Key Points
verITI-8 is the first prospective study of first-time rFVIIIFc ITI for severe hemophilia A and high historical peak titers.
A rFVIIIFc offered rapid time to tolerization with durable responses in almost two-thirds of patients, no recurrence, and good tolerability.
Abstract
Inhibitor development remains a major challenge in factor VIII (FVIII) replacement therapy. verITI-8 is the first prospective study of a recombinant FVIII Fc fusion protein (rFVIIIFc; efmoroctocog alfa) for first-time immune tolerance induction (ITI) in males with severe hemophilia A and high-titer inhibitors (historical peak ≥5 Bethesda units [BU]/mL). In this single-arm, open-label, multicenter study, screening was followed by ITI (rFVIIIFc 200 IU/kg per day until tolerization or maximum of 48 weeks). Those who achieved ITI success entered a tapering period, returning to standard prophylaxis, and then entered follow-up. Primary end point was time to tolerization with rFVIIIFc defined by inhibitor titer <0.6 BU/mL, incremental recovery (IR) ≥66% of expected IR (IR ≥1.32 IU/dL per IU/kg), and half-life (t½) ≥7 hours within 48 weeks. Sixteen patients received ≥1 rFVIIIFc dose. Twelve (75%), 11 (69%), and 10 patients (63%), respectively, achieved negative inhibitor titers, an IR ≥66%, and a t½ ≥7 hours (ie, tolerance) within 48 weeks. Median times in weeks to achieve these markers of success were 7.4 (interquartile range [IQR], 2.2-17.8), 6.8 (IQR, 5.4-22.4), and 11.7 (IQR, 9.8-26.2), respectively. All patients experienced ≥1 treatment-emergent adverse event (TEAE), and 1 reported ≥1 related TEAE (injection site pain). Nine patients experienced ≥1 treatment-emergent serious AE. No thrombotic events, discontinuations because of AEs, or deaths were reported during the study. As the first extended half-life rFVIII with prospective data in ITI, rFVIIIFc offered short time to tolerization with durable responses in almost two-thirds of patients and was well tolerated. This trial was registered at www.clinicaltrials.gov as #NCT03093480.
Introduction
Prophylactic factor VIII (FVIII) replacement remains the standard treatment for severe hemophilia A (<1 IU/dL [1%] endogenous FVIII activity) to prevent bleeds, maintain musculoskeletal health, and promote quality of life.1 However, development of inhibitors (immunoglobulin G alloantibodies to exogenous FVIII that neutralize the clotting factor’s function, compromising treatment efficacy) continues to be a major challenge, resulting in increased hemophilia-related morbidities, including pain and physical limitations.1-4
Immune tolerance induction (ITI) is the standard of care for inhibitor eradication in patients with high-titer inhibitors (>5 Bethesda units [BU]/mL).2 ITI involves frequent exposure to FVIII over an extended period, typically from a minimum of 9 months to 2 or more years.4 Intensive infusion schedules impose a high patient and caregiver burden, although, ultimately, successful ITI restores FVIII pharmacokinetic (PK) and sensitivity to FVIII treatment, allowing regular prophylactic therapy to be resumed.1,5,6 However, ITI success rates are variable, and no clear consensus exists regarding the optimal dose or the most appropriate product.1,3-5,7,8
Data from the prospective International ITI (I-ITI) study, which focused on patients with a favorable risk profile, compared high-dose (HD) (200 IU/kg/day) and low-dose (LD) (50 IU/kg, 3 times per week) standard half-life (t½) FVIII ITI treatment regimens.7 The overall success rate was 70% (∼40% in the intent-to-treat population), which was similar for HD and LD regimens.7 Tolerization was achieved more quickly and bleed rates were lower in those patients on HD vs LD.7 However, even in the HD group, median time to tolerance (from start of ITI, progressing through negative titer, first normal recovery, and finally, tolerance) was almost 2 years in the 66 of 115 patients who reached the defined study end points.7
Reports of clinical cases employing an extended half-life (EHL) recombinant FVIII Fc fusion protein (efmoroctocog alfa, referred to in this article as rFVIIIFc; Eloctate/Elocta, Sanofi, Cambridge, MA, and Sobi, Stockholm, Sweden) for ITI suggest rapid time to negative inhibitor titer, and it has been hypothesized that this may be partially attributed to its immunomodulatory Fc portion.2,9,10 For example, 1 series included 2 first-time ITI patients who achieved negative inhibitor titer at 11 and 12 weeks at initial rFVIIIFc ITI doses of 100 IU/kg every other day and 200 IU/kg every other day, respectively.9 A further case study achieved a titer of 0.7 BU/mL over the course of 10 months with an rFVIIIFc regimen of 50 IU/kg 3 times weekly, after a brief trial of another product.10 A retrospective chart review including 10 first-time ITI patients suggested that rapid tolerization and an 80% success rate could be achieved with rFVIIIFc ITI.3 In that chart review, dosing regimens for rFVIIIFc ITI were median dose of 100 IU/kg (range, 50-200 IU/kg) and median weekly dose of 700 IU/kg (range, 150-1400 IU/kg).3 The median time to achieve tolerization was 30 weeks (range, 3-99 weeks).3 Furthermore, a recent interim assessment of a retrospective chart review showed that use of rFVIIIFc ITI in first-time ITI was successful in nearly 50% (10/22) of patients; the remaining 10 patients were still receiving ITI at the time of cutoff for the interim analysis.11 None of these reports highlighted any additional safety signals associated with using rFVIIIFc in a high-intensity regimen.2,3,9,10
Although case reports and chart reviews provide an indication of the potential benefit of rFVIIIFc, they are limited by small patient numbers, their retrospective nature, heterogeneous treatment regimens (including dose and frequency), and differing response criteria used for ITI. Against this background, 2 phase 4 studies were designed to prospectively evaluate ITI outcomes with rFVIIIFc. ReITIrate (www.clinicaltrials.gov, #NCT03103542) evaluated rFVIIIFc-based rescue ITI (ie, for those who had previously failed at least 1 ITI attempt).12 The present work reports findings from verITI-8 (#NCT03093480), the first prospective study of rFVIIIFc in first-time ITI in patients with severe hemophilia A and inhibitors.
Methods
Study design and participants
verITI-8 (#NCT03093480) was a phase 4, open-label, single-arm, interventional study conducted across Europe, United States, and Canada (supplemental Table 1, available on the Blood website). The protocol was approved by institutional review boards and/or ethics committees at participating institutions. Written informed consent was obtained from patients or parents/legal guardians. verITI-8 was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.13
The primary objective of the study was to describe the time to tolerization (ITI success) in patients within a maximum of 48 weeks of ITI treatment with rFVIIIFc. Secondary objectives included describing the outcome of ITI treatment, the relapse rate over the 48-week period following successful ITI performed with rFVIIIFc, bleed rates during the ITI period and during the 48-week period after successful ITI performed with rFVIIIFc, and finally the safety and tolerability of rFVIIIFc when used for ITI.
Enrolled patients were male, of any age, with severe hemophilia A and high-titer inhibitors (historical peak ≥5 BU/mL based on medical records). Patients had to have received prior treatment with any plasma-derived or recombinant conventional or EHL FVIII. Key exclusion criteria included the presence of other coagulation disorder(s) in addition to hemophilia A or receipt of previous ITI therapy. Concomitant immunomodulation or emicizumab were not permitted. Additional eligibility criteria are listed in the supplemental Methods.
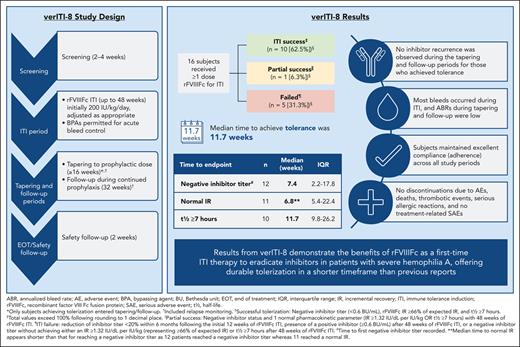
verITI-8 consisted of consecutive study periods: screening, ITI, tapering, follow-up, and safety follow-up (Figure 1). The initial screening period lasted up to 4 weeks and was followed by an ITI period, which had a maximum duration of 48 weeks. During the ITI period, rFVIIIFc was administered at a starting dose of 200 IU/kg per day. In the event of FVIII activity levels rising above 200 IU/dL, after reaching a negative inhibitor titer but before complete ITI success, the dose was to be adjusted on an individual basis according to investigator judgment to maintain peak FVIII activity levels between 100 and 200 IU/dL. Administration of bypassing agents was permitted during ITI to control acute bleeds.
verITI-8 study design. ∗Indicates only patients who achieved tolerization entered the tapering and follow-up periods; † indicates patients with bleeding episodes during the tapering and follow-up periods were tested for recurrence of inhibitors and monitored for relapse. In the event of relapse, patients were to proceed to the end of treatment visit.
verITI-8 study design. ∗Indicates only patients who achieved tolerization entered the tapering and follow-up periods; † indicates patients with bleeding episodes during the tapering and follow-up periods were tested for recurrence of inhibitors and monitored for relapse. In the event of relapse, patients were to proceed to the end of treatment visit.
ITI success was defined as achieving an inhibitor titer <0.6 BU/mL and rFVIIIFc incremental recovery (IR) ≥1.32 IU/dL per IU/kg (representing ≥66% of the expected IR 2 IU/dL per IU/kg); each criterion had to be met at 2 consecutive visits taking place 2 weeks (±3 days) apart, in addition to a reported t½ ≥7 hours. Partial success was also recorded in patients completing 48 weeks of ITI and was defined as achieving negative inhibitor titer and 1 of the PK parameters of ITI success (IR ≥1.32 IU/dL per IU/kg or t½ ≥7 hours). Treatment failure was defined as fulfilling one of the following criteria: no downward trend of ≥20% in inhibitor titer in a 24-week period after the initial 12 weeks of the ITI period, presence of a sustained positive inhibitor (≥0.6 BU/mL) after 48 weeks of ITI treatment, or negative inhibitor titer without achieving either of the 2 PK parameters.
Only patients who achieved ITI success within the maximum 48-week timeframe entered tapering and follow-up periods lasting ≥16 weeks and 32 weeks, respectively, followed by a final safety follow-up visit or call within a further 2 weeks. During tapering, doses were adjusted to achieve effective prophylaxis.
Patients with successful ITI were monitored during the tapering and follow-up periods for the occurrence of relapse. Relapse was defined as positive inhibitor titer (≥0.6 BU/mL) and IR <1.32 IU/dL (representing <66% of the expected IR), each reported on 2 consecutive assessments performed within 2 to 4 weeks, and a t½ <7 hours. In the event of relapse, patients were to proceed to the end of treatment visit.
End points
The primary end point of the verITI-8 study was time to tolerization (ITI success) with rFVIIIFc ITI within a maximum of 48 weeks.
Secondary end points included the number of participants achieving ITI success. Additional secondary end points included the number of bleeding episodes during the ITI period and during the 48-week period after successful ITI with rFVIIIFc, adverse events (AEs) and serious AEs, and rFVIIIFc consumption.
Data collection and analysis
Samples for inhibitor testing, IR, and t½, were analyzed at the local laboratory as well as centrally. A washout period of at least 24 hours was required before assessments for IR and t½. Analysis of ITI outcomes was based on the investigator’s assessment of local laboratory data unless results could not be interpreted, in which case central laboratory results were used.
Statistical analysis
No formal sample size calculation was performed based on the limited population. All analyses describing efficacy and safety during ITI treatment were based on the ITI full analysis set including all participants receiving ≥1 infusion of rFVIIIFc. The tapering period and follow-up period full analysis sets included all patients entering the tapering and follow-up phases, respectively. All analyses describing efficacy and safety during the tapering or follow-up periods used these analysis sets.
Because of the sample size, descriptive statistics were performed rather than inferential hypothesis testing.
Results
Patient characteristics
A total of 16 patients with a median age of 2.1 years (range, 0.8-16) were enrolled into and completed the study. Baseline characteristics and demographics demonstrated presence of risk factors for poor ITI outcome in many patients, including extended time from inhibitor diagnosis to ITI treatment, high historical peak titers, and high-risk mutations (Table 1).5,7,14 The median time from inhibitor diagnosis to the start of rFVIIIFc ITI was 23.1 weeks (range, 3.8-744.9), with 1 patient having a 14-year gap from diagnosis to start of ITI. Median historical peak inhibitor titer (pre-ITI) was 22.4 BU/mL (range, 6.2-256); 2 patients had peak titers >200 BU/mL. F8 genotyping showed that over half (n = 10) of the cohort had a large structural change involving >50 base pairs, most of which were intron 22 inversions. Medical and surgical histories of enrolled patients were characteristic of individuals with severe hemophilia A. The most common surgery among patients before the study was central venous catheterization placement (n = 11, 69%).
Baseline demographics and characteristics of patients enrolled in verITI-8 (ITI full analysis set)
| Characteristic . | Enrolled patients (N = 16) . |
|---|---|
| Median (range) age at informed consent, y | 2.1 (0.8-16) |
| Median (range) time from inhibitor to start of rFVIIIFc ITI, wk | 23.1 (3.8-744.9∗) |
| Median (range) historical peak inhibitor titer (pre-ITI), BU/mL | 22.4 (6.2-256) |
| Median (range) inhibitor titer at rFVIIIFc ITI start, BU/mL | 9.7 (0.7-144) |
| No. of FVIII exposure d at first confirmed inhibitor development, n (%) | |
| <25 | 8 (50.0) |
| 25-50 | 5 (31.3) |
| 51-100 | 2 (12.5) |
| >100 | 1 (6.3) |
| Family history of inhibitor, n (%) | |
| Yes | 3 (18.8) |
| No | 13 (81.3) |
| Race, n (%) | |
| White | 12 (75.0) |
| Black or African American | 2 (12.5) |
| Other | 2 (12.5) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 2 (12.5) |
| Not Hispanic or Latino | 14 (87.5) |
| F8 genotype, n (%) | |
| Frameshift | 2 (12.5) |
| Large structural change (>50 base pairs) | 10 (62.5) |
| Intron 22 inversion | 7 (43.8) |
| Intron 1 inversion | 1 (6.3) |
| Deletion | 2 (12.5) |
| Small structural change (in-frame, <50 base pairs) | 1 (6.3) |
| Nonsense | 2 (12.5) |
| Missing genotype data | 1 (6.3) |
| Characteristic . | Enrolled patients (N = 16) . |
|---|---|
| Median (range) age at informed consent, y | 2.1 (0.8-16) |
| Median (range) time from inhibitor to start of rFVIIIFc ITI, wk | 23.1 (3.8-744.9∗) |
| Median (range) historical peak inhibitor titer (pre-ITI), BU/mL | 22.4 (6.2-256) |
| Median (range) inhibitor titer at rFVIIIFc ITI start, BU/mL | 9.7 (0.7-144) |
| No. of FVIII exposure d at first confirmed inhibitor development, n (%) | |
| <25 | 8 (50.0) |
| 25-50 | 5 (31.3) |
| 51-100 | 2 (12.5) |
| >100 | 1 (6.3) |
| Family history of inhibitor, n (%) | |
| Yes | 3 (18.8) |
| No | 13 (81.3) |
| Race, n (%) | |
| White | 12 (75.0) |
| Black or African American | 2 (12.5) |
| Other | 2 (12.5) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 2 (12.5) |
| Not Hispanic or Latino | 14 (87.5) |
| F8 genotype, n (%) | |
| Frameshift | 2 (12.5) |
| Large structural change (>50 base pairs) | 10 (62.5) |
| Intron 22 inversion | 7 (43.8) |
| Intron 1 inversion | 1 (6.3) |
| Deletion | 2 (12.5) |
| Small structural change (in-frame, <50 base pairs) | 1 (6.3) |
| Nonsense | 2 (12.5) |
| Missing genotype data | 1 (6.3) |
14.3 years.
Time to tolerization and ITI success
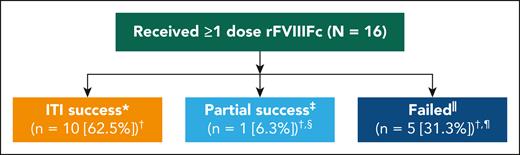
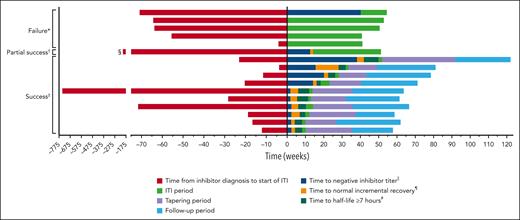
Twelve patients (75%) achieved a negative inhibitor, of which 11 (69%) achieved an IR of ≥66% of expected, and 10 patients (63%) a t½ ≥7 hours within 48 weeks. Median times in weeks to achieve these markers of success were 7.4 (interquartile range [IQR], 2.2-17.8), 6.8 (IQR, 5.4-22.4), and 11.7 (IQR, 9.8-26.2), respectively, meaning patients achieved tolerance in a median of 11.7 weeks (Table 2). All patients who achieved a normal recovery had first achieved a negative Bethesda titer, and those patients who achieved t½ ≥7 hours had to first achieve a negative titer and IR ≥66% (supplemental Table 2). Complete success was achieved by 10 patients (63%), 1 patient (6%) achieved partial success (negative inhibitor titer and IR ≥66% of expected), and 5 patients (31%) failed ITI (Figure 2). The patient who achieved partial success had a negative inhibitor titer and normal IR. Of those who failed, 2 (13%) had a reduction in titer from weeks 12 to 36 of <20%, 2 (13%) completed 48 weeks of rFVIIIFc ITI and had positive inhibitor, and 1 (6%) had completed 48 weeks of ITI and had a negative inhibitor titer but did not reach normal IR or t½. The Kaplan-Meier estimate of ITI success rate was 67.2%, with an estimated median time to tolerization of 29 weeks (95% confidence interval, 10.3 to not estimable) (supplemental Figure 1). No relapses were reported during the tapering or follow-up periods (during which time rFVIIIFc was tapered to a prophylactic dose, supplemental Table 3) in patients who achieved ITI success. The time course of milestone achievement in individual patients is represented graphically in Figure 3. The detailed information regarding ITI for individual patients is included in supplemental Table 2.
Time to achieve ITI success end points from start of ITI (ITI full analysis set)
| Time to end point . | n . | Median (wk) . | IQR . |
|---|---|---|---|
| Negative inhibitor titer∗ | 12 | 7.4 | 2.2-17.8 |
| Normal IR | 11 | 6.8 | 5.4-22.4 |
| t½ ≥ 7 h | 10 | 11.7 | 9.8-26.2 |
| Time to end point . | n . | Median (wk) . | IQR . |
|---|---|---|---|
| Negative inhibitor titer∗ | 12 | 7.4 | 2.2-17.8 |
| Normal IR | 11 | 6.8 | 5.4-22.4 |
| t½ ≥ 7 h | 10 | 11.7 | 9.8-26.2 |
Defined as time to first negative inhibitor titer recorded.
Success rates among patients undergoing rFVIIIFc ITI. ∗Successful tolerization was defined as attaining negative inhibitor titer (<0.6 BU/mL), rFVIIIFc IR ≥66% of expected IR, and t1/2 ≥7 hours; †total values exceed 100% following rounding; ‡partial success was defined as receiving 48 weeks of ITI treatment and achieving a negative inhibitor status and 1 normal PK parameter (IR ≥1.32 IU/dL per IU/kg OR t1/2 ≥7 hours); §patient achieved a negative inhibitor titer and normal IR; ||patients were considered to have failed ITI if there was reduction of inhibitor titer <20% within a 6-month period following the initial 12 weeks of rFVIIIFc ITI, presence of a positive inhibitor (≥0.6 BU/mL) after 48 weeks of ITI with rFVIIIFc, or a negative-inhibitor titer without achieving either an IR ≥1.32 IU/dL per IU/kg (representing ≥66% of expected IR) or t1/2 ≥7 hours after 48 weeks of rFVIIIFc ITI; and ¶2 (12.5%) had a titer change of <20% from weeks 12 to 36, 2 (12.5%) had completed 48 weeks of ITI and maintained a positive inhibitor titer, and 1 (6.3%) completed 48 weeks of ITI and achieved a negative inhibitor titer without attaining either normal PK parameter (IR or t1/2 ≥7 hours).
Success rates among patients undergoing rFVIIIFc ITI. ∗Successful tolerization was defined as attaining negative inhibitor titer (<0.6 BU/mL), rFVIIIFc IR ≥66% of expected IR, and t1/2 ≥7 hours; †total values exceed 100% following rounding; ‡partial success was defined as receiving 48 weeks of ITI treatment and achieving a negative inhibitor status and 1 normal PK parameter (IR ≥1.32 IU/dL per IU/kg OR t1/2 ≥7 hours); §patient achieved a negative inhibitor titer and normal IR; ||patients were considered to have failed ITI if there was reduction of inhibitor titer <20% within a 6-month period following the initial 12 weeks of rFVIIIFc ITI, presence of a positive inhibitor (≥0.6 BU/mL) after 48 weeks of ITI with rFVIIIFc, or a negative-inhibitor titer without achieving either an IR ≥1.32 IU/dL per IU/kg (representing ≥66% of expected IR) or t1/2 ≥7 hours after 48 weeks of rFVIIIFc ITI; and ¶2 (12.5%) had a titer change of <20% from weeks 12 to 36, 2 (12.5%) had completed 48 weeks of ITI and maintained a positive inhibitor titer, and 1 (6.3%) completed 48 weeks of ITI and achieved a negative inhibitor titer without attaining either normal PK parameter (IR or t1/2 ≥7 hours).
Swimmer plot of patients' time to criteria of success and time in each period during the study. ∗Patients were considered to have failed ITI if there was a reduction of inhibitor titer <20% within a 6-month period following the initial 12 weeks of rFVIIIFc ITI, presence of a positive inhibitor (≥0.6 BU/mL) after 48 weeks of ITI with rFVIIIFc, or a negative inhibitor titer without achieving either an IR ≥1.32 IU/dL per IU/kg (representing ≥66% of expected IR) or t1/2 ≥7 hours after 48 weeks of rFVIIIFc ITI; †partial success was defined as receiving 48 weeks of ITI treatment and achieving a negative inhibitor status and 1 normal PK parameter (IR ≥1.32 IU/dL per IU/kg or t1/2 ≥7 hours); ‡successful tolerization was defined as attaining negative inhibitor titer (<0.6 BU/mL), rFVIIIFc IR ≥66% of expected IR, and t1/2 ≥7 hours; §>202 weeks; ||time to negative inhibitor titer was the time interval (in weeks) from the start date of ITI treatment with rFVIIIFc to date of first negative inhibitor titer, which was subsequently confirmed by a sample from a consecutive visit; ¶time to FVIII normal recovery was the time interval (in weeks) from the date of ITI treatment with rFVIIIFc to date of first normal IR (≥66%), which was subsequently confirmed by a sample from a consecutive visit; and #time to FVIII t1/2 of ≥7 hours was the time interval (in weeks) from start date of ITI treatment with rFVIIIFc to date of t1/2 ≥7 hours.
Swimmer plot of patients' time to criteria of success and time in each period during the study. ∗Patients were considered to have failed ITI if there was a reduction of inhibitor titer <20% within a 6-month period following the initial 12 weeks of rFVIIIFc ITI, presence of a positive inhibitor (≥0.6 BU/mL) after 48 weeks of ITI with rFVIIIFc, or a negative inhibitor titer without achieving either an IR ≥1.32 IU/dL per IU/kg (representing ≥66% of expected IR) or t1/2 ≥7 hours after 48 weeks of rFVIIIFc ITI; †partial success was defined as receiving 48 weeks of ITI treatment and achieving a negative inhibitor status and 1 normal PK parameter (IR ≥1.32 IU/dL per IU/kg or t1/2 ≥7 hours); ‡successful tolerization was defined as attaining negative inhibitor titer (<0.6 BU/mL), rFVIIIFc IR ≥66% of expected IR, and t1/2 ≥7 hours; §>202 weeks; ||time to negative inhibitor titer was the time interval (in weeks) from the start date of ITI treatment with rFVIIIFc to date of first negative inhibitor titer, which was subsequently confirmed by a sample from a consecutive visit; ¶time to FVIII normal recovery was the time interval (in weeks) from the date of ITI treatment with rFVIIIFc to date of first normal IR (≥66%), which was subsequently confirmed by a sample from a consecutive visit; and #time to FVIII t1/2 of ≥7 hours was the time interval (in weeks) from start date of ITI treatment with rFVIIIFc to date of t1/2 ≥7 hours.
Bleeds
In an analysis including patients with ≥90 days of data within a study period, most bleeds occurred in the ITI period when median annualized bleed rates (n = 13) were 3.8 (IQR, 0-10.1) overall. Corresponding annualized bleed rates for tapering and follow-up in patients with successful ITI were low (Table 3). No bleeds occurred in 7 patients during ITI, and in patients in whom bleeds occurred, the counts suggested a trend toward reduced bleeds from months 1 to 12. Bleeding episodes during ITI are presented by type (overall, spontaneous, trauma, and unknown) (supplemental Figure 2) and by location (overall, joint, muscle, and other) (supplemental Figure 3).
Summary of annualized bleed rates during each period of the study
| Period∗,†,‡ . | n . | Overall,§ median (IQR) . | Spontaneous, median (IQR) . | Traumatic, median (IQR) . | Joint, median (IQR) . |
|---|---|---|---|---|---|
| During ITI‖ | 13 | 3.8 (0-10.1) | 0 (0-2.6) | 1 (0-4) | 0 (0-3.1) |
| During tapering¶ | 10 | 0 (0-2.4) | 0 (0-0) | 0 (0-1.3) | 0 (0-0) |
| During follow-up# | 10 | 0 (0-1.5) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| Period∗,†,‡ . | n . | Overall,§ median (IQR) . | Spontaneous, median (IQR) . | Traumatic, median (IQR) . | Joint, median (IQR) . |
|---|---|---|---|---|---|
| During ITI‖ | 13 | 3.8 (0-10.1) | 0 (0-2.6) | 1 (0-4) | 0 (0-3.1) |
| During tapering¶ | 10 | 0 (0-2.4) | 0 (0-0) | 0 (0-1.3) | 0 (0-0) |
| During follow-up# | 10 | 0 (0-1.5) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
Annualized bleed rate is defined as the number of bleeding episodes divided by length of the noted period in days × 365.25.
Excludes patients observed <90 days within the period.
Only patients who achieved tolerization entered the tapering and follow-up periods.
Overall includes those with 0 ABR.
There were 7 patients (44%) with no bleeds during the ITI period.
There were 6 patients (60%) with no bleeds during the tapering period.
There were 7 patients (70%) with no bleeds during the follow-up period.
rFVIIIFc consumption and bypassing agent use
Median annualized rFVIIIFc consumption was, as expected, highest during the ITI period (70 677 IU/kg; IQR, 59 996-72 466 IU/kg; n = 13). Lower values were reported for the tapering (22 342 IU/kg; IQR, 19 106-27 220 IU/kg; n = 10) and follow-up periods (9210 IU/kg; IQR, 8205-12 544 IU/kg; n = 10) (Table 4). Across the ITI, tapering, and follow-up periods, patients were able to maintain excellent adherence (supplemental Table 4). Half of all participants (n = 8) used bypassing agents during the ITI period to treat bleeding events. Four of 16 patients (25%) received activated prothrombin complex concentrate, and 5 (31%) received rFVIIa within the ITI period, during which bypassing agent use was permitted to treat active bleeds (Table 4). Individual listings of bypassing agent use during the ITI period are presented in supplemental Table 5.
rFVIIIFc consumption on study and bypassing agent use during the ITI period
| . | Total∗, † (N = 16) . | ITI period∗ (N = 16) . | Tapering period‡ (N = 10) . | Follow-up period§ (N = 10) . |
|---|---|---|---|---|
| Annualized rFVIIIFc consumption‖,¶,#n | ||||
| 16 | 13 | 10 | 10 | |
| Median (IQR), IU/kg per y | 36 717 (25 646-62 841) | 70 677 (59 996-72 466) | 22 342 (19 106-27 220) | 9 210 (8 205-12 544) |
| Patients receiving bypassing agents,∗∗n (%) | ||||
| aPCC | 4 (25.0) | 4 (25.0) | 0 | 0 |
| rFVIIa | 5 (31.3) | 5 (31.3) | 0 | 0 |
| Median (IQR) annualized bypassing agent consumption¶,†† | ||||
| aPCC, U/kg per y | 4 866 (211-26 437) | 9 463 (154-43 411) | N/A | N/A |
| rFVIIa, μg/kg per y | 2 345 (229-23 642) | 5 691 (714-23 642) | N/A | N/A |
| . | Total∗, † (N = 16) . | ITI period∗ (N = 16) . | Tapering period‡ (N = 10) . | Follow-up period§ (N = 10) . |
|---|---|---|---|---|
| Annualized rFVIIIFc consumption‖,¶,#n | ||||
| 16 | 13 | 10 | 10 | |
| Median (IQR), IU/kg per y | 36 717 (25 646-62 841) | 70 677 (59 996-72 466) | 22 342 (19 106-27 220) | 9 210 (8 205-12 544) |
| Patients receiving bypassing agents,∗∗n (%) | ||||
| aPCC | 4 (25.0) | 4 (25.0) | 0 | 0 |
| rFVIIa | 5 (31.3) | 5 (31.3) | 0 | 0 |
| Median (IQR) annualized bypassing agent consumption¶,†† | ||||
| aPCC, U/kg per y | 4 866 (211-26 437) | 9 463 (154-43 411) | N/A | N/A |
| rFVIIa, μg/kg per y | 2 345 (229-23 642) | 5 691 (714-23 642) | N/A | N/A |
aPCC, activated prothrombin complex concentrate; N/A, not applicable.
Total study and ITI period based on the ITI full analysis set.
The total column includes a patient’s entire study period for annualized consumption calculations.
Tapering period is based on the tapering period full analysis set.
Follow-up period is based on the follow-up period full analysis set.
Annualized rFVIIIFc consumption for a treatment period is the (total nominal rFVIIIFc [IU/kg] per length of period in days) × 365.25.
Annualized analysis excludes patients who were observed for <90 days in the period.
Surgical-related consumption was not included.
Bypassing agents were used only during the ITI period.
Annualized bypassing agent consumption is the (total consumption/length of period in days) × 365.25.
Safety
A total of 220 treatment-emergent AEs (TEAEs) occurred in 16 patients. All participants experienced ≥1 TEAE. The most frequently reported TEAEs (incidence ≥15%) are reported in supplemental Table 6. All but 3 TEAEs were assessed as mild to moderate in severity; the 3 assessed as severe were vascular device infection, mouth injury, and arthropathy (all considered serious). Twenty-eight treatment-emergent serious AEs were reported in 9 patients (56%); none were considered related to rFVIIIFc ITI treatment by the investigator. The most common treatment-emergent serious AEs were vascular device infection in 4 of 16 (25%), contusion in 2 of 16 (13%), and hemarthrosis in 2 of 16 patients (13%) within the study. No thrombotic events or serious allergic reactions were reported. No AEs led to treatment discontinuation, and no deaths occurred. One mild nonserious TEAE of injection site pain was reported and assessed as related by the investigator.
Discussion
The present work describes findings from verITI-8, the first prospective study of rFVIIIFc in first-time ITI therapy. Although this study used strict success criteria, requiring all 3 end points to be met, rFVIIIFc had a notable success rate of 63% in patients with severe hemophilia A with high historical peak inhibitor titers and a rapid time to tolerization, with a median time to tolerance of 11.7 weeks. rFVIIIFc was well tolerated as ITI treatment. The timeframe for achieving tolerance was shorter than observed in the I-ITI study, where similar success rates were observed despite the I-ITI study only including patients with a favorable risk profile.7 In total, 70% achieved tolerance in I-ITI, although the success rate appears lower (40%) when considering success among the intent-to-treat population of the I-ITI study.7 In addition, 6 tolerized I-ITI study patients experienced a partial relapse, 2 of which were transient and 4 were permanent.7 Of the I-ITI study patients with partial relapse, 3 originated from the group that received HD therapy, and 3 patients had received LD therapy.7 Relapse in the I-ITI study was defined as inhibitor recurrence during the 12-month follow-up period on prophylaxis after tolerance was achieved, as evidenced by recurrent positive Bethesda titer or a decline in FVIII recovery or t½ below study limits.7 This contrasts with the absence of relapse observed in successfully tolerized patients in verITI-8. However, differences in study design make direct comparisons difficult. The duration of cutoff for ITI success (48 weeks) in verITI-8 was much shorter than in previous ITI study designs, and it is conceivable that the success rate could have been higher if the timeframe had been extended. Of note, 1 patient did achieve Bethesda negativity within the 48-week period but had not shown a normal recovery or t½ before study termination at week 48. It is possible that this patient could have subsequently achieved success with longer treatment.
The rationale for dose selection included maintaining consistency with current US ITI guidance in which supportive evidence for use of 200 IU/kg per day is strongest in patients <8 years with adequate venous access and favorable risk factors.15 The present dose regimen also aligns with that employed in the HD arm of the I-ITI study. Although the I-ITI study predated the introduction of EHL products and therefore used standard t½, it provided a historical comparator.7 The current dose is also consistent with but less intensive than the 300 IU/kg per day advocated by the Bonn protocol.16 LD ITI has been explored and is associated with higher bleed rates and longer time to tolerance than HD regimens, with cumulative median time from start of ITI through negative titer, first normal recovery, and tolerance extending for ∼3 years.7 HD ITI regimens, although more intensive, may be associated with greater patient acceptability based on their shorter duration. This in turn may be reflected in high adherence throughout the verITI-8 study when calculated relative to the prescribed daily dose of rFVIIIFc.
The t½ threshold of 7 hours exceeds the 6 hours recommended by World Federation of Hemophilia guidelines for standard FVIII clotting factor concentrates;1 however, using 7 hours reduces the burden in young children and has been considered the lower limit of normal in the pediatric population.15,17 However, updated guidelines from UK Haemophilia Centre Doctors Organization and pediatric working party on the management of FVIII inhibitors in congenital hemophilia recommend a threshold of the lower end of normal for the age of the child undergoing ITI for the particular EHL product used during ITI.18 In light of these recommendations, 7 hours may be an underestimate; however, for rFVIIIFc, because it was the first EHL FVIII at the time of verITI-8 study design, evidence suggested that use of 7-hour or greater t½ as an indicator of tolerance would be applicable.
When attempting to draw comparisons between verITI-8 and I-ITI study outcomes, it is important to note that participants in the I-ITI were described by the authors as a “good-risk cohort.”7 Some of the patients failing ITI in the verITI-8 study would not have been included in earlier studies, in particular 2 patients with a historical peak inhibitor titer >200 BU/mL, and notably the I-ITI study reported an inverse association between historical peak inhibitor titer and successful ITI, although this was not significant upon multivariate analysis.7 Regarding historical peak titer, all 10 patients who were successfully tolerized in verITI-8 had values ≤40 BU/mL. In comparison, the results from a recent retrospective chart review of rFVIIIFc ITI in first-time patients showed that 50% of rFVIIIFc first-time ITI patients who were successfully tolerized had historical peak inhibitor titer values ≤40 BU/mL.3 One patient with a historical peak of 1126 BU/mL was also successfully tolerized, with ITI starting within 1 week of inhibitor diagnosis.3
Most of the verITI-8 study population harbored high-risk mutations for inhibitor development. Furthermore, the time from inhibitor diagnosis to start of ITI was >6 months in half of the participants (although 1 patient with an interval of 744.9 weeks [∼14 years] achieved ITI success and another patient with an interval of >202 weeks achieved partial success). Retrospective evidence from 2 US treatment centers suggests that a long interval between inhibitor diagnosis and start of ITI can negatively influence the outcome of ITI by permitting the accumulation of long-lived plasma cells.5
In the context of patient and caregiver treatment burden associated with ITI, it is interesting to note the shorter time to tolerization observed in verITI-8 than in the I-ITI study. Times to achieve each sequential milestone toward tolerization in the I-ITI study were longer than the overall time to tolerization in verITI-8. Whereas in verITI-8, median time to tolerization was 11.7 weeks (IQR, 9.8-26.2), corresponding to 2.7 months (IQR, 2.3-6.0), and the I-ITI study median time to negative titer in the HD group was 4.6 months (IQR, 2.8-13.8) (n = 31), negative titer to normal recovery was 6.9 months (IQR, 3.5-12.0) (n = 23), and normal recovery to tolerance was 10.6 months (IQR, 6.3-20.5) (n = 22).7 With the advent of nonfactor therapies such as emicizumab, the burden associated with ITI has come into focus. Eradication of inhibitors is still considered an appropriate goal,17 and the use of emicizumab concurrently with ITI is being investigated (#NCT04303572,19,20 #NCT02196207, and #NCT04030052). Considering that emicizumab is licensed for bleed prevention in patients with hemophilia A with and without inhibitors,1 ITI treatment regimen durations similar to those employed in the I-ITI study are less likely to be favored. Indeed, it will be critical to select the best ITI regimen and corresponding factor product.17 Selecting a regimen that provides for a more rapid time to tolerization, such as that observed in the verITI-8 trial, could contribute to a reduction in ITI treatment burden. Prospective studies of first-time ITI using recombinant standard half-life or EHL FVIII apart from I-ITI are lacking, although ObsITI, a prospective observational study, reported a median time to success of 11.25 months using plasma-derived products.21 Registry and retrospective studies were not considered appropriate for comparison; however, the efficacy observed in achieving tolerance and favorable safety profiles in verITI-8 are consistent with published chart review data with rFVIIIFc ITI.2,3,11 No new safety concerns were identified, despite the intensity of the ITI treatment. Strengths of the study include the prospective design and broad inclusion criteria that increase generalizability of the findings. For example, limits were not imposed on potential risk factors such as time from inhibitor to start of rFVIIIFc, historical peak titer, or inhibitor titer at start of ITI. A major limitation of the study includes the small study population.
In conclusion, the final data from verITI-8 demonstrate benefit of rFVIIIFc as first-time ITI to eradicate inhibitors in patients with severe hemophilia A. Despite the limited number of participants enrolled in the study (N = 16), rFVIIIFc is the first EHL product with data available for this therapeutic application. As evaluated within a 48-week timeframe, rFVIIIFc offered a rapid time to tolerization in a shorter timeframe than previous reports,7 combined with durable responses in almost two-thirds of patients. Furthermore, recurrence of inhibitors was not observed after successful tolerization during the tapering and follow-up periods, lasting ≥16 and 32 weeks, respectively. Optimizing ITI to eradicate inhibitors remains a priority for people with severe hemophilia A and inhibitors.
Acknowledgments
The investigators, Sanofi, and Sobi thank the patients and their families and all investigators and their teams for participating in the verITI-8 study. Editorial assistance for the development of this manuscript was provided by Shelley Morris and Ashleigh Pulkoski-Gross, Fishawack Communications Ltd, part of Fishawack Health, and was funded by Sanofi and Sobi.
This study was funded by Sanofi (Cambridge, MA) and Swedish Orphan Biovitrum AB (Sobi; Stockholm, Sweden).
Authorship
Contribution: L.M., A.K.C.C., N.J., J.D., S.L., and M.C. contributed substantially to the design of the work and/or the preparation of the manuscript for publication; L.M., A.V.D., A.K.C.C., M.S., J.D., S.L., M.C., and F.P. contributed substantially to data acquisition; L.M., A.V.D., A.K.C.C., C.S., J.D., S.L., M.C., and F.P. contributed substantially to data analysis and interpretation of data; and all authors provided critical revision of the manuscript and had final approval of the manuscript for publication.
Conflict-of-interest disclosure: L.M. has presented in advisory boards and/or received consultancy fees or investigator-initiated grant funding from Bioverativ, a Sanofi company, Shire, and/or Bayer. A.V.D. has received honoraria for advisory board participation from Bayer, Pfizer, and Shire outside the submitted work. A.K.C.C. has participated in an advisory board for Bioverativ, a Sanofi company. M.S. has received honoraria for advisory board participation from Bayer, Sobi, and Roche outside the submitted work. N.J. is a former employee of Sanofi and a current employee of Takeda and may hold shares and/or stock options in the company. C.S. and J.D. are employees of Sanofi and may hold shares and/or stock options in the company. S.L. is an employee of Sobi and may hold shares and/or stock options in the company. M.C. has received research support and/or honoraria for speaking/participating in advisory boards from Bayer, CSL Behring, LFB, Novartis, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, and/or Takeda. F.P. has received personal fees from Ablynx, Grifols, Kedrion, Novo Nordisk, Roche, Shire, and Sobi outside the submitted work.
The current affiliation for N.J. is Takeda, Cambridge, MA.
Correspondence: Lynn Malec, Versiti Blood Research Institute, 8733 Watertown Plank Rd, Milwaukee, WI 53226-4874; e-mail: lmalec@versiti.org.
References
Author notes
Qualified researchers may request access to data and related documents (including, eg, the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Any patient-level data provided will be anonymized, and study documents will be redacted, including to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal