Abstract
Within the first months of the COVID-19 vaccination campaign, previously healthy recipients who developed severe thrombosis (often cerebral and/or splanchnic vasculature) and thrombocytopenia typically after adenoviral vector-based vaccination were identified. Similarities between this syndrome, vaccine-induced immune thrombotic thrombocytopenia (VITT), and heparin-induced thrombocytopenia prompted recognition of the role of antiplatelet factor 4 (PF4) antibodies and management strategies based on IV immunoglobulin and nonheparin anticoagulants, which improved outcome. We update current understanding of VITT and potential involvement of anti-PF4 antibodies in thrombotic disorders.
Introduction
Biotechnology and pharmaceutical companies responded to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic with unprecedented speed, generating adenovirus-based vaccines (ChAdOx1/nCoV-19 from AstraZeneca/Covishield and Ad26 COV2.S from Johnson and Johnson/Janssen) and mRNA-based vaccines (mRNA-1273 from Moderna and tozinameran/Comirnaty from Pfizer-BioNTech). Outside the European Union and the United States, SputnikV, Sinopharm, Sinovac-CoronaVac and Bharat Biotech BBV152 Covaxin vaccines are also used. However, by March 2021, safety signals concerning vaccines that use adenovirus-based vectors emerged involving previously healthy recipients who developed thromboses and thrombocytopenia, typically in the second week after initial vaccination.1-5
The term thrombosis and thrombocytopenia syndrome (TTS) refers to all thrombocytopenia and thrombotic complications after vaccination. Based on clinical similarities to “autoimmune” or “spontaneous” heparin-induced thrombocytopenia,6,7 Immunoglobulin G (IgG) antibodies cross-reacting with “unbound” platelet factor 4 (PF4) in assays for heparin-induced thrombocytopenia (HIT) were soon identified in a subgroup of patients with TTS. This syndrome, vaccine-induced immune thrombotic thrombocytopenia (VITT), was the subject of a Blood Spotlight in 20218; several national and international guidelines for diagnosis and management have been published,9-14 which will be updated in 2023 by the World Health Organization.15 VITT remains highly relevant in low- and middle-income countries that can only afford adenoviral vector-based vaccines and where the vaccination campaign is ongoing. It is also important to understand which vaccine constituent(s) trigger(s) the immune response to PF4, in order to design safer delivery systems. Here we update our understanding of VITT based on recent findings.
Clinical presentation
The incidence of VITT is estimated to be 3 to 15 cases per million initial vaccinations,16,17 depending on recognition and case definition. Fewer than 50 patients with VITT have been reported from Asia, Africa and Latin America combined. Potential under-recognition18 is worrisome, as early recognition and start of treatment can reduce mortality by nearly 90%. This has been demonstrated by the reduction in mortality from about 50% in the first 3 case series1,2,19 to about 5% to 6% in Australia where the vaccination campaign was combined with an educational program informing the public and especially physicians about symptoms of VITT and appropriate treatment.20
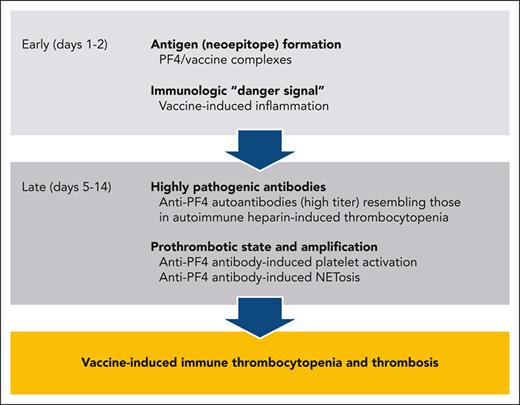
Patients typically present 4 to 30 days after first vaccination with an adenoviral vector-based vaccine (irrespective of whether another type of COVID-19 vaccine had been given before),19 with rare cases developing after a second vaccination with an adenovirus vector-based vaccine.18,21 There is a predilection for cerebral venous sinus thrombosis (CVST) and cerebral venous thrombosis, with ∼50% to 70% of patients presenting with persistent headache or other neurologic complaints, and for thrombi developing within the splanchnic and portal venous systems, with 10% to 20% of patients presenting with abdominal complaints; the remaining patients present with symptoms resulting from deep venous thrombosis, pulmonary embolism, and arterial thrombosis, including stroke and myocardial infarction. Thrombocytopenia is almost universal by the time of presentation, but occasionally precedes thromboembolism. An unexplained fall in platelet count >50% also indicates VITT independent of the absolute platelet count.17 A marked elevation in plasma D-dimers (>4000 fibrinogen equivalent units) is common, and so is a reduction in plasma fibrinogen,1,2,19 indicative of disseminated intravascular coagulation. Imaging reveals one or more sites of thrombosis in over 90% of patients, but neurovascular imaging may be negative initially in some who present on or after postvaccination day 4 with severe headache or with incipient microvascular CVST, necessitating treatment for presumed VITT and repeat imaging22 (“pre-VITT syndrome”23).
Diagnosis
VITT is a life-threatening condition, especially when CVST is present,21 and should be the foremost consideration in any patient who presents with severe headache and/or thrombosis with thrombocytopenia starting 4 to 30 days after adenoviral vector-based vaccination against SARS-CoV-2. Criteria have been published to help assess the likelihood of VITT (definite, probable, possible, unlikely) based on clinical features alone and along with the results of enzyme-linked immunosorbent assays (ELISAs) to detect anti-PF4 antibodies (Table 1).17,19 Routine postvaccine screening for PF4/heparin antibodies after vaccination is discouraged, as low levels of clinically irrelevant anti-PF4/heparin antibodies are detected in 5% to 10% of healthy recipients of adenoviral and mRNA-based vaccines.24 However, the clinical diagnosis of VITT should always be confirmed by PF4/heparin ELISA, as recent COVID-19 vaccination does not exclude coincidental thrombotic complications caused by HIT, cancer associated thrombosis, catastrophic antiphospholipid syndrome, HELLP syndrome in pregnancy, and thrombotic thrombocytopenic purpura, among others,25,26 especially if not all the clinical criteria presented in Table 1 are present.
The UK Haematology Expert Group developed consensus diagnostic criteria for VITT
| Case definition criteria |
| Onset of symptoms 5-30 days after COVID-19 vaccine (or up to 42 days if isolated DVT/PE) |
| Presence of thrombosis |
| Thrombocytopenia (platelet count <150 × 109/L) |
| D-dimer >4000 μg/mL (FEU) |
| Positive anti-PF4 Abs ELISA |
| Definite VITT |
| Meets all 5 criteria |
| Probable |
| D-dimer >4000 FEU but one criterion not fulfilled (timing, thrombosis, thrombocytopenia, anti-PF4 Abs), or |
| D-dimer unknown or 2000-4000 FEU with all other criteria present |
| Possible |
| D-dimer unknown or 2000-4000 FEU with one other criterion not fulfilled, or |
| Two other criteria not fulfilled (timing, thrombosis, thrombocytopenia, anti-PF4 Abs) |
| Unlikely |
| Platelet count <150 × 109/L without thrombosis with D-dimer <2000 FEU, or |
| Thrombosis with platelet count >150 × 109/L and D-dimer <2000 FEU, regardless of anti-PF4 Ab result, and/or |
| Case definition criteria |
| Onset of symptoms 5-30 days after COVID-19 vaccine (or up to 42 days if isolated DVT/PE) |
| Presence of thrombosis |
| Thrombocytopenia (platelet count <150 × 109/L) |
| D-dimer >4000 μg/mL (FEU) |
| Positive anti-PF4 Abs ELISA |
| Definite VITT |
| Meets all 5 criteria |
| Probable |
| D-dimer >4000 FEU but one criterion not fulfilled (timing, thrombosis, thrombocytopenia, anti-PF4 Abs), or |
| D-dimer unknown or 2000-4000 FEU with all other criteria present |
| Possible |
| D-dimer unknown or 2000-4000 FEU with one other criterion not fulfilled, or |
| Two other criteria not fulfilled (timing, thrombosis, thrombocytopenia, anti-PF4 Abs) |
| Unlikely |
| Platelet count <150 × 109/L without thrombosis with D-dimer <2000 FEU, or |
| Thrombosis with platelet count >150 × 109/L and D-dimer <2000 FEU, regardless of anti-PF4 Ab result, and/or |
Reproduced from “Vaccine induced immune thrombocytopenia and thrombosis: summary of NICE guidance,” Pavord S, Hunt BJ, Horner D, Bewley S, Karpusheff J; Guideline Committee. BMJ. 375:n2195, © 2021, with permission from BMJ Publishing Group Ltd.84
Microtiter plate ELISAs that measure antibodies to PF4/polyanion complexes used to diagnose HIT also detect anti-PF4 antibodies that cause VITT,17,19,27-29 most likely because noncomplexed PF4 is also coated on the solid phase.30 However, assays differ in sensitivity, and no single assay detects all possible cases. In case of strong clinical suspicion, treatment (see below) should be started immediately. Importantly most “rapid” and automated assays used to diagnose HIT give false negative results in VITT.19,28,30-32 The diagnosis can be confirmed with a functional assay showing PF4-dependent platelet activation by patient plasma or serum; activation is blocked by a monoclonal antibody to FcRγIIA and typically by high concentrations (100 IU/mL) of heparin. In contrast to HIT, low concentrations of heparin (0.2-1.0 IU) do not enhance, and indeed often inhibit, platelet activation by patient sera; instead, addition of PF4 (10 μg/mL) is needed to optimize platelet activation by VITT antibodies. The combination of a strongly positive PF4/heparin ELISA with PF4-dependent, heparin-independent platelet-activating antibodies has a sensitivity approaching 100% in the absence of recent administration of IVIG.33 In one study, the specificity of combined testing is ∼80% because of positive serology in patients with pre-VITT without documented thrombosis who might indeed have VITT.33 The sensitivity of the functional assay can be enhanced by testing undiluted and, if negative, diluted serum, which optimizes the stoichiometric ratio between PF4 and high titer antibodies that form pathogenic immune complexes.34 However, functional testing is not widely available in time for clinical decisions. The duration of antibody positivity is quite variable. Similar to HIT, platelet activating antibodies are generally no longer detected by 5 months, but positive ELISAs (albeit with falling optical densities) often persist.17,35-37 Rarely thrombocytopenia and high D-dimers do not resolve within 2-3 weeks after the onset of VITT, especially in patients with persistent high titer platelet-activating antibodies.35,36
Pathophysiology
VITT is the most recently described prothrombotic disorder mediated by anti-PF4 antibodies, ranging in decreasing frequency from “classic HIT,” to “persistent HIT” triggered by heparin that continues beyond drug exposure, to “autoimmune HIT” or “spontaneous HIT” that occurs without heparin exposure after orthopedic knee replacement surgery or after viral or bacterial infections.6,7,38
VITT antibodies recognize an epitope on PF4 that overlaps with the binding site for heparin, distinct from those recognized by HIT antibodies.39-41 This may enable VITT antibodies to bypass the template function served in HIT by heparin, DNA and polyphosphates42,43 by clustering PF4 directly.44 Binding of VITT antibodies is not restricted to this site,40 allowing them to also form immune complexes with PF4 bound to glycosaminoglycans expressed on cell surfaces. By analogy to the other anti-PF4 antibody-mediated disorders, the subsequent downstream prothrombotic cascade of VITT with activation of multiple cells including NETosis, is reasonably well understood and is summarized in Figure 1B.45
Current concept of the pathogenesis of VITT. The schematic presentation shown in A is speculative and in large part inferred from experiments in HIT. The schematic presentation of the downstream prothrombotic process shown in Panel B is largely substantiated by experimental data, some performed with VITT antibodies, others with HIT antibodies. Modified from Greinacher et al.45 (A) After vaccination, PF4 comes in contact with vaccine constituents and activates B-cells. Left side: It has been proposed that a direct inadvertent breach in the microvasculature at the vaccination site by IV injection or by disruption of vascular endothelial-cadherin tight junctions by EDTA in ChAdOx1, allows vaccine constituents to enter the circulation.45 Within the circulation, adenovirus particles can bind to platelets and can also bind PF4 released by activated platelets or from the matrix coating the microvascular endothelium.46,47 Platelets may become activated by vessel injury caused by injection of vaccine, after binding of the virions to the cell surface, or by immune complexes formed between contaminating host cell-line proteins in the vaccine and natural IgG antibodies against these proteins. Whether the virions themselves or another yet unknown constituent in the vaccine causes a conformational change in PF4 is unknown. Middle: once complexes with PF4 have formed, natural IgM antibodies activate complement (as it has been shown for PF4/heparin complexes),48 which enhances their proximity to B-cell receptors. In a mouse model, IV injection of ChAdOx-1, platelet-bound adenoviral particles are transported to the marginal zone of the spleen where B-cells are activated upon direct contact.49 However, electron microscopy and super resolution microscopy revealed complexes between PF4 and anti-PF4 VITT antibodies with amorphous constituents of the vaccine rather than virus particles.50 Beside the virions, other potential partners for PF4 include unassembled hexons51 and host cell-line proteins, although there is little overlap in the proteins contaminating ChAdOx1 and Ad26.COV2 vaccines.51 Right side: eventually, complexes of PF4 and vaccine (constituents) come in contact with B-cells expressing a cognate Ig receptors for PF4, either as fluid phase complexes, as virion-PF4 complexes, or the complexes are presented by platelets. (B) From right to left. After clonal expansion and isotype switching of one or a few B-cell clones, high titer IgG anti-PF4 antibodies are released into the circulation.52,53 Immune complexes containing PF4 and anti-PF4 IgG cluster and signal through FcRγIIA,50,54 which generates procoagulant platelets, induces platelet/neutrophil aggregates,55 and stimulates NETosis by neutrophils.50,54,56 DNA released by NETosis amplifies immune injury and activates complement, which deposits on the endothelium.57,58 Endothelial cells become activated, expressing tissue factor and releasing von Willebrand factor (VWF). VWF binds PF4 and subsequently anti-PF4 antibodies, which in turn further activates neutrophils and further propagates thrombin generation.59 Professional illustration by Patrick Lane, ScEYEnce Studios.
Current concept of the pathogenesis of VITT. The schematic presentation shown in A is speculative and in large part inferred from experiments in HIT. The schematic presentation of the downstream prothrombotic process shown in Panel B is largely substantiated by experimental data, some performed with VITT antibodies, others with HIT antibodies. Modified from Greinacher et al.45 (A) After vaccination, PF4 comes in contact with vaccine constituents and activates B-cells. Left side: It has been proposed that a direct inadvertent breach in the microvasculature at the vaccination site by IV injection or by disruption of vascular endothelial-cadherin tight junctions by EDTA in ChAdOx1, allows vaccine constituents to enter the circulation.45 Within the circulation, adenovirus particles can bind to platelets and can also bind PF4 released by activated platelets or from the matrix coating the microvascular endothelium.46,47 Platelets may become activated by vessel injury caused by injection of vaccine, after binding of the virions to the cell surface, or by immune complexes formed between contaminating host cell-line proteins in the vaccine and natural IgG antibodies against these proteins. Whether the virions themselves or another yet unknown constituent in the vaccine causes a conformational change in PF4 is unknown. Middle: once complexes with PF4 have formed, natural IgM antibodies activate complement (as it has been shown for PF4/heparin complexes),48 which enhances their proximity to B-cell receptors. In a mouse model, IV injection of ChAdOx-1, platelet-bound adenoviral particles are transported to the marginal zone of the spleen where B-cells are activated upon direct contact.49 However, electron microscopy and super resolution microscopy revealed complexes between PF4 and anti-PF4 VITT antibodies with amorphous constituents of the vaccine rather than virus particles.50 Beside the virions, other potential partners for PF4 include unassembled hexons51 and host cell-line proteins, although there is little overlap in the proteins contaminating ChAdOx1 and Ad26.COV2 vaccines.51 Right side: eventually, complexes of PF4 and vaccine (constituents) come in contact with B-cells expressing a cognate Ig receptors for PF4, either as fluid phase complexes, as virion-PF4 complexes, or the complexes are presented by platelets. (B) From right to left. After clonal expansion and isotype switching of one or a few B-cell clones, high titer IgG anti-PF4 antibodies are released into the circulation.52,53 Immune complexes containing PF4 and anti-PF4 IgG cluster and signal through FcRγIIA,50,54 which generates procoagulant platelets, induces platelet/neutrophil aggregates,55 and stimulates NETosis by neutrophils.50,54,56 DNA released by NETosis amplifies immune injury and activates complement, which deposits on the endothelium.57,58 Endothelial cells become activated, expressing tissue factor and releasing von Willebrand factor (VWF). VWF binds PF4 and subsequently anti-PF4 antibodies, which in turn further activates neutrophils and further propagates thrombin generation.59 Professional illustration by Patrick Lane, ScEYEnce Studios.
It is less clear how the anti-PF4 response is initiated. VITT appears to be a class effect of adenovirus vector-based vaccines. The few cases of platelet activating PF4-dependent antibodies attributed to mRNA vaccines probably reflect the background rate of spontaneous or autoimmune HIT.34 However, which constituent(s) in the vaccines might cause(s) conformational changes in PF4 through electrostatic interactions to trigger the immune response46,50,51 is unresolved. The observation of a VITT-like syndrome after human-papilloma virus 60 vaccination suggests a range of factors may trigger conformational changes in PF4, similar to the HIT antigen that can be induced by diverse polyanions.61Figure 1A summarizes some of the current hypotheses on the generation of VITT antibodies.
Anti-PF4 antibodies in VITT do not cross-react with spike protein,62,63 nor does COVID-19 infection increase anti-PF4 antibody titers,64 although high titer anti-PF4 antibodies have also been observed in patients with COVID-19.65 The development of high avidity isotype-switched IgG antibodies by day 5 postvaccination, also seen in HIT, suggests activation of preexisting PF4-reactive marginal zone memory B-cells in the spleen and elsewhere posited to be involved in the innate immune response to microbial infection50,66 in the setting of inflammatory costimulatory signals.50,56
At least some VITT antibodies are oligoclonal52 (in contrast to HIT antibodies) and the reported skewing in the expression of the genes that encode the hypervariable region of the immunoglobulin light chain53 might point to a genetic predisposition to respond to vaccination by generating anti-PF4 antibodies. Few individuals have preexisting antibodies against ChAdOx-1 and or Ad26 COV2.S before vaccination.67,68 Neutralizing antibodies to adenoviral proteins that develop after the initial vaccination may thereafter limit exposure to viral proteins entering the circulation or inhibit formation of viral protein-PF4 complexes, reducing the risk of developing VITT after subsequent vaccinations. VITT-like antibodies are not restricted to COVID-19 vaccination. In one patient with monoclonal gammopathy and recurrent venous and arterial thromboses, PF4-dependent platelet activating antibodies binding to the same binding site on PF4 as VITT antibodies were identified.34 The frequency of such VITT-like antibodies in patients with recurrent thrombosis is currently unresolved as is the predilection for thrombosis involving the cerebral and splanchnic-portal vasculatures.69
Treatment
Treatment should be initiated with IVIG (1 mg/kg IV × 2 days),70 which inhibits FcRγIIA-mediated platelet activation70-72 and a nonheparin anticoagulant (direct thrombin inhibitors, oral anti-Xa agents, fondaparinux). Although >95% of patients with VITT develop thrombotic complications, in a few patients, clinical symptoms, especially headache, can precede thrombosis, so-called pre-VITT. In case of strong clinical suspicion, treatment with IVIG and therapeutic dose anticoagulation should be started immediately, even in the absence of overt thrombosis. Heparin appears to be safe and effective in most patients and may be the only anticoagulant available in some jurisdictions.4,9,73 However, nonheparin anticoagulants are preferred as concurrent heparin-dependent antibodies might be present in a small subset of patients. Vitamin K antagonists should be avoided during the acute phase of VITT, as the induced protein C deficiency can worsen microvascular thrombosis in severe prothrombotic disorders.74 In patients with extensive CVST, anticoagulation after IVIG is generally indicated,75 but timing and intensity depends on severity of thrombocytopenia and presence of extensive intracerebral hemorrhage. Prophylactic platelet transfusions are discouraged; however, supplementation with fibrinogen17 and transfusion of platelets may be indicated for bleeding especially during surgical procedures.76 Patients with platelet counts <30 × 109/L and CVST may benefit from plasma exchange.17,19,77,78 The role of corticosteroids19 and inhibition of complement79,80 in nonresponsive or critically ill patients remains to be established. The potential of blocking FcRγIIA-mediated procoagulant pathways with inhibitors of the Bruton kinase pathway81 has not been tested clinically. Platelet inhibitors may have a role in long term secondary prophylaxis in patients with arterial thrombosis. However, in acute VITT, inhibition of thrombin generation is more important and dual anticoagulation may enhance the bleeding risk.
The platelet count and D-dimer levels should be used to guide duration of therapeutic dose anticoagulation rather than the results of anti-PF4 antibody tests. If platelet counts decrease and D-dimer levels increase after stopping anticoagulation, treatment should be resumed as recurrences of thrombocytopenia/thrombosis requiring retreatment with IVIG have been reported.17,19,35,71 The optimal duration of thromboprophylaxis is uncertain, but generally guided by the location and severity of the initial thrombotic complication(s).17 Revaccination using mRNA-based vaccines is safe and has not led to a recrudescence of platelet activating anti-PF4 antibodies.64,82
Conclusions, unknowns and future directions
Fortunately, VITT is very rare. However, it can be life-threatening, especially if the diagnosis and treatment are delayed. Adenovirus vectors provide an affordable framework for highly effective vaccines. Unravelling the mechanisms of the anti-PF4 response in VITT has the potential to provide the basis for a more rational approach to developing safer vaccine delivery systems. The finding of VITT-like antibodies in patients with recurrent thrombosis raises the possibility that platelet-activating anti-PF4 antibodies might contribute to as yet unexplained recurrent venous and arterial thrombotic disorders. This question can be addressed once assays that distinguish HIT-like and VITT-like anti-PF4 antibodies are developed.83
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL151730 and HL142122 (D.B.C.).
Authorship
Contribution: D.B.C. and A.G. contributed equally to this work, from conceptualization through the development of the manuscript.
Conflict-of-interest disclosure: A.G.'s employer, Universitätsmedizin Greifswald, holds a patent for a laboratory assay detecting VITT-like anti-PF4 antibodies. D.B.C. declares no competing financial interests.
Correspondence: Douglas B. Cines, Department of Pathology and Laboratory Medicine, 513 Stellar-Chance, 422 Curie Blvd, Philadelphia, PA 19104; e-mail: dcines@pennmedicine.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal