Key Points
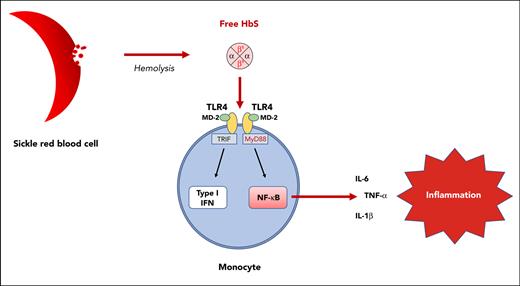
Hemoglobin S, unlike hemoglobin A or heme, is responsible for TLR4-mediated monocyte activation.
Interaction between hemoglobin S and the TLR4/MD-2 complex results in the activation of both the NF-κB and type I interferon pathways.
Abstract
Monocytes are considered crucial actors of inflammation in sickle cell disease (SCD), being responsible for an increased production of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and IL-6. Although a role of free heme released by intravascular hemolysis has been suspected, the mechanisms underlying monocyte activation in patients with SCD remain unknown. Using purified human hemoglobin (Hb), we demonstrate herein, that cell-free HbS, unlike HbA or heme, is responsible for a major enhancement in the expression of proinflammatory cytokines by human monocytes. This effect was found mediated by direct interaction with the Toll-like receptor 4 (TLR4)/myeloid differentiation factor 2 (MD-2) complex, resulting in the activation of both the nuclear factor-κB (NF-κB) and type I interferon pathways. In Townes SCD mice, injection of HbS, unlike HbA, was responsible for an increased production of proinflammatory cytokines, which was prevented by the TLR4 inhibitor, TAK-242. Our results reveal a novel mechanism of monocyte activation and systemic inflammation in SCD, which opens new promising therapeutic perspectives targeting the HbS-TLR4 interaction.
Introduction
Sickle cell disease (SCD) is a severe hemoglobin (Hb) disorder and is considered the most common monogenic disease worldwide.1 It is characterized by hemolytic anemia, recurrent painful vaso-occlusive crises (VOC), and progressive multiple organ damage, together with a systemic inflammatory state.2 At the molecular level, SCD originates from a single mutation in the HBB gene, leading to the replacement of a hydrophilic glutamic acid by a hydrophobic valine at position 6 in the β-globin chain (Glu6Val, βS). The inheritance of 2 βS alleles by patients with homozygous HbSS results in an abnormal α2βS2 tetramer of HbS instead of the normal α2β2 tetramer of HbA. Intracellular polymerization of deoxygenated HbS induces sickling of the red blood cells (RBCs), thereby promoting hemolysis and vaso-occlusion of the small vessels with ischemia-reperfusion injuries. Over the last 2 decades, the understanding of SCD pathophysiology has improved considerably and highlighted a major role for cells other than RBCs, including monocytes.3 Monocytes from patients with SCD display an activated profile with increased production of proinflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α) as compared with healthy controls,4 but the mechanisms underlying their activation remain unclear.3 A role for free heme released by intravascular hemolysis has legitimately been suspected. Indeed, a high expression of Toll-like receptor 4 (TLR4) by monocytes from patients with SCD has been reported, with a close correlation to IL-6 expression,5 and heme is a potent activator of TLR4 expressed by endothelial cells in SCD.6 However, contrary to lipopolysaccharide (LPS), heme was found insufficient to induce IL-6 production by monocytes from patients with SCD.7 Because SCD is associated with increased chronic inflammation compared with thalassemia,8 another hemoglobinopathy with a high rate of hemolysis, and because Hb/heme exposure within a hematoma or during hemolysis is classically not responsible for systemic inflammation in patients without SCD,9 we hypothesized a specific role for cell-free HbS as compared with HbA or heme. Using purified human Hb, we demonstrate here that cell-free HbS, unlike HbA or heme, induces a major increase in the expression of proinflammatory cytokines by human monocytes. Furthermore, using cultured human monocytes and murine macrophages, including KO-TLR4 THP1 and CRISPR-engineered KO-myeloid differentiation factor 2 (MD-2) RAW cell lines, we demonstrate that the action of HbS is mediated by the TLR4/MD-2 complex and results in the activation of both the nuclear factor-κB (NF-κB) and type I interferon pathways.
Methods
Additional methods relative to Hb purification, surface plasmon resonance analysis, RAW murine macrophagic and THP1 human monocytic cell lines, chemotaxis assays, and CRISPR-Cas9 editing are provided in supplemental Methods.
Patients
A prospective observational study was performed between October and December 2018 in our French university-hospital SCD reference center. Eligibility criteria were SS or S/β0 SCD and age ≥1 year. Exclusion criteria were other diseases possibly modifying TLR4 expression by monocytes (eg, infections and inflammatory or autoimmune diseases such as systemic lupus erythematosus) and immunosuppressive or anti-inflammatory treatment. All patients were recruited during follow-up in the SCD reference center (n = 19) (supplemental Table 1). Controls (n = 10) were recruited among unaffected siblings (HbAA) of patients with SCD. Blood was collected in EDTA, and the plasma obtained by centrifugation (10 minutes, 3500 × g, 4°C) was stored at −80°C. Informed consent was obtained from adult patients and from parents or legal guardians for children (aged <18 years). The study was approved by a medical ethics committee (GR-Ex/CPP-DC2016-2618/CNIL-MR01).
Reagents
Bovine serum albumin (BSA), catalase, ferrous-stabilized human HbS and HbA, haptoglobin, hemopexin, L-NAME, LPS, NONOate, polymyxin B, proteinase K, superoxide dismutase (SOD), sodium dithionite, and TAK-242 were obtained from Sigma-Aldrich and were prepared by diluting in phosphate-buffered saline (PBS). Hemin (Ferric PPIX; Sigma-Aldrich) was prepared by dissolving in 0.25 M NaOH, before pH neutralization with HCl and concentration adjustment with PBS. The TLR4/MD-2 complex was obtained from R&D Systems (3146-TM/CF) and was prepared by diluting in PBS. N-acetylcysteine (NAC) was obtained from Sigma-Aldrich and was prepared by diluting in RNase-free water. ODQ (1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one) was obtained from Sigma-Aldrich and was prepared by diluting in dimethyl sulfoxide. Endotoxin levels were monitored on HbS and HbA (HbS lot# SLBV0862, SLBV0392, and SLCC7764; HbA lot# SLCB6683, SLCB7096, and SLCB9785) using a Limulus amebocyte lysate test (Pierce Chromogenic Endotoxin Quant Kit, Thermo Fisher Scientific). All preparations contained <0.1 (0.020-0.068) endotoxin units per mL and <0.005 (0.0015-0.0050) endotoxin units per mg.
Isolation of PBMCs by Ficoll-Paque density gradient centrifugation
Peripheral blood mononuclear cells (PBMCs) obtained by cytapheresis were isolated using Ficoll-Paque. Briefly, blood obtained by cytapheresis from healthy donors of the Etablissement Français du Sang (the French Blood Establishment) was diluted with an equal volume of PBS (pH 7.4) containing 2% BSA. Diluted blood (30 mL) was layered over 15 mL of the Ficoll-Paque PLUS (GE Healthcare). Gradients were centrifuged at 1600 × g for 20 minutes at room temperature in a swinging-bucket rotor without the brake applied. The PBMC interface was carefully removed by pipetting and then washed twice with PBS-BSA by centrifugation at 300 × g for 5 minutes. Cell number and viability were determined using a Countess Automated Cell Counter (Invitrogen). Nonviable cells were identified by staining with trypan blue, and cell viability was calculated using the total cell count and the count of nonviable cells. PBMCs were cryopreserved in liquid nitrogen in fetal calf serum (Invitrogen) containing 10% dimethyl sulfoxide (Thermo Fisher Scientific) and stored until required for downstream analyses.
Isolation of human monocytes by magnetic sorting
Human monocytes were obtained from PBMCs by using the MojoSort Human CD14 Nanobeads protocol (Biolegend) following the manufacturer’s instructions. Monocytes were cultured in Mononuclear Cell Medium (Promocell) with penicillin/streptomycin (1%, Life Technologies).
Cell culture conditions
Cells were cultured at a concentration of 106 cells per mL. HbA, HbS, or hemin were added at a concentration of 20 μM (unless otherwise stated) to culture medium, which was incubated for 4 hours (for messenger RNA [mRNA] expression measurement) or 18 hours (for cytokine/chemokine measurement in the cell culture supernatant). TAK-242 (TLR4 inhibitor) was added to culture medium at a concentration of 1 μM, 1 hour before HbA, HbS, or hemin. HbS and LPS were pretreated with polymyxin B at a concentration of 100 μg/mL for 2 hours at 37°C. HbS was pretreated with proteinase K at a concentration of 5 mg/mL for 3 hours at 37°C.
Quantitative PCR analysis
Total RNA was extracted (RNEasyPlus Mini or Micro kit, Qiagen) and quantified with a NanoDrop system. RNA quality was assessed by an absorbance ratio of 260:280 nm. Reverse transcription was carried out with 100 to 300 ng total RNA using the iScript Reverse Transcription Supermix (Bio-Rad). Real-time polymerase chain reaction (PCR) was performed using SsoFast EvaGreen supermix (Bio-Rad). The sequences of primers obtained from Eurofins MWG Operon (Germany) are detailed in supplemental Methods. All data are expressed relative to matched controls (nontreated cells). Gene quantification was performed in duplicate using CFX96 PCR Detection System (Bio-Rad). Data were normalized to β-actin and/or glyceraldehyde-3-phosphate dehydrogenase values.
NanoString
Human monocytes from healthy donors (n = 4) were incubated for 4 hours with HbA (20 μM), HbS (20 μM), or control (culture medium). Total RNA was extracted using the RNeasy Plus Micro Kit (Qiagen France SAS). RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific); RNA quality was analyzed using a Qubit4 fluorometer and the Qubit RNA Integrity and Quality Assay Kit (Thermo Fisher Scientific). The RNA samples for NanoString analysis were chosen as per the following qualitative criteria: absorbance ratio of 260:280 nm > 1.8, absorbance ratio of 260:230 nm > 2.0, and RNA Integrity and Quality > 7. RNA (100 ng) from Hb-stimulated human monocytes was analyzed using the nCounter Human Inflammatory Panel (NanoString Technologies, Seattle, WA), which included 255 inflammation-related genes and 6 internal reference controls. The hybridization assay was performed on the nCounter MAX system, and raw gene expression data were supplied for further analysis. Experiments were performed by Canopy Biosciences (St. Louis, MO). Data analysis was performed on Rosalind Platform (OnRamp BioInformatics, San Diego, CA) by Canopy Biosciences.
Cytokine/chemokine measurement
Cytokines and chemokines produced by human monocytes after incubation with HbA, HbS, hemin, or control (culture medium) were measured by either enzyme-linked immunosorbent assay (ELISA) (Human TNF-α DuoSet ELISA, Human IL-1β DuoSet ELISA, and Human IL-6 DuoSet ELISA; R&D Systems) or with a membrane-based antibody array, allowing for simultaneous detection of 105 different cytokines and chemokines (Proteome Profiler Human XL Cytokine Array Kit; R&D Systems), following the manufacturer’s instructions.
Heat map representations
Heat map representations were done with ClustVis webtool (http://biit.cs.ut.ee/clustvis/),10 using either a logarithmic or a linear scale color bar.
Spectral flow cytometry
Hyperspectral cytometry was performed on fresh whole blood obtained from patients (n = 19) and controls (n = 10) in a spectral cell analyzer SP 6800 (Sony). Fluorochrome-conjugated monoclonal antibodies used for spectral flow cytometry included Alexa Fluor 532 anti-CD45 (clone HI30, Life Technologies), BV570 anti-CD14 (clone M5E2, Sony), BV711 anti-CD11b (clone ICRF44, Sony), and BV421 anti-CD284/TLR4 (clone HTA125, Sony). The gating strategy is detailed in supplemental Figure 3.
Mice and procedures
All animal experiments were performed in compliance with the local ethics committee. These studies used male and female Townes-AA (HbAA) and Townes-SS (HbSS) mice on a 129/B6 mixed genetic background, obtained from the Jackson Laboratories (strain #013071). Mouse genotypes were assessed by PCR, using protocols established by the Jackson Laboratories (United States). All animals were aged between 12 and 14 weeks. They were housed in specific pathogen-free rooms to limit infections and kept on a 12-hour light/dark cycle at 21°C. All animals were monitored daily for health problems, food and water levels, and cage conditions. Mice were injected with freshly prepared solutions of HbS (2 μmol/kg IV, batches SLBV0862 and SLCC7764) or HbA (2 μmol/kg IV, batch SLCB7096). The same batch of HbS was injected into HbSS and HbAA mice in each experiment. In some groups, mice received TAK-242 (1 mg/kg intraperitoneal) 1 hour before administration of Hb. For hypoxia/reoxygenation experiments, mice were exposed to 8% oxygen for 3 hours in a hypoxia chamber (Biospherix-ProOx P360), followed by reoxygenation for 1 hour in room air.
For RNA analysis, blood samples (500-700 μL) were obtained by retro-orbital sinus puncture with capillary tubes internally coated with heparin/EDTA anticoagulant, 4 hours after Hb injection. RBC lysis was performed for 5 minutes according to the manufacturer’s protocol (Red Blood Cell Lysis Solution, Miltenyi Biotec). Monocytes were isolated using an EasySep Mouse Monocytes Isolation Kit (STEMCELL, Grenoble, France). Total RNA was extracted using the RNeasy Plus Micro Kit (Qiagen, France SAS) and quantified with a NanoDrop system. RNA quality was assessed by an absorbance ratio of 260:280 nm. Reverse transcription was carried out with 500 ng total RNA using the iScript Reverse Transcription Supermix (Bio-Rad 170-8891). Real-time PCR was performed using SsoAdvanced Universal Sybr Green Supermix (Bio-Rad 172-5274). The sequences of primers obtained from Eurofins MWG Operon (Germany) are detailed in supplemental Methods. Gene quantification was performed in duplicate using the CFX96 PCR Detection System (Bio-Rad). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase and β-actin values.
For plasma cytokine evaluation, blood samples were obtained by retro-orbital sinus puncture with capillary tubes and transferred to tubes internally coated with EDTA anticoagulant, 18 hours after Hb injection. Plasma was obtained by centrifugation (10 minutes, 2000 × g, 4°C) and stored at −80°C. Quantification of IL-6 and TNF-α was performed in 25 μL of plasma using a LEGENDplex assay (Biolegend) following the manufacturer’s instructions. Data were acquired on a Gallios flow cytometer (Beckman Coulter) and analyzed on LEGENDplex software (Qognit Inc, San Carlos, CA).
Statistical analysis
Values are expressed as mean ± standard error of the mean (unless otherwise specified), representative of at least 2 independent experiments. Differences between groups were assessed with Mann-Whitney test, unpaired Student t test, or one-way analysis of variance (ANOVA) with post hoc test, as appropriate. Statistical significance threshold was set at a P value of .05. Data were analyzed using GraphPad Prism version 9 (GraphPad Software).
Results
HbS, unlike HbA or heme, is responsible for monocyte activation
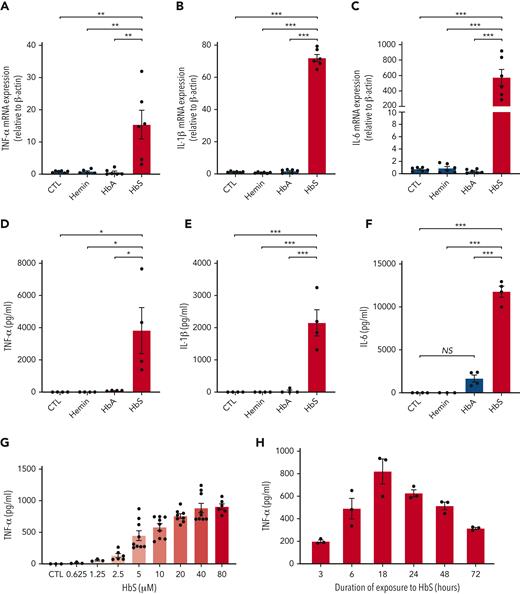
To test the hypothesis that HbS may have a specific action on monocytes, we first added purified ferrous human HbS, HbA, or hemin, the oxidized form of heme that is found in extracellular environments, to the culture medium of human monocytes obtained from healthy blood donors and incubated for 4 hours. Unlike HbA or hemin, HbS induced a dramatic increase in mRNA expression of TNF-α, IL-1β, and IL-6 in human monocytes (Figure 1A-C). As previously reported, we did not observe any effect of hemin on IL-6 mRNA expression.7 Next, to confirm that gene overexpression of TNF-α, IL-1β, and IL-6 was responsible for an enhanced production of these proinflammatory cytokines by human monocytes, we measured these cytokine levels in culture medium after 18 hours of coincubation with HbS, HbA, or hemin. As expected, a major increase in the levels of these proinflammatory cytokines was observed with HbS as compared with HbA, and no effect was observed with hemin (Figure 1D-F). Dose-response and time-course experiments revealed that cytokine secretion was maximal after 18 hours of incubation with HbS and led us to choose a concentration of 20 μM for all experiments thereafter (Figure 1G-H). Importantly, these results obtained with ferrous-stabilized human HbS (from Sigma-Aldrich) were further confirmed with fresh Hb preparations obtained from HbAA and HbSS murine and human red cell lysates (supplemental Figures 1 and 2).
HbS induces proinflammatory cytokine production by human monocytes. (A-C) mRNA expression (relative to β-actin and compared with control) of TNF-α (A), IL-1β (B), and IL-6 (C) by human monocytes from healthy blood donors (n = 6), in culture with HbS (20 μM), HbA (20 μM), or control, after 4 hours (n = 6 per group). (D-F) Levels of TNF-α (D), IL-1β (E), and IL-6 (F) in the cell culture supernatant of human monocytes from healthy blood donors (n = 4), in culture with HbS (20 μM), HbA (20 μM), or control, after 18 hours (n = 4 per group). (G-H) Levels of TNF-α in the cell culture supernatant of human monocytes from healthy blood donors (n = 3 to 6 per group), in culture with HbS or control, after 18 hours, at increasing HbS concentrations of 0.625, 1.25, 2.5, 5, 10, 20, 40, and 80 μM (G) and after increasing time of culture with HbS at a concentration of 20 μM (H). ∗∗∗P < .001; ∗∗P < .01; ∗P < .05 by one-way ANOVA. CTL, control; NS, nonsignificant.
HbS induces proinflammatory cytokine production by human monocytes. (A-C) mRNA expression (relative to β-actin and compared with control) of TNF-α (A), IL-1β (B), and IL-6 (C) by human monocytes from healthy blood donors (n = 6), in culture with HbS (20 μM), HbA (20 μM), or control, after 4 hours (n = 6 per group). (D-F) Levels of TNF-α (D), IL-1β (E), and IL-6 (F) in the cell culture supernatant of human monocytes from healthy blood donors (n = 4), in culture with HbS (20 μM), HbA (20 μM), or control, after 18 hours (n = 4 per group). (G-H) Levels of TNF-α in the cell culture supernatant of human monocytes from healthy blood donors (n = 3 to 6 per group), in culture with HbS or control, after 18 hours, at increasing HbS concentrations of 0.625, 1.25, 2.5, 5, 10, 20, 40, and 80 μM (G) and after increasing time of culture with HbS at a concentration of 20 μM (H). ∗∗∗P < .001; ∗∗P < .01; ∗P < .05 by one-way ANOVA. CTL, control; NS, nonsignificant.
HbS induces activation of the NF-κB and type I interferon signaling pathways
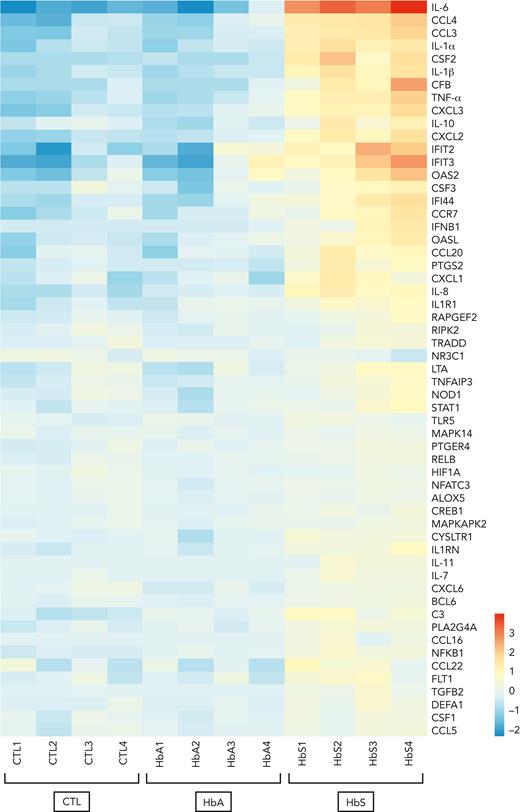
To investigate which inflammatory signaling pathways may be activated in response to monocyte stimulation by HbS, mRNA expression profiles of human monocytes from healthy donors was evaluated with an nCounter human mRNA expression assay (NanoString Inflammation Panel, Canopy Biosciences) after incubation with HbS or HbA for 4 hours. Functional analysis of gene ontology revealed that HbS, unlike HbA, was mainly responsible for the activation of the NF-κB and type I interferon signaling pathways with a predominant overexpression of IL-6, together with other cytokines and chemokines, including TNF-α, IL-1α, IL-1β, IL-8, CCL3, CCL4, CXCL2, CXCL3, and CSF2 (granulocyte-macrophage colony-stimulating factor [GM-CSF]) (Figure 2). The main activated genes belonging to the type I interferon signaling pathway were IFIT2, IFIT3, OAS2, CSF3, IFI44, IFNB1, and OASL.
HbS is responsible for the activation of the NF-κB and type I interferon signaling pathways. mRNA expression profile of human monocytes from healthy blood donors (n = 4) after 4-hour incubation with HbS (20 μM), HbA (20 μM), or control (NanoString Inflammation Panel, Canopy Biosciences). Presented genes (n = 57 out of 255) are those with a statistically significant >1.5-increase in mRNA expression after incubation with HbS compared with HbA. Heat map representation of the 57 genes is ordered by hierarchical clustering, using a logarithmic scale color bar. IL-6, CCL3, CCL4, IL-1α, IL-1β, TNF-α, CXCL2, CXCL3, and IL-10 belong to the NF-κB signaling pathway, whereas IFIT2, IFIT3, OAS2, CSF3, IFI44, IFNB1, and OASL belong to the type I signaling pathway.
HbS is responsible for the activation of the NF-κB and type I interferon signaling pathways. mRNA expression profile of human monocytes from healthy blood donors (n = 4) after 4-hour incubation with HbS (20 μM), HbA (20 μM), or control (NanoString Inflammation Panel, Canopy Biosciences). Presented genes (n = 57 out of 255) are those with a statistically significant >1.5-increase in mRNA expression after incubation with HbS compared with HbA. Heat map representation of the 57 genes is ordered by hierarchical clustering, using a logarithmic scale color bar. IL-6, CCL3, CCL4, IL-1α, IL-1β, TNF-α, CXCL2, CXCL3, and IL-10 belong to the NF-κB signaling pathway, whereas IFIT2, IFIT3, OAS2, CSF3, IFI44, IFNB1, and OASL belong to the type I signaling pathway.
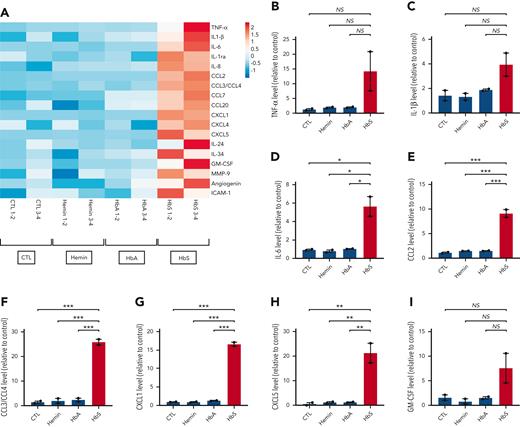
To further investigate which cytokines and chemokines may be secreted by human monocytes after stimulation by HbS, we used a membrane-based antibody array that allowed the simultaneous detection of 105 different cytokines and chemokines in the culture supernatant of human monocytes from healthy donors after incubation with HbS, HbA, or hemin for 18 hours. Unlike HbA or hemin, HbS was responsible for an enhanced production of several major cytokines and chemokines, including TNF-α, IL-1β, IL-6, CCL2, CCL3/CCL4, CXCL1, CXCL5, and GM-CSF (Figure 3 A-I). However, the trend to an increased production of TNF-α, IL-1β, and GM-CSF was not statistically significant, which may be explained by the limited number of samples in this specific assay (pooled samples, n = 2 per group). With the exception of CXCL5, increased levels of these different mediators have previously been reported in patients with SCD,11-14 including GM-CSF, whose plasma levels were found positively correlated with the total number of leukocytes, especially monocytes.15 A role for activated mast cells in producing GM-CSF has been reported,16 but our findings suggest that monocytes might also be important actors of leukocytosis in SCD.
Main cytokines and chemokines produced by human monocytes in response to HbS. (A) Heat map representation (linear scale color bar) of the main cytokines/chemokines secreted by human monocytes from healthy donors (n = 4) after incubation with HbS (20 μM), HbA (20 μM), hemin (20 μM), or control for 18 hours. Presented cytokines/chemokines (n = 18 out of 105) are those with a >1.5–increased level in the cell culture supernatant (measured in pooled samples with a membrane-based antibody array, n = 2 per group) after incubation with HbS compared with HbA. (B-I) Relative level of TNF-α (B), IL-1β (C), IL-6 (D), CCL2 (E), CCL3/CCL4 (F), CXCL1 (G), CXCL5 (H), and GM-CSF (I) in the culture medium. ∗∗∗P < .001; ∗∗P < .01; ∗P < .05 by one-way ANOVA.
Main cytokines and chemokines produced by human monocytes in response to HbS. (A) Heat map representation (linear scale color bar) of the main cytokines/chemokines secreted by human monocytes from healthy donors (n = 4) after incubation with HbS (20 μM), HbA (20 μM), hemin (20 μM), or control for 18 hours. Presented cytokines/chemokines (n = 18 out of 105) are those with a >1.5–increased level in the cell culture supernatant (measured in pooled samples with a membrane-based antibody array, n = 2 per group) after incubation with HbS compared with HbA. (B-I) Relative level of TNF-α (B), IL-1β (C), IL-6 (D), CCL2 (E), CCL3/CCL4 (F), CXCL1 (G), CXCL5 (H), and GM-CSF (I) in the culture medium. ∗∗∗P < .001; ∗∗P < .01; ∗P < .05 by one-way ANOVA.
Activation of monocytes by HbS is mediated by TLR4
Because the activation of the NF-κB and type I interferon signaling pathways in monocytes may be mediated by TLR4 and because high mRNA expression of TLR4 by monocytes from patients with SCD was reported, with strong correlation to IL-6 expression,5 we hypothesized that the action of HbS might be mediated by TLR4. To explore this hypothesis, we first confirmed that surface expression of TLR4 by human monocytes was increased in patients with SCD (n = 19) as compared with controls (n = 10) (Figure 4A; supplemental Figure 3). We also found that TLR4 mRNA expression by human monocytes from healthy blood donors was increased after coincubation with HbS as compared with HbA (Figure 4B).
Monocyte activation by HbS is mediated by TLR4. (A) Surface expression of TLR4 on human monocytes from patients with SCD (n = 19) and controls (n = 10), measured by flow cytometry. (B) mRNA expression (relative to β-actin and compared with control) of TLR4 by human monocytes from healthy blood donors (n = 5), in culture with HbS (20 μM) or HbA (20 μM), after 4 hours (n = 5 per group). (C-E) Levels of TNF-α (C), IL-1β (D), and IL-6 (E) in the cell culture supernatant of human monocytes from healthy blood donors (n = 4), in culture with HbS (20 μM), HbS (20 μM) with the TLR4 inhibitor TAK-242 (1 μM) added to culture medium 1 hour before HbS, or control, after 18 hours (n = 4 per group). (F-G) Levels of TNF-α (F) and IL-6 (G) in the cell culture supernatant of THP1-Dual and THP1-Dual KO-TLR4 cells in culture with HbA (20 μM), HbS (20 μM), LPS (0.1 μg/mL), or control, after 18 hours (n = 3 per group). (H-I) The NF-κB pathway activation level (H) and type I interferon pathway activation level (I) relative to control, of THP1-Dual and THP1-Dual KO-TLR4 cells in culture with HbA (20 μM), HbS (20 μM), LPS (0.1 μg/mL), or control, after 18 hours (n = 6 per group). ∗∗∗P < .001; ∗P < .05 by Mann-Whitney test (A) or one-way ANOVA (B-I). MFI, mean fluorescence intensity.
Monocyte activation by HbS is mediated by TLR4. (A) Surface expression of TLR4 on human monocytes from patients with SCD (n = 19) and controls (n = 10), measured by flow cytometry. (B) mRNA expression (relative to β-actin and compared with control) of TLR4 by human monocytes from healthy blood donors (n = 5), in culture with HbS (20 μM) or HbA (20 μM), after 4 hours (n = 5 per group). (C-E) Levels of TNF-α (C), IL-1β (D), and IL-6 (E) in the cell culture supernatant of human monocytes from healthy blood donors (n = 4), in culture with HbS (20 μM), HbS (20 μM) with the TLR4 inhibitor TAK-242 (1 μM) added to culture medium 1 hour before HbS, or control, after 18 hours (n = 4 per group). (F-G) Levels of TNF-α (F) and IL-6 (G) in the cell culture supernatant of THP1-Dual and THP1-Dual KO-TLR4 cells in culture with HbA (20 μM), HbS (20 μM), LPS (0.1 μg/mL), or control, after 18 hours (n = 3 per group). (H-I) The NF-κB pathway activation level (H) and type I interferon pathway activation level (I) relative to control, of THP1-Dual and THP1-Dual KO-TLR4 cells in culture with HbA (20 μM), HbS (20 μM), LPS (0.1 μg/mL), or control, after 18 hours (n = 6 per group). ∗∗∗P < .001; ∗P < .05 by Mann-Whitney test (A) or one-way ANOVA (B-I). MFI, mean fluorescence intensity.
We next added the TLR4 inhibitor TAK-242 (resatorvid) to culture medium of human monocytes from healthy donors 1 hour before adding HbS, which led to a complete inhibition of the enhanced proinflammatory cytokine expression induced by HbS (Figure 4C-E). To further demonstrate the crucial role of TLR4, we used the human monocyte-like THP1 and KO-TLR4 THP1 cell lines. As expected, HbS induced an increased production of TNF-α and IL-6 in THP1 cells, whereas no effect was observed in KO-TLR4 THP1 cells (Figure 4F-G; supplemental Figure 4). A chemotactic effect of HbS on THP1 cells was also observed, which was mediated by TLR4 (supplemental Figure 5). TLR4 belongs to the TLR4/MD-2 complex, which plays an essential role in mediating inflammatory responses to damage-associated molecular patterns.17 Depending on the ligand interacting with MD-2, activation of the TLR4/MD-2 complex may induce the activation of 2 distinct signal transduction pathways: the MyD88-dependent pathway leading to the expression of proinflammatory cytokines through NF-κB and the TRIF-dependent pathway leading to the expression of type I interferon, through interferon regulatory factors (IRFs).18 To investigate the involvement of these 2 signaling pathways, we used THP1-Dual cells, allowing for measurement of reporter proteins secreted in the cell culture supernatant under activation of NF-κB or IRFs. HbS was found to induce activation of both MyD88- and TRIF-dependent pathways (Figure 4H-I). These results were consistent with the fact that both MyD88 and TRIF are required for TLR4-mediated TNF-α expression.19 These experiments were repeated in RAW and KO-TLR4 RAW cell line murine macrophages, with similar results (supplemental Figure 6A-B). To further confirm the role of MD-2 in HbS-dependent TLR4 signaling, we decided to use CRISPR-Cas9 to knock out MD-2 in RAW-Dual murine macrophages. As expected, coincubation of KO-MD-2 murine macrophages with HbS did not result in any activation of NF-κB or IRFs (supplemental Figure 6C-D), thereby demonstrating that MD-2 is mandatory for HbS-dependent TLR4 signaling.
HbS has a strong affinity for the TLR4/MD-2 complex
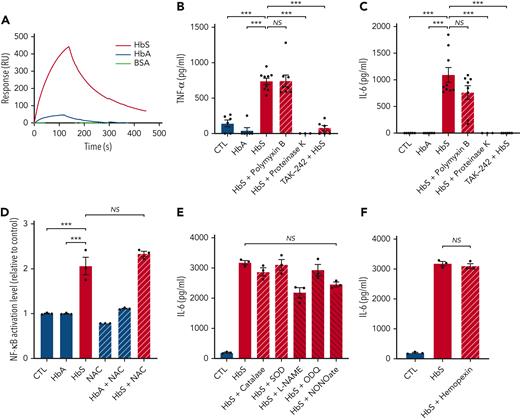
Then, to investigate whether activation of the TLR4/MD-2 complex was mediated by direct molecular interaction with HbS, we used the SPR/Biacore technology, allowing the measurement of affinities between HbS or HbA and TLR4/MD-2. HbS was found to strongly interact with TLR4/MD-2 with a dissociation constant (Kd) of 5 × 10−8 M, whereas only a slight interaction was observed with HbA (Figure 5A).
HbS interacts with the TLR4/MD-2 complex and is responsible for TLR4-mediated monocyte activation, independently of LPS, ROS, NO, or heme. (A) SPR analysis of the binding between HbS (50 nM), HbA (50 nM), or BSA (200 nM) and the TLR4/MD-2 complex. (B-C) Levels of TNF-α (B) and IL-6 (C) in the cell culture supernatant of human monocytes from healthy blood donors (n = 9), in culture with HbA (20 μM), HbS (20 μM), HbS (20 μM) pretreated with polymyxin B (100 μg/mL) for 2 hours, HbS (20 μM) pretreated with proteinase K (5 mg/mL) at 37°C for 3 hours, HbS (20 μM) with TAK-242 (1 μM) added to culture medium 1 hour before HbS, or control, after 18 hours (n = 3 to 9 per group). (D) NF-κB activation level of THP1-Blue cells in culture with HbA (20 μM), HbS (20 μM), NAC (50 μM), HbA (20 μM) with NAC (50 μM), HbS (20 μM) with NAC (50 μM), or control, after 18 hours (n = 3 per group). (E-F) IL-6 levels in the cell culture supernatant of human monocytes from healthy blood donors (n = 3), in culture with HbS (20 μM), HbS (20 μM) with catalase (500 U/mL), SOD (100 U/mL), L-NAME (5 mM), ODQ (100 μM), NONOate (1 mM), hemopexin (100 μg/mL), or control, after 18 hours (n = 3 per group). ∗∗∗P < .001 by one-way ANOVA. NO, nitric oxide; ROS, reactive oxygen species.
HbS interacts with the TLR4/MD-2 complex and is responsible for TLR4-mediated monocyte activation, independently of LPS, ROS, NO, or heme. (A) SPR analysis of the binding between HbS (50 nM), HbA (50 nM), or BSA (200 nM) and the TLR4/MD-2 complex. (B-C) Levels of TNF-α (B) and IL-6 (C) in the cell culture supernatant of human monocytes from healthy blood donors (n = 9), in culture with HbA (20 μM), HbS (20 μM), HbS (20 μM) pretreated with polymyxin B (100 μg/mL) for 2 hours, HbS (20 μM) pretreated with proteinase K (5 mg/mL) at 37°C for 3 hours, HbS (20 μM) with TAK-242 (1 μM) added to culture medium 1 hour before HbS, or control, after 18 hours (n = 3 to 9 per group). (D) NF-κB activation level of THP1-Blue cells in culture with HbA (20 μM), HbS (20 μM), NAC (50 μM), HbA (20 μM) with NAC (50 μM), HbS (20 μM) with NAC (50 μM), or control, after 18 hours (n = 3 per group). (E-F) IL-6 levels in the cell culture supernatant of human monocytes from healthy blood donors (n = 3), in culture with HbS (20 μM), HbS (20 μM) with catalase (500 U/mL), SOD (100 U/mL), L-NAME (5 mM), ODQ (100 μM), NONOate (1 mM), hemopexin (100 μg/mL), or control, after 18 hours (n = 3 per group). ∗∗∗P < .001 by one-way ANOVA. NO, nitric oxide; ROS, reactive oxygen species.
Because the TLR4/MD-2 complex is classically activated by LPS from Gram-negative bacteria, we used polymyxin B, an antibiotic that inactivates LPS, and proteinase K, a broad-spectrum protease that has no action on LPS, to confirm the absence of contamination of our purified human HbS by LPS (Figure 5B-C; supplemental Figure 7). As TLR4-dependent activation of NF-κB can be modulated by ROS,20 we used NAC, catalase, and SOD to confirm that our results with human HbS were not mediated by ROS (Figure 5D-E). Furthermore, we used L-Name, ODQ, and NONOate to confirm the absence of involvement of NO (Figure 5E). Finally, hemopexin was used to exclude any involvement of heme in HbS-mediated TLR4/MD-2 activation (Figure 5F). As expected, no effect of hemopexin was observed, whereas haptoglobin significantly decreased the HbS-induced production of proinflammatory cytokines (supplemental Figure 8).
HbS induces the production of proinflammatory cytokines in SCD mice
To investigate the effect of HbS in vivo, we administrated HbS or HbA to Townes HbAA and HbSS mice, pretreated or not with TAK-242. Consistent with our in vitro findings, an increased mRNA expression of TNF-α and IL-6 by sorted monocytes of HbSS mice was observed after IV injection of HbS, contrary to HbA (supplemental Figure 9). Moreover, HbS, unlike HbA, was responsible for an increase in the plasma level of these proinflammatory cytokines in HbSS mice, which was prevented by the administration of TAK-242 (Figure 6). This effect of HbS was independent of the batch used. It was not observed in HbAA mice, and this was not due to a lower TLR4 expression on monocytes from HbAA mice compared with HbSS mice (supplemental Figure 10) but was possibly reflecting effective inhibition of HbS by haptoglobin, which is depleted in HbSS mice unlike in HbAA mice.21 Notably, the plasma levels of TNF-α and IL-6 were only slightly increased at steady state in HbSS mice compared with HbAA mice, which was consistent with rather low plasma levels of free Hb in HbSS mice at steady state (0.29 ± 0.08 mg/mL) compared with the levels observed during VOC (2.34 ± 0.31 mg/mL) induced by hypoxia/reoxygenation (supplemental Figure 11).
HbS induces the production of proinflammatory cytokines in SCD mice. (A-B) Levels of TNF-α (A) and IL-6 (B) in the plasma of Townes HbAA and HbSS mice, 18 hours after IV injection with purified human HbS (2 μmol/kg, batches SLBV0862 [data represented as triangles] and SLCC7764 [data represented as dots]) or HbA (2 μmol/kg, batch SLCB7096), preceded or not by intraperitoneal injection with TAK-242 (1 mg/kg), 1 hour before (n = 3-11 mice per group). In each experiment, the same batch of HbS was injected into HbSS and HbAA mice. ∗∗∗P < .001; ∗∗P < .01; ∗P < .05 by Mann-Whitney test.
HbS induces the production of proinflammatory cytokines in SCD mice. (A-B) Levels of TNF-α (A) and IL-6 (B) in the plasma of Townes HbAA and HbSS mice, 18 hours after IV injection with purified human HbS (2 μmol/kg, batches SLBV0862 [data represented as triangles] and SLCC7764 [data represented as dots]) or HbA (2 μmol/kg, batch SLCB7096), preceded or not by intraperitoneal injection with TAK-242 (1 mg/kg), 1 hour before (n = 3-11 mice per group). In each experiment, the same batch of HbS was injected into HbSS and HbAA mice. ∗∗∗P < .001; ∗∗P < .01; ∗P < .05 by Mann-Whitney test.
Discussion
In patients with SCD, plasma HbS levels have been reported to increase 1.5-fold to threefold during VOC,22 secondary to intravascular hemolysis of sickle RBCs promoted by intracellular polymerization of deoxygenated HbS. This observation is consistent with our findings of monocyte activation by HbS, because increased plasma levels of proinflammatory cytokines and chemokines, including TNF-α, IL-1β, and IL-6, have been reported in patients with SCD during VOC compared with steady state.11,23,24 The level of these cytokines also tends to be higher at steady state than in healthy controls, and discrepancies between publications might reflect varying degrees of baseline hemolysis in patients with SCD, with a potential benefit for hydroxyurea.11,23,25,26 In SCD mice, we observed rather low levels of free HbS at steady state, reflecting mild chronic hemolysis, that were increased eightfold during VOC induced by hypoxia/reoxygenation. Free HbS levels during VOC were comparable with the amount of exogenous HbS injected into SCD mice in our experiments. Intravascular hemolysis is considered a fundamental pathophysiological mechanism of SCD, actively contributing to morbidity and mortality.27 Its role in promoting monocyte activation has been suggested by the positive correlation between monocytosis and hemolysis markers in patients with SCD.28 More recently, upregulation of the type I interferon signaling pathway has been reported in CD14+ monocytes from patients with SCD, with a positive correlation between hemolysis markers and plasma interferon alpha levels.29,30 Cell-free HbS released in plasma during chronic and acute intravascular hemolysis undergoes oxidation from the ferrous (Fe2+) to the ferric (Fe3+) state through both NO scavenging by dioxygenation and reactions with several oxidizing agents such as H2O2.31 Free heme released by ferric Hb is a potent activator of endothelial cells via TLR4 and was shown to induce vaso-occlusion and acute chest syndrome in SCD mice.6,32 Over the last years, heme has been increasingly recognized as an important mediator of both adhesion and inflammation in SCD.33-35 However, its ability to activate monocytes has not been demonstrated, and unlike LPS, it was found insufficient to induce TLR4-mediated IL-6 production by monocytes from patients with SCD.7 Even if oxidation occurs faster for HbS than HbA,36 the potential role of cell-free ferrous Hb should not be underestimated because plasma concentrations of ferrous HbS in patients with SCD are high enough to deplete haptoglobin, the Hb scavenger, and to abrogate endothelial NO bioactivity.31 Intravascular hemolysis induced by hypotonic water infusion in non-SCD mice was shown to trigger rapid systemic inflammation, and this effect was abolished by the concomitant administration of an NO donor or haptoglobin but not by hemopexin, the hemin scavenger.37 This effect was possibly mediated by vascular NO consumption, and cell-free Hb may exert some of its inflammatory action via endothelial activation, but it could also reflect an activation of monocytes by Hb. Consistent with this hypothesis, we observed a trend, although nonstatistically significant, of increased production of proinflammatory cytokines by human monocytes after coincubation with HbA, and a slight interaction was also found between HbA and TLR4/MD-2.
Here, we describe, to the best of our knowledge, for the first time, a mechanism of monocyte activation by HbS, mediated by the TLR4/MD-2 complex. LPS, the main ligand of TLR4/MD-2, has been shown to interact with a large hydrophobic pocket in MD-2,38 and a variety of hydrophobic molecules, including endogenous ligands, have also been reported to bind to this complex.39 Because HbS structure differs from that of HbA by the replacement of a hydrophilic glutamic acid with a hydrophobic valine on the sixth position of the β-globin chains, we hypothesize that HbS hydrophobic properties may induce its binding to MD-2, thereby promoting TLR4-mediated monocyte activation. Future in silico binding affinity studies are required to confirm this hypothesis.
Altogether, our findings demonstrate that HbS is responsible for monocyte activation with enhanced production of proinflammatory cytokines and chemokines in patients with SCD. This may explain the proinflammatory condition of SCD as compared with other hemolytic diseases and is consistent with the recent identification of a unique monocyte transcriptome involving interferon signaling in SCD compared with other hereditary hemolytic anemias.29 The activation of the NF-κB signaling pathway was however predominant compared with the type I interferon pathway, which deserves further investigation. This novel mechanism of cell activation is likely to concern not only monocytes but also other innate immune cells expressing TLR4. In favor of this assumption, HbS was previously found to induce a higher expression of P-selectin on endothelial cells exposed to hypoxia/reoxygenation, compared with HbA, and HbS infused to SCD mice also induced significantly more stasis than HbA.6 Our results may open new, exciting therapeutic perspectives, especially through inhibition of the HbS-TLR4 interaction. Whereas therapeutically targeting TLR4 would probably increase susceptibility to infections, a particularly high risk in the context of SCD, specifically targeting the HbS-TLR4 interaction should not be deleterious and may prevent the vicious cycle of inflammation in SCD. The forthcoming screen for small molecules that inhibit the HbS-TLR4 interaction may lead to the development of innovative treatments for patients with SCD.
Acknowledgment
This work was supported by state funding from the Agence Nationale de la Recherche under the Investissements d’avenir program (ANR-10-IAHU-01).
Authorship
Contribution: S.A., R.R.-B., T.T.M., and O.H. designed the study; S.A., M.d.M., and M.T. were responsible for patient recruitment; S.A., R.R.-B., and T.T.M. performed the experiments; T.B. performed Biacore experiments; S.A., R.R.-B., T.T.M., and O.H. analyzed all experiments and drafted the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Slimane Allali, Department of Pediatrics, Necker-Enfants Malades Hospital, 149 rue de Sèvres, 75015 Paris, France; e-mail: slimane.allali@aphp.fr.
References
Author notes
∗O.H. and T.T.M. contributed equally to this study.
Data are available on request from the corresponding author, Slimane Allali (slimane.allali@aphp.fr).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![HbS induces the production of proinflammatory cytokines in SCD mice. (A-B) Levels of TNF-α (A) and IL-6 (B) in the plasma of Townes HbAA and HbSS mice, 18 hours after IV injection with purified human HbS (2 μmol/kg, batches SLBV0862 [data represented as triangles] and SLCC7764 [data represented as dots]) or HbA (2 μmol/kg, batch SLCB7096), preceded or not by intraperitoneal injection with TAK-242 (1 mg/kg), 1 hour before (n = 3-11 mice per group). In each experiment, the same batch of HbS was injected into HbSS and HbAA mice. ∗∗∗P < .001; ∗∗P < .01; ∗P < .05 by Mann-Whitney test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/18/10.1182_blood.2021014894/6/m_blood_bld-2021-014894-gr6.jpeg?Expires=1765990227&Signature=ytomNVzfHqcQgZ~hPpiCLZI3CjJDSkOEa5a4H2VY3qJ-QBeaWcFbeP1qlTbxv7mqlrJe~9SQFQaZ4wkMZsVL9bbOZXJa6k6jvDNBnM3qorGGdeVMQ26fJ9svxy8I-Sh28Y5aIoOqTjEK1LFYIS8h5LCOv3T2QCPk3He6ceIxH0JMLXRAS8BwYq6RtLvl6r7yEhbprScmaROKIH5TXG1Q7GEYF7GrYnLJwC7Z-4faD3nmUGEWaHixiUjFMxpSo9SWQIwCuzDqOuZKXhp-4rKQD4mqAWtsF90V5htj6H-dvpGPjVcdPGzObEteXUB2UbIYh~qbzVk-FkYDLAVjWCt~Pw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal