Sphk2 expression than CD150low oHSCs and CD41– oHSCs, respectively (Figure 3B). These suggested a potential role of Sphk2 during HSC aging. Indeed, the numbers of HSCs, especially CD150low HSCs and CD41– HSCs, were higher in aged Sphk2-deficient mice than in littermates (Figure 3C). The oHSCs from Sphk2-deficient mice showed increased quiescence and reduced DNA damage and ROS concentrations than from littermates (Figure 3D-F). Furthermore, aged Sphk2-deficient mice showed improved lymphopoiesis, which was compromised in control aged mice32 (Figure 3G).
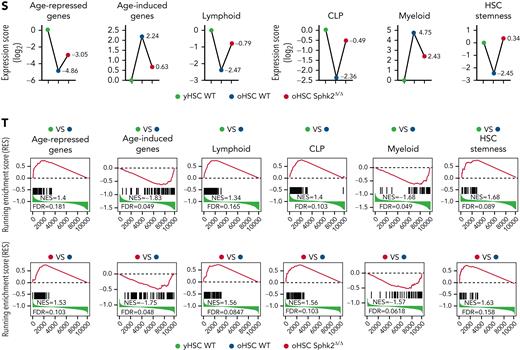
Sphk2 deletion prevents the accumulation of HSC aging phenotypes. (A) Western blots (left) and quantification analyses (right) of Sphk2 expression in yHSCs and oHSCs. β-Actin was used as a loading control; 1# and 2# indicated 2 individual mice (n = 4 mice, 2 replicates were presented, and 4 replicates were quantified). (B) Western blots (left) and quantification analyses (right) of Sphk2 expression in the HSC subpopulations from 22- to 24-month-old mice as indicated. β-Actin was used as a loading control (n = 1-2 mice per replicate). (C) The absolute number of HSC subpopulations from 22- to 24-month-old Sphk2Δ/Δ or control mice (n = 7 mice per group). (D–F) The frequency of (D) cell cycle, (E) γH2AX+ cells, and (F) Cell ROX Deep (ROShigh) cells in HSCs from 22- to 24-month-old Sphk2Δ/Δ or control mice (WT n = 8 mice and Sphk2Δ/Δ n = 7 mice). (G) PB analysis for the percentage of B, T, and myeloid lineage cells from young or old Sphk2Δ/Δ or control mice as indicated (2-month-old WT n = 5 mice, 22- to 24-month-old WT n = 8 mice, and 22- to 24-month-old Sphk2Δ/Δ n = 7 mice). (H) Scheme for quantification of functional HSCs by transplantation assay. A total of 2 × 105 BM cells from 22- to 24-month-old Sphk2Δ/Δ or control mice were transplanted into irradiated mice along with 2 × 105 recipient BM cells. A total of 1 × 106 BM cells from primary recipient mice were transplanted into irradiated mice in secondary transplantation. (I,K) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (J,L) the percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (primary transplantation n = 6-8 mice per group, secondary transplantation WT n = 3-4 mice, and Sphk2Δ/Δ n = 5-6 mice). 1°, primary transplantation; 2°, secondary transplantation. (M) Experimental strategy and (N) Western blots for Sphk2 overexpression in yHSCs. (O) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (P) the percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (vector n = 5 mice and Sphk2 OE n = 5 mice). Overexpression (OE). (Q) t-SNE plot depicting the distribution of yHSCs, oHSCs from WT mice, or oHSCs from Sphk2Δ/Δ mice (oHSC Sphk2Δ/Δ) as indicated. (R) Heatmap, (S) gene expression score, and (T) signature enrichment plots from GSEA for age-repressed, age-induced, lymphoid, CLP, myeloid, and HSC stemness genes in yHSCs, oHSCs, and Sphk2Δ/Δ oHSCs. yHSCs indicate SLAM HSCs obtained from 2-month-old mice. oHSCs indicate SLAM HSCs obtained from 22- to 24-month-old mice. Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. One-way ANOVA with Tukey's multiple comparison post hoc test, †P < .05, ††P < .01, and †††P < .001. N.S., not significant.
Sphk2 deletion prevents the accumulation of HSC aging phenotypes. (A) Western blots (left) and quantification analyses (right) of Sphk2 expression in yHSCs and oHSCs. β-Actin was used as a loading control; 1# and 2# indicated 2 individual mice (n = 4 mice, 2 replicates were presented, and 4 replicates were quantified). (B) Western blots (left) and quantification analyses (right) of Sphk2 expression in the HSC subpopulations from 22- to 24-month-old mice as indicated. β-Actin was used as a loading control (n = 1-2 mice per replicate). (C) The absolute number of HSC subpopulations from 22- to 24-month-old Sphk2Δ/Δ or control mice (n = 7 mice per group). (D–F) The frequency of (D) cell cycle, (E) γH2AX+ cells, and (F) Cell ROX Deep (ROShigh) cells in HSCs from 22- to 24-month-old Sphk2Δ/Δ or control mice (WT n = 8 mice and Sphk2Δ/Δ n = 7 mice). (G) PB analysis for the percentage of B, T, and myeloid lineage cells from young or old Sphk2Δ/Δ or control mice as indicated (2-month-old WT n = 5 mice, 22- to 24-month-old WT n = 8 mice, and 22- to 24-month-old Sphk2Δ/Δ n = 7 mice). (H) Scheme for quantification of functional HSCs by transplantation assay. A total of 2 × 105 BM cells from 22- to 24-month-old Sphk2Δ/Δ or control mice were transplanted into irradiated mice along with 2 × 105 recipient BM cells. A total of 1 × 106 BM cells from primary recipient mice were transplanted into irradiated mice in secondary transplantation. (I,K) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (J,L) the percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (primary transplantation n = 6-8 mice per group, secondary transplantation WT n = 3-4 mice, and Sphk2Δ/Δ n = 5-6 mice). 1°, primary transplantation; 2°, secondary transplantation. (M) Experimental strategy and (N) Western blots for Sphk2 overexpression in yHSCs. (O) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (P) the percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (vector n = 5 mice and Sphk2 OE n = 5 mice). Overexpression (OE). (Q) t-SNE plot depicting the distribution of yHSCs, oHSCs from WT mice, or oHSCs from Sphk2Δ/Δ mice (oHSC Sphk2Δ/Δ) as indicated. (R) Heatmap, (S) gene expression score, and (T) signature enrichment plots from GSEA for age-repressed, age-induced, lymphoid, CLP, myeloid, and HSC stemness genes in yHSCs, oHSCs, and Sphk2Δ/Δ oHSCs. yHSCs indicate SLAM HSCs obtained from 2-month-old mice. oHSCs indicate SLAM HSCs obtained from 22- to 24-month-old mice. Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. One-way ANOVA with Tukey's multiple comparison post hoc test, †P < .05, ††P < .01, and †††P < .001. N.S., not significant.
We then performed a competitive repopulation analysis to investigate the impact of Sphk2 deficiency on the function of oHSCs (Figure 3H). During the 2 rounds of 16-week transplantation observation, BM cells from aged Sphk2-deficient mice showed significantly higher reconstitution than those from control littermates (Figure 3I,K). More importantly, mice transplanted with old Sphk2-deficient cells showed improved lymphopoiesis and reduced myeloid skew (Figure 3J-L). In addition, the overexpression of Sphk2 accelerated HSC aging, as evidenced by the reduced reconstitution and lymphopoietic capacities of HSCs (Figure 3M-P).
To further explore the role of Sphk2 in HSC aging, we analyzed the transcriptomes of yHSCs, oHSCs from control mice, and oHSCs from aged Sphk2-deficient mice. The t-SNE analysis revealed that Sphk2-deficient oHSCs clustered separately from control oHSCs, and instead clustered closer to yHSCs (Figure 3Q). Unsupervised hierarchical clustering of the top differentially expressed genes revealed that the Sphk2-deficient oHSCs expressed signature genes similar to those of yHSCs (supplemental Figure 3A). Furthermore, Sphk2-deficient oHSCs had a remarkable restoration of HSC age-related signature genes, including HSC aging, lymphoid- and myeloid- lineage determination, and HSC stemness genes, approaching the yHSCs concentrations (Figure 3R-T). The restoration of HSC age-related signature genes in Sphk2-deficient oHSCs was also confirmed in other published oHSC datasets33-35 (supplemental Figure 3B-D).
These data demonstrate that Sphk2 deficiency prevents the accumulation of oHSC molecular signature and functionally rejuvenates oHSCs.
Sphk2 deletion increases hypoxic response and metabolic fitness in HSCs
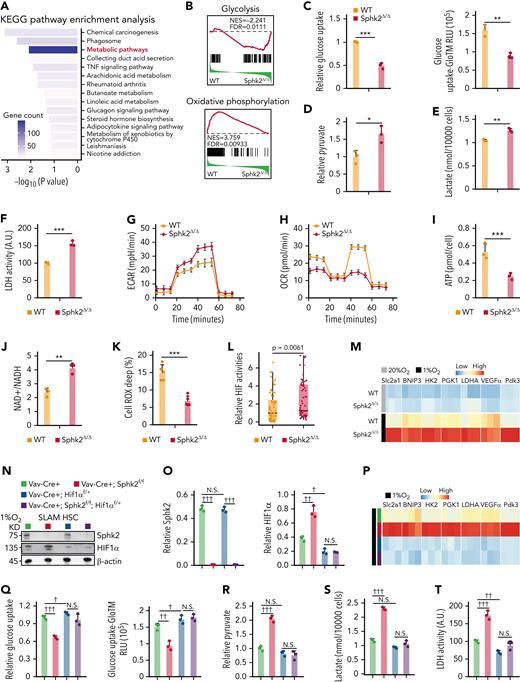
To understand how Sphk2 deletion affects HSC function, we analyzed the transcriptional data and found that metabolic pathways were among the most significantly altered pathways in Sphk2-deficient HSCs, which had decreased genes in OXPHOS and increased genes in anaerobic glycolysis (Figure 4A-B). We then explored the carbohydrate metabolic profiles of HSCs. Sphk2-deficient HSCs showed reduced glucose uptake (Figure 4C) but increased concentrations of pyruvate and lactate (Figure 4D-E), accompanied by increased intracellular glycolytic lactate dehydrogenase (LDH) activity (Figure 4F) and elevated extracellular acidification rate (ECAR) (Figure 4G; supplemental Figure 4A). Furthermore, Sphk2-deficient HSCs had a lower oxygen consumption rate (OCR) (Figure 4H; supplemental Figure 4B), lower ATP production (Figure 4I), and a higher NAD+/NADH ratio (Figure 4J). In addition, Sphk2 deletion dramatically reduced ROS concentrations in HSCs (Figure 4K). Overall, our data showed that Sphk2 deletion repressed mitochondrial OXPHOS and increased anaerobic glycolysis, thereby improving the metabolic fitness of HSCs.
Sphk2 deletion increases hypoxic response and metabolic fitness in HSCs. (A) Enriched pathways of differentially expressed genes in HSCs from Sphk2Δ/Δ or control littermates. The enriched P value is derived from Fisher's exact test. (B) Signature enrichment plots from GSEA using glycolysis and oxidative phosphorylation gene sets in HSCs from WT control mice or Sphk2Δ/Δ mice. (C-K) (C) Relative glucose uptake, (D) relative intracellular pyruvate concentration, (E) lactate production, (F) lactate dehydrogenase (LDH) activity, (G) extracellular acidification rate (ECAR), (H) oxygen consumption rate (OCR), (I) intracellular ATP concentration, (J) intracellular NAD+/NADH ratio, and (K) Cell ROX Deep (ROShigh) cells in HSCs from Sphk2Δ/Δ or control mice (C-J: n = 5 mice in 3 replicates; K: n = 5 mice). (L) Relative HIF activity in HSCs from Sphk2Δ/Δ or control mice. The relative expression values of 64 differentially expressed HIF targeted genes are presented. Each dot represents the mean level of 3 replicates (n = 3 mice per genotype). (M) Relative expression of hypoxia response genes in HSCs from Sphk2Δ/Δ or control mice under hypoxia or normoxia culture for 24 hours (n = 5 mice in 3 replicates). (N-P) (N) Western blots, (O) quantification of HIF1α and Sphk2 protein expression, and (P) relative expression of hypoxia response genes in sorted HSCs from indicated mice under hypoxia culture for 24 hours (n = 5 mice in 3 replicates). (Q-Y) (Q) Relative glucose uptake, (R) relative intracellular pyruvate concentration, (S) lactate production, (T) LDH activity, (U) ECAR, (V) OCR, (W) intracellular ATP concentration, (X) intracellular NAD+/NADH ratio, and (Y) Cell ROX Deep (ROShigh) cells in HSCs from indicated mice (Q-X: n = 5 mice in 3 replicates; Y: n = 5 mice). (Z-AA) The (Z) absolute number and (AA) cell cycle of HSCs from indicated mice (n = 5 mice per group). (AB-AC) One hundred purified HSCs from indicated donor mice were transplanted into irradiated mice with 2 × 105 recipient BM cells. (AB) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (AC) percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (n = 5 mice per group). Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. One-way ANOVA with Tukey's multiple comparison post hoc test, †P < .05, ††P < .01, and †††P < .001. N.S., not significant.
Sphk2 deletion increases hypoxic response and metabolic fitness in HSCs. (A) Enriched pathways of differentially expressed genes in HSCs from Sphk2Δ/Δ or control littermates. The enriched P value is derived from Fisher's exact test. (B) Signature enrichment plots from GSEA using glycolysis and oxidative phosphorylation gene sets in HSCs from WT control mice or Sphk2Δ/Δ mice. (C-K) (C) Relative glucose uptake, (D) relative intracellular pyruvate concentration, (E) lactate production, (F) lactate dehydrogenase (LDH) activity, (G) extracellular acidification rate (ECAR), (H) oxygen consumption rate (OCR), (I) intracellular ATP concentration, (J) intracellular NAD+/NADH ratio, and (K) Cell ROX Deep (ROShigh) cells in HSCs from Sphk2Δ/Δ or control mice (C-J: n = 5 mice in 3 replicates; K: n = 5 mice). (L) Relative HIF activity in HSCs from Sphk2Δ/Δ or control mice. The relative expression values of 64 differentially expressed HIF targeted genes are presented. Each dot represents the mean level of 3 replicates (n = 3 mice per genotype). (M) Relative expression of hypoxia response genes in HSCs from Sphk2Δ/Δ or control mice under hypoxia or normoxia culture for 24 hours (n = 5 mice in 3 replicates). (N-P) (N) Western blots, (O) quantification of HIF1α and Sphk2 protein expression, and (P) relative expression of hypoxia response genes in sorted HSCs from indicated mice under hypoxia culture for 24 hours (n = 5 mice in 3 replicates). (Q-Y) (Q) Relative glucose uptake, (R) relative intracellular pyruvate concentration, (S) lactate production, (T) LDH activity, (U) ECAR, (V) OCR, (W) intracellular ATP concentration, (X) intracellular NAD+/NADH ratio, and (Y) Cell ROX Deep (ROShigh) cells in HSCs from indicated mice (Q-X: n = 5 mice in 3 replicates; Y: n = 5 mice). (Z-AA) The (Z) absolute number and (AA) cell cycle of HSCs from indicated mice (n = 5 mice per group). (AB-AC) One hundred purified HSCs from indicated donor mice were transplanted into irradiated mice with 2 × 105 recipient BM cells. (AB) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (AC) percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (n = 5 mice per group). Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. One-way ANOVA with Tukey's multiple comparison post hoc test, †P < .05, ††P < .01, and †††P < .001. N.S., not significant.
Our transcriptional profile analysis revealed that Sphk2-deficient HSCs had elevated activity of hypoxia-inducible factor (HIF), a master regulator of anaerobic glycolysis12,13 (Figure 4L). In line with this, Sphk2-deficient HSCs had increased hypoxia-induced gene response and elevated concentrations of HIF1α protein and its downstream target PDK3,36 but not HIF2α concentrations than control HSCs under hypoxia (Figure 4M; supplemental Figure 4C-D). Furthermore, Sphk2 deficiency did not increase the HIF1α protein and hypoxia responses in committed progenitor cells (supplemental Figure 4E-J), potentially owning to the exclusive high Sphk2 expression in HSCs (Figure 1D).
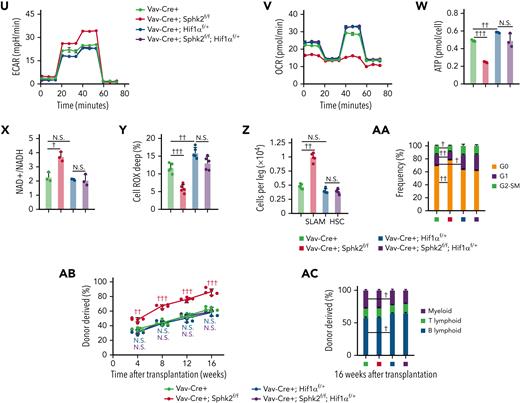
To investigate the role of HIF1α-mediated hypoxia responses in Sphk2-deficient HSCs, we employed Hif1α floxed mice and found that Hif1α heterozygotes (Vav-Cre+; Sphk2f/f; Hif1αf/+) efficiently attenuated the increase in HIF1α protein concentrations and hypoxic responses in Sphk2-deficient HSCs (Vav-Cre+; Sphk2f/f) (Figure 4N-P). We then evaluated the carbohydrate metabolic profile of HSCs from Vav-Cre+; Sphk2f/f; Hif1αf/+ and various control mice. Our data indicated that the Hif1α heterozygote (Vav-Cre+; Sphk2f/f; Hif1αf/+) compromised the increase in metabolic fitness, which was observed in Sphk2-deficient HSCs (Vav-Cre+; Sphk2f/f), as evidenced by the glucose uptake (Figure 4Q), pyruvate (Figure 4R), lactate (Figure 4S), intracellular glycolytic LDH activity (Figure 4T), ECAR (Figure 4U; supplemental Figure 4K), OCR (Figure 4V; supplemental Figure 4L), ATP production (Figure 4W), and NAD+/NADH ratio (Figure 4X). In addition, HSCs from Vav-Cre+; Sphk2f/f; Hif1αf/+ mice did not exhibit reduced ROS concentrations (Figure 4Y). More importantly, the inhibition of HIF1α compromised the increased number (Figure 4Z; supplemental Figure 4M), quiescence (Figure 4AA), and repopulation capacity of HSCs in Sphk2-deficient mice (Figure 4AB-AC). Similarly, Hif1α homozygotes (Vav-Cre+; Sphk2f/f; Hif1αf/f) compromised the increased hypoxic responses, metabolic fitness, and pool size of HSCs, which was observed in Sphk2-deficient HSCs (Vav-Cre+; Sphk2f/f) (supplemental Figure 4N-Y).
Sphk2 bridges the association between PHD2 and VHL to repress HIF1α activity in HSCs
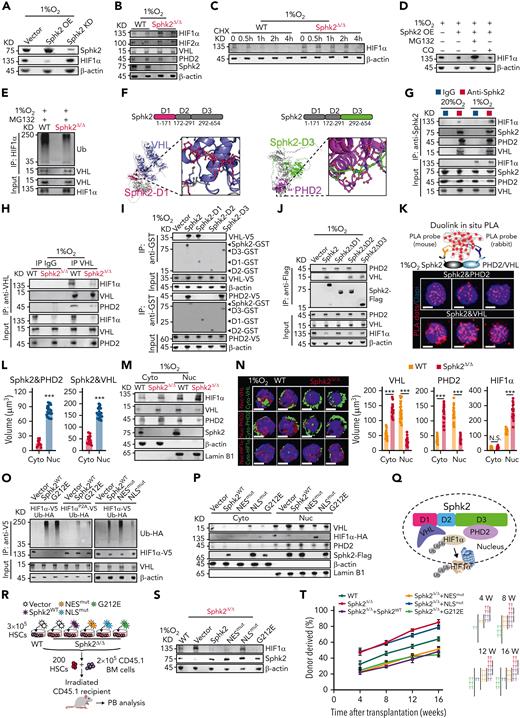
To explore the mechanistic link between Sphk2 and HIF1α, we employed a reporter to measure the activity of HIF by binding hypoxia response elements to the target genes.37 Sphk2 suppressed HIF1α expression and HIF activity in 293T cells under hypoxia conditions (supplemental Figure 5A). Moreover, overexpression of Sphk2 downregulated HIF1α protein; conversely, Sphk2 silencing increased HIF1α expression in HSCs under hypoxia (Figure 5A; supplemental Figure 5B). In contrast to HIF2α, HIF1α protein was increased in purified Sphk2-deficient HSCs recovered by hypoxia culture for 24 hours (Figure 5B; supplemental Figure 5C). We did not observe a noticeable change in HIF1α mRNA or HIF2α mRNA in Sphk2-deficient HSCs (supplemental Figure 5D). In addition, Sphk1 deletion did not affect the expression of HIF1α or PDK3 in HSCs (supplemental Figure 5E). These observations indicate that Sphk2 may regulate the stability of HIF1α protein. Indeed, the half-life of HIF1α protein was longer in Sphk2-deficient HSCs than that in control HSCs under hypoxia (Figure 5C). Consistently, Sphk2 silencing increased the half-life of HIF1α protein in 293T cells under hypoxia, whereas overexpression decreased it (supplemental Figure 5F). More importantly, Sphk2 overexpression triggered proteasome-dependent, but not lysosome-dependent, degradation of HIF1α protein in HSCs (Figure 5D), and Sphk2 deletion reduced the poly-ubiquitination concentrations of HIF1α in HSCs (Figure 5E). These results indicate that Sphk2 deletion stabilizes HIF1α protein in HSCs.
Sphk2 bridges the association between PHD2 and VHL to repress HIF1α activity in HSCs. (A) Western blots of Sphk2 and HIF1α in HSCs with Sphk2 overexpression (OE) or knockdown (KD) as indicated under hypoxia (quantified in supplemental Figure 5B). (B) Western blots of the HIF1α, HIF2α, VHL, PHD2, and Sphk2 in sorted HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours. β-Actin was used as a loading control (n = 4 mice, replicates from 2 individual mice were shown and were quantified in supplemental Figure 5C). (C) Western blots of HIF1α concentrations in HSCs purified from Sphk2Δ/Δ or control mice at indicated time after cycloheximide (CHX) treatment under hypoxia. (D) Western blots of HIF1α concentrations in control HSCs or HSCs with Sphk2 overexpression with or without proteasome inhibitor MG132 or lysosome inhibitor chloroquine (CQ) treatment under hypoxia culture for 24 hours. β-Actin was used as a loading control. (E) Western blots of Ub and VHL in the input and immunoprecipitate with anti-HIF1α antibody in HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours. (F) Representation of Sphk2–VHL–PHD2 interacting surfaces predicted by AlphaFold2 and GRAMM-X. (G) Western blots of HIF1α, Sphk2, PHD2, and VHL in the input and immunoprecipitate with anti-Sphk2 antibody in sorted HSCs under normoxia and hypoxia culture for 24 hours. (H) Western blots of HIF1α, VHL, and PHD2 in the input and immunoprecipitate with anti-VHL antibody in HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours. (I) The interaction of VHL-V5 (upper) or PHD2-V5 (lower) with Sphk2-GST, Sphk2-D1-GST, Sphk2-D2-GST, and Sphk2-D3-GST in GST-pulldown assay. (J) Western blots of PHD2, VHL, and Sphk2-Flag in the input and immunoprecipitate with anti-Flag antibody in 293T cells under hypoxia. (K-L) (K) Representative staining images and (L) quantification of the proximity ligation assays (PLAs) of indicated proteins in HSCs under hypoxia culture for 24 hours (n = 25 cells from 3 mice). (M) Western blots of HIF1α, VHL, PHD2, and Sphk2 in cytoplasmic and nuclear-sorted HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours. (N) Representative images (left) and quantification (right) of VHL, PHD2, and HIF1α in sorted HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours (n = 25 cells from 3 mice). (O) Western blots of Ub-HA, HIF1α-V5, and VHL in the input and immunoprecipitate with anti-V5 antibody in 293T cells. (P) Western blots of the HIF1α-HA, VHL, PHD2, and Sphk2-Flag in the nucleus (Nuc) and cytoplasm (Cyto) after overexpression of Sphk2 or Sphk2 mutants as indicated under hypoxia. (Q) Illustration of Sphk2-PHD2-VHL complex. (R) Experimental strategy. (S) Western blots of HIF1α and Sphk2 in HSCs from Sphk2Δ/Δ or control mice after overexpression of Sphk2 or Sphk2 mutants as indicated under hypoxia culture for 24 hours (quantified in supplemental Figure 5P). (T) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation (n = 5 mice per group). (K,N) Scale bars, 5 μm. Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗∗∗P < .001. One-way ANOVA with Tukey's multiple comparison post hoc test, †P < .05, ††P < .01, and †††P < .001. N.S., not significant.
Sphk2 bridges the association between PHD2 and VHL to repress HIF1α activity in HSCs. (A) Western blots of Sphk2 and HIF1α in HSCs with Sphk2 overexpression (OE) or knockdown (KD) as indicated under hypoxia (quantified in supplemental Figure 5B). (B) Western blots of the HIF1α, HIF2α, VHL, PHD2, and Sphk2 in sorted HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours. β-Actin was used as a loading control (n = 4 mice, replicates from 2 individual mice were shown and were quantified in supplemental Figure 5C). (C) Western blots of HIF1α concentrations in HSCs purified from Sphk2Δ/Δ or control mice at indicated time after cycloheximide (CHX) treatment under hypoxia. (D) Western blots of HIF1α concentrations in control HSCs or HSCs with Sphk2 overexpression with or without proteasome inhibitor MG132 or lysosome inhibitor chloroquine (CQ) treatment under hypoxia culture for 24 hours. β-Actin was used as a loading control. (E) Western blots of Ub and VHL in the input and immunoprecipitate with anti-HIF1α antibody in HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours. (F) Representation of Sphk2–VHL–PHD2 interacting surfaces predicted by AlphaFold2 and GRAMM-X. (G) Western blots of HIF1α, Sphk2, PHD2, and VHL in the input and immunoprecipitate with anti-Sphk2 antibody in sorted HSCs under normoxia and hypoxia culture for 24 hours. (H) Western blots of HIF1α, VHL, and PHD2 in the input and immunoprecipitate with anti-VHL antibody in HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours. (I) The interaction of VHL-V5 (upper) or PHD2-V5 (lower) with Sphk2-GST, Sphk2-D1-GST, Sphk2-D2-GST, and Sphk2-D3-GST in GST-pulldown assay. (J) Western blots of PHD2, VHL, and Sphk2-Flag in the input and immunoprecipitate with anti-Flag antibody in 293T cells under hypoxia. (K-L) (K) Representative staining images and (L) quantification of the proximity ligation assays (PLAs) of indicated proteins in HSCs under hypoxia culture for 24 hours (n = 25 cells from 3 mice). (M) Western blots of HIF1α, VHL, PHD2, and Sphk2 in cytoplasmic and nuclear-sorted HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours. (N) Representative images (left) and quantification (right) of VHL, PHD2, and HIF1α in sorted HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours (n = 25 cells from 3 mice). (O) Western blots of Ub-HA, HIF1α-V5, and VHL in the input and immunoprecipitate with anti-V5 antibody in 293T cells. (P) Western blots of the HIF1α-HA, VHL, PHD2, and Sphk2-Flag in the nucleus (Nuc) and cytoplasm (Cyto) after overexpression of Sphk2 or Sphk2 mutants as indicated under hypoxia. (Q) Illustration of Sphk2-PHD2-VHL complex. (R) Experimental strategy. (S) Western blots of HIF1α and Sphk2 in HSCs from Sphk2Δ/Δ or control mice after overexpression of Sphk2 or Sphk2 mutants as indicated under hypoxia culture for 24 hours (quantified in supplemental Figure 5P). (T) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation (n = 5 mice per group). (K,N) Scale bars, 5 μm. Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗∗∗P < .001. One-way ANOVA with Tukey's multiple comparison post hoc test, †P < .05, ††P < .01, and †††P < .001. N.S., not significant.
To explore how Sphk2 promotes HIF1α degradation, we investigated the association of Sphk2 with the HIF1α degradation complex. AlphaFold238 predicted that Sphk2 interacted with prolyl hydroxylase 2 (PHD2), which hydroxylates HIF1α, and Von Hippel-Lindau (VHL), which binds hydroxylated HIF1α for ubiquitylation and degradation39 through different domains (Figure 5F). Our immunoprecipitation assay demonstrated that Sphk2 complexed with VHL and PHD2 in HSCs under normoxia and hypoxia (Figure 5G). Moreover, VHL interacted with PHD2 and HIF1α in an Sphk2-dependent manner in HSCs (Figure 5H). In addition, Sphk2 interacted with VHL independent of PHD2 in HSCs under hypoxia (supplemental Figure 5G). Our in vitro assay showed that Sphk2 directly interacted with VHL in domain 1 (D1) and PHD2 in domain 3 (D3) (Figure 5I). We further confirmed that the deletion of D1 or D3 in Sphk2 impaired its interaction with VHL and PHD2, respectively, in 293T cells (Figure 5J). Moreover, the proximity ligation assay showed that Sphk2 directly interacted with VHL and PHD2 in the nuclei of HSCs (Figure 5K-L). Furthermore, Sphk2 deletion dramatically inhibited the nuclear localization of VHL and PHD2 in HSCs (Figure 5M-N), although the total concentrations of VHL and PHD2 remained unchanged (Figure 5B; supplemental Figure 5C).
To determine whether Sphk2 enzymatic activity is required to regulate HIF1α, we adapted a previously described catalytically inactive Sphk2 (G212E) mutant that does not generate S1P.40 Sphk2 (G212E) promoted polyubiquitination and degradation of HIF1α protein and inhibited HIF activity as efficiently as wild-type Sphk2 in 293T cells (Figure 5O; supplemental Figure 5H), indicating that Sphk2 regulates HIF1α independent of Sphk2 catalytic activity. Consistently, the administration of exogenous S1P did not affect HIF1α protein concentrations (supplemental Figure 5I) in Sphk2-deficient or control HSCs, and it did not influence HIF1α activity (supplemental Figure 5J) or PDK3 expression (supplemental Figure 5K) in 293T cells. Because Sphk2 is expressed in the nucleus, cytoplasmic mitochondria, and endoplasmic reticulum (ER),41 we further employed a nucleus-excluded form of Sphk2, in which the nuclear localization signal (NLS) is mutated (Sphk2 [NLSmut])28; and a cytoplasm-excluded form of Sphk2, in which the nuclear export signal (NES) is mutated (Sphk2 [NESmut])42 to investigate how subcellular Sphk2 compartments regulate HIF1α protein. In contrast to Sphk2 (NLSmut), Sphk2 (NESmut) efficiently promoted polyubiquitination and degradation of HIF1α protein and inhibited HIF activity in 293T cells (Figure 5O). In line with this, Sphk2 (G212E) and Sphk2 (NESmut), but not Sphk2 (NLSmut), promoted VHL and PHD2 nuclear localization and inhibited HIF activity as efficiently as wild-type Sphk2 in 293T cells (Figure 5P; supplemental Figure 5H). These results suggested that nuclear Sphk2 regulated HIF1α protein degradation, but Sphk2 in the mitochondria or ER did not influence HIF1α. Although Sphk2-S1P regulates mitochondrial permeability transition pore (PTP) in cardiovascular and cancer cells,43,44 we did not observe a direct influence on mitochondrial PTP or the number of mitochondrial cristae in Sphk2-deficient HSCs (supplemental Figure 5L-O), potentially because of the dominant nuclear localization of Sphk2 in HSCs (Figure 1F). In addition, we noticed a reduced mitochondrial number in Sphk2-deficient HSCs (supplemental Figure 5O), potentially because of the increased HIF1α activity, which inhibits mitochondrial biogenesis.45 As nuclear localization of VHL and PHD is important for HIF1α degradation,46,47 our observations indicate that Sphk2 bridges the association between PHD2 and VHL in the nucleus to facilitate HIF1α degradation, independent of Sphk2 catalytic activity (Figure 5Q).
We then transduced wild-type Sphk2 or its various mutants into Sphk2-deficient HSCs for function rescue assays (Figure 5R). In contrast to cytoplasmic Sphk2 (NLSmut), wild-type Sphk2, nuclear Sphk2 (NESmut), and catalytic activity-inactive Sphk2 (G212E) reduced the HIF1α concentrations in Sphk2-deficient HSCs (Figure 5S; supplemental Figure 5P). We further transplanted the HSCs with overexpression of Sphk2 or its mutants into lethally irradiated mice to evaluate their self-renewal capacity. In contrast to cytoplasmic Sphk2 (NLSmut), wild-type Sphk2, Sphk2 (NESmut), and Sphk2 (G212E) compromised the function of Sphk2-deficient HSCs throughout a 16-week observation (Figure 5T; supplemental Figure 5Q). Furthermore, wild-type Sphk2, Sphk2 (NESmut), and Sphk2 (G212E), but not Sphk2 (NLSmut), recovered the number, quiescence, apoptosis, and metabolic fitness of Sphk2-deficient HSCs in recipients (supplemental Figure 5R-AA), although Sphk2 (G212E) did not rescue the S1P concentrations in Sphk2-deficient HSCs (supplemental Figure 5A-B).
Sphk2 deletion activates the HIF1α–PDK3 axis to improve the metabolic fitness and function of HSCs
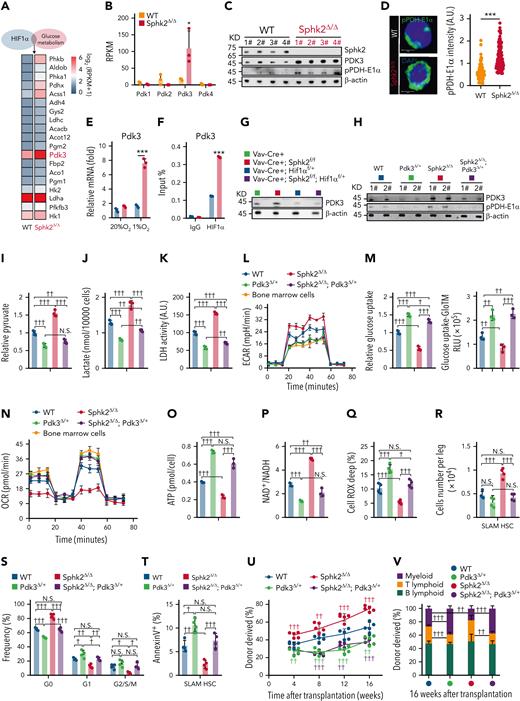
We further examined the HIF1α-targeted metabolic genes to explore how HIF1α regulates metabolic fitness in Sphk2-deficient HSCs. We found that Pdk 3 (pyruvate dehydrogenase kinase 3) was dramatically upregulated (Figure 6A). PDK is a group of metabolic checkpoints for HSC quiescence and the maintenance of stem cell capacity.5 Among the 4 members of the Pdk family, Pdk3 was selectively upregulated in Sphk2-deficient HSCs (Figure 6B), which had increased phosphorylation of pyruvate dehydrogenase-E1α (pPDH-E1α), a target of PDK48 (Figure 6C-D; supplemental Figure 6A). PDK3 expression was dramatically increased in Sphk2-deficient HSCs under hypoxia (Figure 6E; supplemental Figure 6B), owing to the increased binding of HIF1α to the promoter region of PDK3 (Figure 6F; supplemental Figure 6C). Furthermore, hypoxia upregulated the expression of other PDK members in both control and Sphk2-deficient HSCs (supplemental Figure 6B-C), which corroborated previous studies.5,49 In contrast to Sphk2 (NLSmut), wild-type Sphk2, Sphk2 (NESmut), and Sphk2 (G212E) reduced PDK3 expression and PDH-E1α phosphorylation while increasing the activity of PDH, which is inactivated by PDK3, in Sphk2-deficient HSCs (supplemental Figure 6D-E). These observations suggested that Sphk2 deletion activated HIF1α, thereby upregulating PDK3. In support of this, Hif1α heterozygotes efficiently compromised the expression of PDK3 in Sphk2-deficient HSCs (Figure 6G; supplemental Figure 6F).
Sphk2 deletion activates the HIF1α–PDK3 axis to improve metabolic fitness and function of HSCs. (A) Heatmap of metabolic genes overlapped with HIF1α targeted genes, which were upregulated in Sphk2-deficient HSCs compared with those in control HSCs. (B) Reads per kilobase per million mapped reads (RPKM) of PDK in HSCs from Sphk2Δ/Δ mice or control littermates (n = 3 mice per group). (C) Western blots of indicated proteins in HSCs from Sphk2Δ/Δ mice or control littermates. β-Actin was used as a loading control; 1#, 2#, 3#, and 4# indicated 4 individual mice. (D) Representative image (left) and quantification (right) of pPDH-E1α in HSCs from Sphk2Δ/Δ mice or control littermates (n = 100 cells from 4 mice). (E) Relative expression of PDK3 in sorted HSCs from Sphk2Δ/Δ or control mice under normoxia or hypoxia culture for 24 hours (n = 5 mice in 3 replicates). (F) ChIP-qPCR of HIF1α at the promoter region of Pdk3 in sorted HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours (n = 5 mice in 3 replicates). (G) Western blots of PDK3 in sorted HSCs from indicated mice (n = 3 replicates with 1-2 mice per replicate). (H) Western blots of PDK3 and pPDH-E1α in HSCs from indicated mice; 1# and 2# indicated 2 individual mice (n = 4 mice, 2 replicates were shown). (I) Relative intracellular pyruvate concentration, (J) lactate production, (K) LDH activity, (L) ECAR, (M) relative glucose uptake, (N) OCR, (O) intracellular ATP concentration, (P) intracellular NAD+/NADH ratio, and (Q) Cell ROX Deep (ROShigh) cells in HSCs from Pdk3Δ/+, Sphk2Δ/Δ, and Sphk2Δ/Δ; Pdk3Δ/+, or control mice, or in BM cells from 2-month-old mice as indicated (I-P: n = 5 mice in 3 replicates; Q: n = 4 mice per group). (R-T) The (R) absolute number of HSCs (n = 4 mice), (S) cell cycle analysis, and (T) annexin-V analysis of HSCs in BM from Pdk3Δ/+, Sphk2Δ/Δ, and Sphk2Δ/Δ; Pdk3Δ/+, or control mice as indicated (n = 4 mice per group). (U) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (V) the percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (n = 5 mice per group). Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗P < .05, and ∗∗∗P < .001. One-way ANOVA with Tukey's multiple comparison post hoc test, †P < .05, ††P < .01, †††P < .001. N.S., not significant.
Sphk2 deletion activates the HIF1α–PDK3 axis to improve metabolic fitness and function of HSCs. (A) Heatmap of metabolic genes overlapped with HIF1α targeted genes, which were upregulated in Sphk2-deficient HSCs compared with those in control HSCs. (B) Reads per kilobase per million mapped reads (RPKM) of PDK in HSCs from Sphk2Δ/Δ mice or control littermates (n = 3 mice per group). (C) Western blots of indicated proteins in HSCs from Sphk2Δ/Δ mice or control littermates. β-Actin was used as a loading control; 1#, 2#, 3#, and 4# indicated 4 individual mice. (D) Representative image (left) and quantification (right) of pPDH-E1α in HSCs from Sphk2Δ/Δ mice or control littermates (n = 100 cells from 4 mice). (E) Relative expression of PDK3 in sorted HSCs from Sphk2Δ/Δ or control mice under normoxia or hypoxia culture for 24 hours (n = 5 mice in 3 replicates). (F) ChIP-qPCR of HIF1α at the promoter region of Pdk3 in sorted HSCs from Sphk2Δ/Δ or control mice under hypoxia culture for 24 hours (n = 5 mice in 3 replicates). (G) Western blots of PDK3 in sorted HSCs from indicated mice (n = 3 replicates with 1-2 mice per replicate). (H) Western blots of PDK3 and pPDH-E1α in HSCs from indicated mice; 1# and 2# indicated 2 individual mice (n = 4 mice, 2 replicates were shown). (I) Relative intracellular pyruvate concentration, (J) lactate production, (K) LDH activity, (L) ECAR, (M) relative glucose uptake, (N) OCR, (O) intracellular ATP concentration, (P) intracellular NAD+/NADH ratio, and (Q) Cell ROX Deep (ROShigh) cells in HSCs from Pdk3Δ/+, Sphk2Δ/Δ, and Sphk2Δ/Δ; Pdk3Δ/+, or control mice, or in BM cells from 2-month-old mice as indicated (I-P: n = 5 mice in 3 replicates; Q: n = 4 mice per group). (R-T) The (R) absolute number of HSCs (n = 4 mice), (S) cell cycle analysis, and (T) annexin-V analysis of HSCs in BM from Pdk3Δ/+, Sphk2Δ/Δ, and Sphk2Δ/Δ; Pdk3Δ/+, or control mice as indicated (n = 4 mice per group). (U) PB analysis for total engrafted donor cells at the indicated number of weeks after transplantation and (V) the percentage of donor-derived B, T, and myeloid lineage cells at 16 weeks after transplantation (n = 5 mice per group). Data represented as mean ± standard deviation. Two-tailed Student t tests were used to assess statistical significance. ∗P < .05, and ∗∗∗P < .001. One-way ANOVA with Tukey's multiple comparison post hoc test, †P < .05, ††P < .01, †††P < .001. N.S., not significant.
We further investigated whether PDK3 upregulation was responsible for the improved metabolic fitness of Sphk2-deficient HSCs. To this end, we employed Pdk3 knockout mice in which Pdk3 heterozygotes (Pdk3Δ/+) efficiently attenuated the increase in PDK3 and pPDH-E1α in Sphk2-deficient HSCs (Figure 6H; supplemental Figure 6G-J). Furthermore, PDK3 inhibition compromised the increased anaerobic glycolysis in Sphk2-deficient HSCs, as evidenced by reduced pyruvate (Figure 6I), lactate (Figure 6J), intracellular glycolytic LDH activity (Figure 6K), and ECAR in Sphk2Δ/Δ; Pdk3Δ/+ mice compared with those in Sphk2Δ/Δ mice (Figure 6L; supplemental Figure 6K). Conversely, the inhibition of PDK3 increased the mitochondrial OXPHOS concentrations in Sphk2-deficient HSCs to similar concentrations as wild-type HSCs, as evidenced by an increase in glucose uptake (Figure 6M), OCR (Figure 6N; supplemental Figure 6L), and ATP production (Figure 6O), and a decrease in the NAD+/NADH ratio (Figure 6P). Inhibition of PDK3 also restored the ROS concentrations in Sphk2-deficient HSCs to similar concentrations as in wild-type HSCs (Figure 6Q). Moreover, Pdk3Δ/+ and Sphk2Δ/Δ; Pdk3Δ/+ HSCs had decreased anaerobic glycolysis and increased mitochondrial OXPHOS compared with wild-type HSCs, which was because of the reduced PDK3 expression in Pdk3Δ/+ HSCs compared with that in wild-type HSCs (Figure 6H).
Because PDK3 overexpression improved the stem cell function of HSCs (supplemental Figure 6M-O), we considered whether the increased PDK3 was responsible for the improved stem cell function of Sphk2-deficient HSCs. Indeed, PDK3 inhibition in Sphk2Δ/Δ; Pdk3Δ/+ mice dramatically compromised the increase in HSC numbers (Figure 6R; supplemental Figure 6P), HSC quiescence (Figure 6S), and decrease in the HSC apoptosis rate (Figure 6T) caused by Sphk2 deletion in Sphk2Δ/Δ mice. More importantly, competitive repopulation analysis showed that the improved reconstitution capacity in Sphk2Δ/Δ mice was inhibited in Sphk2Δ/Δ; Pdk3Δ/+ mice (Figure 6U-V). Also, Pdk3Δ/+ mice and Sphk2Δ/Δ; Pdk3Δ/+ mice had reduced HSC self-renewal potential compared with wild-type mice (Figure 6U), which was potentially because of the reduced PDK3 expression in HSCs from Pdk3 heterozygous mutants (Figure 6H).
Overall, our data demonstrated that Sphk2 deletion increases HSC function through an HIF1α-PDK3 axis, which improves the metabolic fitness of HSCs.
Discussion
Plasma S1P and sphingolipid metabolites regulate zebrafish development, human embryonic stem cells, and hematopoiesis through S1P membrane receptors.50-53 S1P regulates the inflammatory networks of HSCs and leukemia stem cells through S1PR3.54 DEGS1 (dihydroceramide desaturase), an enzyme that regulates the sphingolipid pathway, controls endoplasmic reticulum stress and proliferation/apoptosis in HSCs.55 However, HSCs might not be a major source of S1P production in the BM owing to their rare cell numbers, indicating that HSCs specifically expressing Sphk2 might have other potential roles rather than generating S1P. In this work, we observed that Sphk2 was highly expressed in HSCs and negatively regulated the hypoxic responses, metabolic fitness, and stem cell function independent of S1P generation. Deletion of Sphk2 did not cause the compensational increase of Sphk1 in HSCs as observed in other tissues.56-58
Metabolism regulates HSC function during homeostasis and aging.59,60 HSCs increase their mitochondrial membrane potential, OXPHOS, and ROS concentrations during proliferation and aging15,61,62; consequently, the accumulated ROS concentrations, replication stress, and mitochondrial dysfunction impair HSC long-term function.63,64 Furthermore, anaerobic metabolism keeps low ROS concentrations to promote HSC maintenance,65,66 whereas exposure to ambient oxygen decreases the recovery of long-term repopulating HSCs.67 Stabilization of HIF1α increases HSC quiescence and function.68,69 We demonstrated that Sphk2 bridged the association between PHD2 and VHL in the nucleus to repress HIF1α activity in HSCs. More importantly, Sphk2 was exclusively expressed in HSCs but not in progenitors, and deletion of Sphk2 specifically increased the hypoxic response in HSCs, thereby improving their metabolic fitness, which indicated the unique role of Sphk2 in HSC regulation. Sphk2 deletion stimulated HIF1α to upregulate PDK3 but not other PDK members,5,49 which suggested the presence of specific HIF1α targets in Sphk2-deficient HSCs. We did not observe a further increase of HIF1α protein in Hif1αf/+ HSCs after Sphk2 deletion, potentially because of the Sphk2-independent or saturated degradation system for HIF1α when its transcription was inhibited. Furthermore, future studies are warranted to investigate the Sphk2-based HSC rejuvenation strategies by inhibiting or deleting Sphk2 in middle-aged mice when HSC aging has already occurred.
Taken together, we identified Sphk2 as a metabolic modifier that regulates metabolic fitness in HSCs. Loss of Sphk2 expands the HSC pool during homeostasis and injury and protects HSC function from aging.
Acknowledgments
The authors thank the National Key Research and Development Program of China (2018YFA0107200), the National Natural Science Foundation of China (82170112, 82100129, and 81900101), the Key Research and Development Program of Guangdong Province (2019B020234002), Guangdong Innovative and Entrepreneurial Research Team Program (2019ZT08Y485), Sanming Project of Medicine in Shenzhen (SZSM201911004), Advanced Medical Technology Center, the First Affiliated Hospital, Zhongshan School of Medicine, and Sun Yat-sen University for generous support.
Authorship
Contribution: C.L., B.W., and Y. Li designed and performed most of the experiments, analyzed the data, and generated figures; Y. Liu and Q.W. contributed to Chipseq; J.W., J.X., and H.Y. contributed to bioinformatic analysis; X.X. contributed to seahorse analysis; J.C. and S.X. contributed to the mouse experiments; Z.Y., B.Z., B.C., J.G., and X.T. joined this project for technical support; T.L. contributed Sphk1/2 knockout mice; C.H.M., X.K., P.Q., L.J., J.M.P., and A.H. contributed for scientific discussion and manuscript preparation; and M.Z. supervised the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Meng Zhao, Sun Yat-sen University, 74 Zhongshan 2nd Rd, Guangzhou, Guangdong 510000, China; e-mail: zhaom38@mail.sysu.edu.cn.
References
Author notes
∗C.L., B.W., Y. Li, Y. Liu, and J.W. contributed equally to this study.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal