In this issue of Blood, Malec et al1 report final results of the verITI-8 study, where immune tolerance induction (ITI) using recombinant factor VIII (FVIII) Fc fusion protein was studied prospectively.
Inhibitor development is today the main challenge to hemophilia treatment. Although reported rates vary, around 30% of patients with severe hemophilia A develop inhibitor antibodies that react with infused FVIII used for replacement therapy. If the inhibitor is present in high titer, it jeopardizes otherwise successful treatment.2,3 The main goal of ITI therapy is to eradicate the inhibitor and, thus, allow the patient to return to ordinary replacement therapy. The basic approach used by ITI is to give massive doses of FVIII, often daily, for months or years. The first successful result was reported by Brackmann and Gormsen in 1977.4 The method was demanding, time-consuming, and excessively expensive. Other protocols have since been developed, such as the van Creveld and Malmö protocols, which use lower doses of FVIII or add immune-suppressive drugs, respectively, to shorten the time and lower cost until tolerance is achieved.5,6 Nonreplacement therapy, to either bypass or mimic FVIII, has shown encouraging results. Most notably, emicizumab has been shown to effectively prevent bleeding.3 Still, inhibitor eradication is the primary goal for many caring for these patients. Inhibitor tolerance induction studies are difficult to perform because of the small patient population and the complexity of inhibitor development. To date, the only reported randomized, prospective ITI trial is the International ITI study.7 In this study, the success rate was 70%, and time to achieve tolerance was a median of almost 2 years. Roughly similar figures have been reported from other retrospective cohort and registry studies.
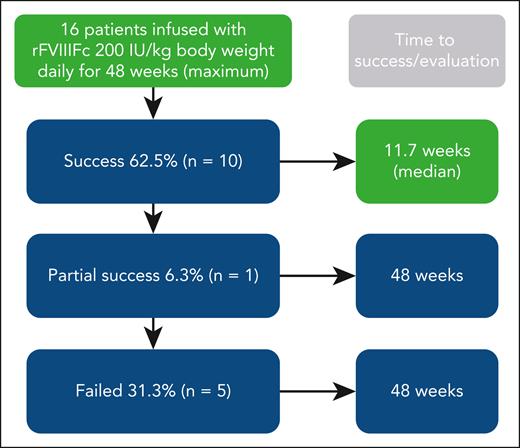
The rationale for the ver8-ITI study is based on clinical cases with rapid achievement of negative inhibitor titers using an extended half-life (EHL) recombinant factor VIII Fc fusion protein. The investigators hypothesized that this may partially be attributed to its immunomodulatory Fc portion. Thus, the verITI-8 trial used an EHL recombinant factor VIII Fc fusion protein (efmoroctocog alfa). The results given in the article (see figure) show rapid inhibitor eradication with a success rate in parity with several other studies. When interpreting the results, some common issues in ITI studies need to be explored: (1) Study material and study design: Remarkably, this is a prospective study that avoids common biases found in retrospective studies. The population is limited (n = 16); thus, only descriptive statistics were performed. F8 genotyping was performed, but it does not add much information in such a small study. There was no control group. ITI was given for 48 weeks, which is shorter than for some other studies and may reduce the true potential of success rate. Follow-up was for 32 weeks and may explain why no relapses were seen during this relatively short period; (2) Treatment: Similar to the International ITI study high-dose arm, 200 IU FVIII/kg was given once daily. This is a reasonable choice, although the science behind the dose is weak. Both higher and lower doses given for longer and shorter intervals have been reported in the literature; (3) Time to tolerization: The definition of tolerization was an internationally accepted definition with strict criteria for half-life of infused factor. The criteria behind this definition do not take pharmacokinetic parameters for an individual patient into account (as pharmacokinetics are not known before inhibitor development) and may lead to wrong classification of tolerance; and (4) Administration of bypassing agents was permitted during ITI to control acute bleeds. It is possible that this additional therapy impacted outcome of tolerance induction.8 This study was just for first-time immune tolerance induction, so previous tolerance attempts do not impact the success rate, which was found to be a favorable parameter in the study by Carlborg et al.8
Overall results of immune tolerance induction using recombinant factor VIII Fc fusion protein (rFVIIIFc) prospectively in 16 patients with severe hemophilia A and high-titer inhibitors (historical peak ≥5 Bethesda units per mL). Dosing was for up to 48 weeks. Patients achieving immune tolerance, according to negative inhibitor titer, in vivo recovery, and half-life, entered tapering and follow-up for ≥16 and 32 weeks, respectively. Boxes in green denote aspects specific for this study (ie, use of rFVIIIFc and short time to success). Treatment was well tolerated.
Overall results of immune tolerance induction using recombinant factor VIII Fc fusion protein (rFVIIIFc) prospectively in 16 patients with severe hemophilia A and high-titer inhibitors (historical peak ≥5 Bethesda units per mL). Dosing was for up to 48 weeks. Patients achieving immune tolerance, according to negative inhibitor titer, in vivo recovery, and half-life, entered tapering and follow-up for ≥16 and 32 weeks, respectively. Boxes in green denote aspects specific for this study (ie, use of rFVIIIFc and short time to success). Treatment was well tolerated.
The median time to tolerance for patients who achieved negative inhibitor titer (n = 12, 75%) was 7.4 weeks: 6.8 weeks for in vivo recovery ≥66% and 11.7 weeks for half-life of infused FVIII ≥7 hours. As pointed out by Malec et al, these are shorter times than observed in the International ITI study, although a direct comparison is not possible because of study differences. In a follow-up of 22 high-responding patients receiving the Bonn protocol, time to achieving normal pharmacokinetic parameters was around 15 months9; and, in a protocol including immunosuppressive drugs, it was as short as 2 to 3 weeks in 9 of 11 patients.5 It seems reasonable to believe that time to tolerization in the study by Malec et al is short for a protocol including only FVIII, especially considering the findings from the International-ITI study. This is the first prospective study using recombinant factor Fc fusion protein, so no trials are available for comparison.10
The verITI-8 study is important as it indicates favorable, but certainly not conclusive, results using an extended half-life product containing Fc fusion protein. As reported in the article, there are ongoing studies investigating ITI with the concomitant use of emicizumab. As therapy is rapidly evolving, future studies using nonreplacement therapy, such as gene or cellular therapy, are to be expected. The burden for the patient and society needs to be addressed, as inhibitors cause substantial cost and suffering. The search for successful and rapid ITI needs to continue.
Conflict-of-interest disclosure: E.B. has acted as a consultant for Bayer, CSL Behring, Novo Nordisk, and Takeda.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal