Abstract
Background
Autologous chimeric antigen receptor (CAR) Ts have advanced the treatment of relapsed/refractory multiple myeloma. Due to access and treatment delays, many patients (pts) may not benefit from these therapies. Allogeneic CAR T cell products may address access challenges and ensure eligible pts benefit. ALLO-715 is a genetically modified anti-B cell maturation antigen (BCMA) AlloCAR T™ cell therapy, which uses Cellectis technology to disrupt the T-cell receptor alpha constant (TRAC) and the CD52 genes to eliminate the risk of graft-versus-host disease (GvHD) and permit the use of ALLO-647, an anti-CD52 monoclonal antibody (mAb), for selective and transitory host lymphodepletion (LD).
Methods
UNIVERSAL is an open-label, Phase 1 trial (NCT04093596) in adults with relapsed/refractory (R/R) multiple myeloma who have received ≥3 prior lines of therapy including a proteasome inhibitor, immunomodulator, and anti-CD38 mAb. Pts must be refractory to their last treatment line. Part A evaluates the safety, efficacy, cellular kinetics, immunogenicity, and pharmacodynamics of a single dose of ALLO-715 following lymphodepletion with an ALLO-647 containing regimen. Pts receive LD followed by ALLO-715 at 1 of 4 dose levels (DLs) in a 3+3 dose escalation design: 40 (DL1), 160 (DL2), 320 (DL3), and 480 (DL4) x 106 CAR+ T cells with varying doses and schedules of LD including ALLO-647 with fludarabine and cyclophosphamide (FC). After the completion of cell dose and LD exploration, two DL3 cohorts were chosen for expansion using lymphodepletion of FC and ALLO-647 (A) at 39mg or 60mg. This publication provides updated safety data on the overall Part A study population and highlights efficacy data from the two expanded DL3 cohorts.
Results
As of June 22, 2022, 53 pts were enrolled; 98% of pts receiving LD were treated with ALLO-715 with one remaining patient (2%) pending ALLO-715 infusion at the time of the data cut-off. Median time from enrollment to LD was 5 days and no pts required bridging therapy. One hundred percent of pts received product manufactured and released per product specifications. Pts were pretreated with a range of 3 to 11 (median:5) prior lines of therapy; 44% were penta refractory. Twenty-three% of pts were international staging system (ISS) Stage III, 42% had high risk cytogenetics, and 19% had extramedullary disease. No GvHD or dose-limiting toxicities were observed. Grade (Gr) 3+ adverse events (AEs) included neutropenia, anemia, thrombocytopenia, and lymphopenia. Cytokine release syndrome (CRS) occurred in 52%, all Gr 1/2 except 1 pt with Gr 3. The use of tocilizumab and steroids across all pts was 19% and 15%, respectively. Potential events of neurotoxicity were identified in 5 (11%) pts, all Gr 1/2. Infections occurred in 56% of pts, with Gr 3+ in 29% of pts. Of all infections, viral infections or low Gr viral reactivation, were most common. No new Gr 5 events occurred.
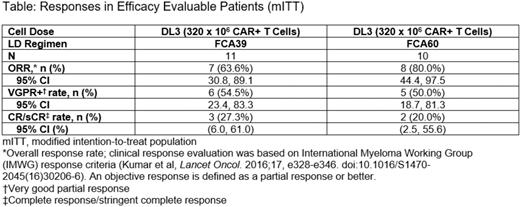
Early results were presented previously (ASH 2021) and this efficacy analysis focuses on pts treated at DL3, which was considered the most active cell dose, using FCA39 (n=11) and FCA60 (n=10) (Table). In these cohorts, the overall response rates (ORR) were 64% and 80%, for FCA39 and FCA60, respectively, with 55% and 50% achieving very good partial response or better (VGPR+) and 27% and 20% achieving complete response/stringent complete response (CR/sCR). Responses were observed at the first disease assessment (14 days) in 9/15 responders. With median follow-up times of 12.1 months in the FCA39 cohort and 16.8 months in the FCA60 cohort, 2 pts are in ongoing response at 12 months in the FCA39 cohort and 3 pts at 12, 19, and 20 months in the FCA60 cohort. Of 11 pts in these cohorts with VGPR+ assessed for minimum residual disease (MRD), 10 (91%) were MRD negative. Safety of these regimens was similar to the overall safety.
Conclusions
UNIVERSAL demonstrates significant and durable responses from allogeneic CAR T therapy. ALLO-715 DL3 with FCA39 and FCA60 was associated with clinically meaningful efficacy, including VGPR+ rates of 55% and 50% without requiring leukapheresis or bridging therapy. Ninety-eight percent of pts receiving LD received ALLO-715 with 100% of product manufactured and released per product specifications. FCA induces a deep and durable window of lymphocyte depletion allowing CAR T cell expansion. The UNIVERSAL trial continues to enroll pts in the FCA60 cohort and updated data will be presented.
Disclosures
Mailankody:Evicore: Consultancy; Janssen Oncology: Consultancy, Research Funding; BioAscend: Consultancy; Optum Oncology: Consultancy; Allogene Therapeutics: Research Funding; Takeda Oncology: Research Funding; Juno Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; Fate Therapeutics: Research Funding; Plexus Communication: Honoraria; OncLive: Honoraria; Physician Education Resource: Honoraria; Legend Biotech: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment. Matous:BeiGene: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Liedtke:Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Caelum: Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam: Membership on an entity's Board of Directors or advisory committees; Allogene: Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Seagen Inc.: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Natera: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees. Sidana:Magenta Therapeutics: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Allogene: Research Funding; Janssen: Consultancy, Research Funding; Sanofi: Consultancy; Prothena: Honoraria; Oncopeptides: Consultancy. Oluwole:Pfizer: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Kite, a Gilead Company: Research Funding; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Curio Science: Consultancy. Nath:Incyte: Consultancy, Honoraria; Actinium: Consultancy, Honoraria. Rossi:adaptive: Consultancy; janssen: Consultancy; gsk: Consultancy; BMS: Consultancy; sanofi: Consultancy. Ghatta:Allogene Therapeutics: Current Employment. Dillon:Allogene Therapeutics: Current Employment. Navale:Allogene, Gamida Cell, Neogene: Consultancy, Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Karski:Crisper Therapeutics: Other: Equity Ownership; Allogene Therapeutics: Current Employment; Nektar Therapeutics: Other: Equity Ownership. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal