Abstract
Introduction: Anti-CD19 chimeric antigen receptor (CAR) T-cells have remarkable efficacy in chemorefractory large B-cell lymphoma (LBCL). Cerebrospinal fluid (CSF) of treated patients shows that CAR T-cells enter the CSF and previous reports have demonstrated anti-lymphoma activity within the central nervous system (CNS). Chemorefractory primary and secondary CNSL have a dismal prognosis and represent an important and urgent unmet need. We conducted a pilot clinical trial of axi-cel in patients with relapsed/refractory CNSL to assess safety and preliminary efficacy in this population. Here we present the results of the 9 patients treated in the CNS disease-only cohort on this study.
Methods: Adult patients with primary or secondary CNSL, refractory to or relapsing after prior CNS directed therapy, were enrolled into 2 cohorts (cohort 1 CNS only disease; cohort 2 CNS and systemic disease). Following T-cell collection, no bridging therapy other than stable steroid dosing was allowed. All patients had an Ommaya reservoir placed prior to axi-cel treatment. Patients received standard lymphodepletion with fludarabine and cyclophosphamide followed by axi-cel infusion at a dose of 2x106 cells/kg. All patients were admitted to the hospital for their axi-cel infusion. The first 3 patients in each cohort had to complete their treatment limiting toxicity (TLT) window (28d) before the subsequent patient could be treated; if 1 TLT was observed, 3 additional patients would be enrolled followed by a safety pause. If there were 2+ TLTs in the first 3-6 patients, the study would be amended with additional safety measures; otherwise, the study would complete enrollment for a total of 9 patients in each cohort. The primary endpoint of the study is safety as measured by the rate of TLTs and grade 3+ adverse events (AEs). Secondary endpoints are those of efficacy, including objective response rate (ORR) and complete response (CR) rate, duration of response (DOR), progression-free survival (PFS) and overall survival (OS).
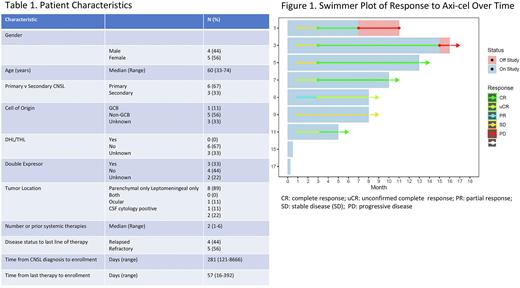
Results: We report the results of the 9 patients treated in cohort 1. Patient characteristics are shown in Table 1. Six of nine patients have primary CNSL (67%), with 89% having parenchymal-only disease. The median number of prior therapies was 2 (range 1-6) and 56% of patients were refractory to their last line of therapy. The median follow-up at the time of the meeting will be 12m (range 4-20m). To date, all 9 patients in cohort 1 have been enrolled and treated, and 7 patients have >1m follow-up. Of these, the ORR is 86% with all 6 responders achieving a CR by 3m (CR rate 86%; Figure 1). The one non-responder has stable disease (SD) through 6m of follow-up. Two of the responders have progressed, at 6 and 15m. DOR, PFS and OS data will be reported at the meeting.
Eight treated patients are evaluable for safety and no TLTs were observed. There were 7 instances of cytokine release syndrome (CRS; 88%). This was gr1-2 in all cases. There were 4 incidences of immune effector cell therapy associated neurologic syndrome (ICANS; 50%), 1 gr1 (12.5%) and 3 gr3 (37.5%). One patient did develop a staphylococcus meningitis related to an infection of the Ommaya reservoir, requiring explant with subsequent recovery.
Axi-cel pharmacokinetics and serial cytokine analysis in the blood and CSF pre- and post-treatment were analyzed and will be presented. Peak serum CAR T-cell levels in the first 7 treated patients were comparable to that seen on ZUMA-1, despite the lack of systemic disease.
Paired CSF and blood samples have undergone flow cytometry and scRNAseq and these results will be submitted in a separate abstract. Cell-free DNA analysis for MRD in the CSF is ongoing and will be reported as part of this abstract.
Conclusion: Based on these results, axi-cel has an acceptable safety profile for the treatment of both primary and secondary CNSL with no increased risk of neurologic events, including high-grade ICANS or cerebral edema. The efficacy is promising, with a CR rate to date of 86%. Nine additional patients with concurrent CNS and systemic lymphoma are still enrolling in cohort 2 with plans to expand enrollment based on further promising results. Ongoing correlative analysis of blood and CSF samples will be completed in order to better characterize safety, efficacy and biomarkers of resistance and/or toxicity of axi-cel in CNSL.
Disclosures
Jacobson:Kite/Gilead: Consultancy, Research Funding; Pfizer: Research Funding; Bluebird Bio: Consultancy; BMS/Celgene: Consultancy; Novartis: Consultancy; Mitlenyi: Consultancy; Abintus Bio: Consultancy; Ipsen: Consultancy; Instil Bio: Consultancy; Epizyme: Honoraria; Daiichi-Sankyo: Consultancy; Morphosys: Consultancy; Caribou Bio: Consultancy; ImmPACT Bio: Consultancy. Kean:Vertex: Consultancy; Magenta: Research Funding; Novartis: Research Funding; Bristol Myers Squibb: Patents & Royalties: clinical trial, Research Funding; EMD-Serono: Research Funding; Mammoth Biosciences: Current equity holder in private company, Current holder of stock options in a privately-held company; HiFiBio: Consultancy; Bluebird Bio: Research Funding. Gerdemann:AlloVir: Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Sholl:AstraZeneca: Consultancy; Bristol Myers Squibb: Research Funding; Genentech: Consultancy, Research Funding; GV20 Therapeutics: Consultancy; Lilly: Consultancy. Poddar:UCLA: Other: intellectual Property, Patents & Royalties; Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company. Davis:Kite/Gilead: Current Employment. Mao:Kite/Gilead: Current Employment. Filosto:Tusk Therapeutics: Patents & Royalties; Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company. Mattie:Kite/Gilead: Current Employment. Armand:AstraZeneca: Consultancy; Epizyme: Consultancy; Regeneron: Consultancy; Enterome: Consultancy; C4 Therapeutics: Consultancy; GenMab: Consultancy; Tessa Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Kite Pharma: Research Funding; IGM Biosciences: Research Funding; Roche/Genentech: Consultancy, Research Funding; Adaptive Biotechnologies: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Merck: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal