Abstract
Essential thrombocythemia (ET) was first described in 1934, and subsequently, progress has been made in better understanding the molecular pathogenesis and which patients may have greatest risk of progression or vascular events. However, it has been more than a decade since a new therapy has been approved for ET. We are beginning to understand more comprehensively both the heterogeneity of this disease, which is largely driven by driver mutation status, as well as the effect of disease-related symptoms, such as fatigue, on patients. In this review we provide a practical overview of diagnosis and management of ET with focus on challenging patient scenarios and some consideration of what comprehensive care might entail. Finally, we also discuss newer therapies and how these might be assessed.
Introduction
Essential thrombocythemia (ET), a classical Philadelphia-negative myeloproliferative neoplasm (MPN), was described by Epstein and Goedel.1 The annual incidence of ET is similar to that of polycythemia vera (PV) at ∼1.5 to 2.0 cases per 100 000 of the population.2,3 Recent data from cancer registries suggest that the incidence of ET may be increasing but, unfortunately, outcome in terms of survival has not improved.3 The median age at onset is 60 to 70 years, with a small second peak in women of reproductive age and, although it can occur at any age, it is rare in childhood. Commonly, ET presents as an asymptomatic chance finding in an otherwise well patient but can also be discovered in a patient with thrombotic or hemorrhagic events. Although diagnostic approaches and treatments have not substantially changed for this condition in the past decade, our understanding of both the heterogeneous nature of this disease and its effect on our patients has.
A separate review in this series covers mutations and pathogenesis of MPN in general, but it is increasingly clear that within the umbrella term ET there are, for the most part, different genomically defined entities with different disease trajectories as clearly illustrated by Grinfeld et al.4 Our medical interpretation of the effect of disease, its treatments, and risk of complications have been challenged by a series of studies focusing on the lived experience of patients with ET, the so-called "landmark studies.”5,6 Common themes included the effect of disease on family relationships, the ability to work, and quality of life with ET, in particular highlighting the issue of fatigue as a common impactful complication. The data from these studies support the re-evaluation of therapeutic approaches and models of care.
Diagnosis
After exclusion of reactive causes of thrombocytosis,7 and in the absence of other abnormalities that would suggest an alternative myeloid neoplasm (Table 1), patients with a persistent thrombocytosis greater than 450 × 109/L should be assessed for ET. Many patients are asymptomatic at diagnosis or, less commonly, present with headaches, other microvascular symptoms such as erythromelalgia, constitutional symptoms, or with vascular (thrombotic or hemorrhagic) complications. Splenomegaly, if present, is usually mild (<5 cm enlargement).
Causes of thrombocytosis
| Reactive |
| Iron deficiency |
| Blood loss |
| Surgery |
| Acute bacterial infection |
| Chronic inflammation (eg, vasculitis, rheumatoid arthritis, chronic infection, inflammatory bowel disease) |
| Nonmyeloid malignancies (eg, lung, colorectal) |
| Splenectomy/hyposplenism |
| Clonal myeloid disorders |
| MPNs |
| ET |
| PV |
| Primary myelofibrosis |
| Chronic myeloid leukemia |
| MPN, not otherwise specified |
| MDS/MPNs |
| MDS/MPN with SF3B1 mutation and thrombocytosis |
| MDS/MPN, not otherwise specified |
| Myelodysplastic neoplasms |
| MDS with low blasts and isolated 5q deletion |
| Reactive |
| Iron deficiency |
| Blood loss |
| Surgery |
| Acute bacterial infection |
| Chronic inflammation (eg, vasculitis, rheumatoid arthritis, chronic infection, inflammatory bowel disease) |
| Nonmyeloid malignancies (eg, lung, colorectal) |
| Splenectomy/hyposplenism |
| Clonal myeloid disorders |
| MPNs |
| ET |
| PV |
| Primary myelofibrosis |
| Chronic myeloid leukemia |
| MPN, not otherwise specified |
| MDS/MPNs |
| MDS/MPN with SF3B1 mutation and thrombocytosis |
| MDS/MPN, not otherwise specified |
| Myelodysplastic neoplasms |
| MDS with low blasts and isolated 5q deletion |
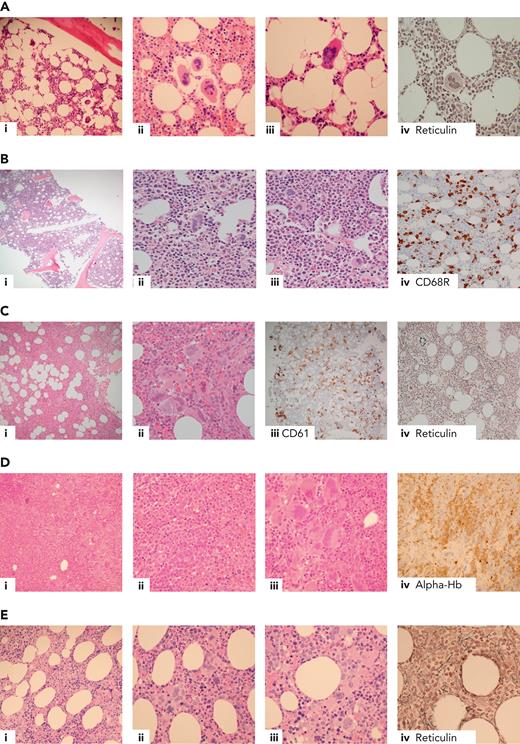
Current diagnostic criteria for ET, including recently revised criteria from the International Consensus Classification8 and the World Health Organization,9 are summarized in Table 2. Most (85%-90%) patients with ET have a mutation in either JAK2 (V617F, 50%-60%), CALR (25%-30%; just over half “type 1/1-like,” and the remainder “type 2/2-like”), or MPL (∼5%).10-15 In the remaining 10% to 15% lacking a canonical driver mutation (“triple negative”), histological diagnosis is mandatory (indeed this is the case for all but the rare, very frail patient with a known driver mutation) but can be challenging (Figure 1A-B, demonstrating morphological features of triple-negative ET [Figure 1A] compared with reactive thrombocytosis [Figure 1B, not considered to be ET]). Although extensive profiling of panels of myeloid genes using next-generation sequencing can be considered in this group and will occasionally identify noncanonical mutations in genes such as JAK2 and MPL,17,18 many cases have no detectable genomic abnormalities and this groups show favorable outcomes,4,19 suggesting that at least some might not represent a clonal disorder.
Diagnostic criteria for ET according to the WHO, the International Consensus Classification, and the British Society for Haematology
| WHO fifth edition . | ICC 2022 . | BSH (2014 modification of 2010 criteria) . | |||
|---|---|---|---|---|---|
| Major | Platelet count ≥450 × 109/L | Major | Platelet count ≥450 × 109/L | A1 | Sustained platelet count ≥450 × 109/L |
| Bone marrow biopsy showing proliferation mainly of the megakaryocytic lineage, with increased numbers of enlarged, mature megakaryocytes with hyperlobulated nuclei; no significant increase or left shift in neutrophil granulopoiesis or erythropoiesis; very rarely a minor (grade 1) increase in reticulin fibers | Bone marrow biopsy showing proliferation mainly of megakaryocytes, with increased numbers of enlarged mature megakaryocytes with hyperlobulated staghorn-like nuclei, infrequently dense clusters; no significant increase or left shift in neutrophil granulopoiesis or erythropoiesis; no relevant BM fibrosis | A2 | Acquired pathogenic mutation (JAK2/CALR/MPL) | ||
| Diagnostic criteria for BCR::ABL1-positive CML, PV, PMF, or other myeloid neoplasms not met | Diagnostic criteria for BCR::ABL1-positive CML, PV, PMF, or other myeloid neoplasms not met | A3 | No other myeloid malignancy, especially PV, PMF, CML, or MDS | ||
| Presence of JAK2, CALR, or MPL mutation | Presence of JAK2, CALR, or MPL mutation | A4 | No reactive cause for thrombocytosis & normal iron stores | ||
| Minor | Presence of a clonal marker | Minor | Presence of a clonal marker | A5 | BM aspirate & trephine biopsy showing increased megakaryocyte numbers displaying a spectrum of morphology with predominant large megakaryocytes with hyperlobated nuclei and abundant cytoplasm. Reticulin generally not increased (grades 0-2/4 or grade 0/3) |
| or | or | ||||
| Exclusion of reactive thrombocytosis | Absence of evidence of reactive thrombocytosis | ||||
| Diagnosis requires either all major criteria or first 3 major criteria + minor criteria | Diagnosis requires either all major criteria or first 3 major criteria + minor criteria | Diagnosis requires A1-A3 or A1 + A3-A5 | |||
| WHO fifth edition . | ICC 2022 . | BSH (2014 modification of 2010 criteria) . | |||
|---|---|---|---|---|---|
| Major | Platelet count ≥450 × 109/L | Major | Platelet count ≥450 × 109/L | A1 | Sustained platelet count ≥450 × 109/L |
| Bone marrow biopsy showing proliferation mainly of the megakaryocytic lineage, with increased numbers of enlarged, mature megakaryocytes with hyperlobulated nuclei; no significant increase or left shift in neutrophil granulopoiesis or erythropoiesis; very rarely a minor (grade 1) increase in reticulin fibers | Bone marrow biopsy showing proliferation mainly of megakaryocytes, with increased numbers of enlarged mature megakaryocytes with hyperlobulated staghorn-like nuclei, infrequently dense clusters; no significant increase or left shift in neutrophil granulopoiesis or erythropoiesis; no relevant BM fibrosis | A2 | Acquired pathogenic mutation (JAK2/CALR/MPL) | ||
| Diagnostic criteria for BCR::ABL1-positive CML, PV, PMF, or other myeloid neoplasms not met | Diagnostic criteria for BCR::ABL1-positive CML, PV, PMF, or other myeloid neoplasms not met | A3 | No other myeloid malignancy, especially PV, PMF, CML, or MDS | ||
| Presence of JAK2, CALR, or MPL mutation | Presence of JAK2, CALR, or MPL mutation | A4 | No reactive cause for thrombocytosis & normal iron stores | ||
| Minor | Presence of a clonal marker | Minor | Presence of a clonal marker | A5 | BM aspirate & trephine biopsy showing increased megakaryocyte numbers displaying a spectrum of morphology with predominant large megakaryocytes with hyperlobated nuclei and abundant cytoplasm. Reticulin generally not increased (grades 0-2/4 or grade 0/3) |
| or | or | ||||
| Exclusion of reactive thrombocytosis | Absence of evidence of reactive thrombocytosis | ||||
| Diagnosis requires either all major criteria or first 3 major criteria + minor criteria | Diagnosis requires either all major criteria or first 3 major criteria + minor criteria | Diagnosis requires A1-A3 or A1 + A3-A5 | |||
CML, chronic myeloid leukemia; BM, bone marrow; BSH, British Society for Haematology; ICC, International Consensus Classification; PMF, primary myelofibrosis; WHO, World Health Organization.
Example cases in the differential diagnosis of ET. (A) Triple-negative ET in a 40-year-old female with thrombocythemia, no mutations detected. Normocellular bone marrow with a mild increase in megakaryocytes (i), occasional loose clusters (ii), and subpopulation of larger megakaryocytes with hypersegmented nuclear morphology (ii-iii) with no evidence of fibrosis (iv). Exclusion of reactive causes of thrombocytosis required to meet diagnostic criteria (Table 2).8,9,16 (B) Reactive thrombocytosis in a 24-year-old female with platelet count of 480 × 109/L, normocellular bone marrow, and no increase in megakaryocytes (i). Occasional large megakaryocytes with hypersegmented nuclei (ii) and most megakaryocytes show normal morphology (iii). CD68R (iv) shows increased macrophage activity. (C) Iron-deficient PV in a 30-year-old female with thrombocytosis, normal hemoglobin, and iron deficient. The combination of hypercellularity (i), megakaryocyte clustering and pleomorphism (ii-iii, respectively), and the WHO classification–based grade-1 fibrosis (iv) favor a diagnosis of iron-deficient PV rather than ET.8,9 (D) MDS/MPN with SF3B1 mutation and thrombocytosis in a 80-year-old female with anemia and thrombocythemia. Hypercellular bone marrow, with erythroid hyperplasia (i, iv, respectively), dyserythropoiesis (ii), and expanded megakaryopoiesis (iii). Megakaryocytes show an MPN-like morphology, and >15% ring sideroblasts on aspirate was observed; JAK2 V617F and SF3B1 mutations detected.8,9 (E) Prefibrotic primary myelofibrosis in a 70-year-old female with leucocytosis and thrombocytosis, JAK2 V617F positive. (i) Hypercellular bone marrow with expanded granulopoiesis and megakaryopoiesis. Megakaryocytes show clustering and include forms with hyperchromatic, hyperlobated nuclei (ii) as well as megakaryocytes with bulbous or cloud-like nuclear morphology (iii), with the WHO classification–based grade 1 reticulin fibrosis (iv).8,9 Hb, hemoglobin; WHO, World Health Organization.
Example cases in the differential diagnosis of ET. (A) Triple-negative ET in a 40-year-old female with thrombocythemia, no mutations detected. Normocellular bone marrow with a mild increase in megakaryocytes (i), occasional loose clusters (ii), and subpopulation of larger megakaryocytes with hypersegmented nuclear morphology (ii-iii) with no evidence of fibrosis (iv). Exclusion of reactive causes of thrombocytosis required to meet diagnostic criteria (Table 2).8,9,16 (B) Reactive thrombocytosis in a 24-year-old female with platelet count of 480 × 109/L, normocellular bone marrow, and no increase in megakaryocytes (i). Occasional large megakaryocytes with hypersegmented nuclei (ii) and most megakaryocytes show normal morphology (iii). CD68R (iv) shows increased macrophage activity. (C) Iron-deficient PV in a 30-year-old female with thrombocytosis, normal hemoglobin, and iron deficient. The combination of hypercellularity (i), megakaryocyte clustering and pleomorphism (ii-iii, respectively), and the WHO classification–based grade-1 fibrosis (iv) favor a diagnosis of iron-deficient PV rather than ET.8,9 (D) MDS/MPN with SF3B1 mutation and thrombocytosis in a 80-year-old female with anemia and thrombocythemia. Hypercellular bone marrow, with erythroid hyperplasia (i, iv, respectively), dyserythropoiesis (ii), and expanded megakaryopoiesis (iii). Megakaryocytes show an MPN-like morphology, and >15% ring sideroblasts on aspirate was observed; JAK2 V617F and SF3B1 mutations detected.8,9 (E) Prefibrotic primary myelofibrosis in a 70-year-old female with leucocytosis and thrombocytosis, JAK2 V617F positive. (i) Hypercellular bone marrow with expanded granulopoiesis and megakaryopoiesis. Megakaryocytes show clustering and include forms with hyperchromatic, hyperlobated nuclei (ii) as well as megakaryocytes with bulbous or cloud-like nuclear morphology (iii), with the WHO classification–based grade 1 reticulin fibrosis (iv).8,9 Hb, hemoglobin; WHO, World Health Organization.
Other challenging diagnostic scenarios, illustrated by selected cases in Figure 1, include distinctions between JAK2 V617F ET vs PV (Figure 1C), ET vs myelodysplastic syndromes (MDS) or MDS/MPN overlap syndromes (Figure 1D), and ET vs earlier forme fruste stages of myelofibrosis, prefibrotic myelofibrosis (Figure 1E).
More extensive molecular and/or cytogenetic profiling can occasionally assist in distinguishing between ET and other myeloid disorders and can add prognostic information in ET (refer to further sections) but is not strictly required in most patients with uncomplicated disease because management recommendations remain unchanged.
In the future, artificial intelligence–based platforms capable of assimilating many thousands of digital images and dimensions might be able to contribute to diagnosis and, possibly, to risk assessment, providing a more unbiased approach to tissue analysis in conjunction with molecular information.20
Prognosis
Few studies have directly addressed survival in ET, and these have reached variable conclusions about life expectancy compared with the general population. A recent multicenter study showed a median survival of 19.8 years (32.7 years if diagnosed before 60 years of age), which was worse than the age- and sex-matched population.21 Sadly, a recent publication using US registry data suggests that prognosis has not improved in recent years.3 Concerning younger patients, in a multicenter cohort of 444 patients with MPN including 318 with ET diagnosed before 26 years of age and with a median follow-up of 9.7 years, using either original or revised international prognostic scores for thrombosis in essential thrombocythemia (IPSET) (see further sections), patients at high risk had an available median-free survival of 28.5 years.22
Although clinical prognostication in ET is focused on vascular risks, rarer later events such as transformation to myelofibrosis are concerning to patients5,6 and represent an unmet need in view of the uncertain effect of therapy. The cumulative risk of occurrence of post-ET myelofibrosis is <10% of patients21,23; for example, it was 7.5% in an entire young patient cohort, with a 5 year rate of 1.89% in ET, being more common in patients with splenomegaly.22 The risk also increases with age and in those with higher white blood cell counts, lower hemoglobin, and higher bone marrow reticulin grade at diagnosis.23-26 Progression to myelodysplasia or acute myeloid leukemia is rare with a cumulative incidence of <5%, although therapy-related disease can occur with alkylating agents and remains a theoretical but not substantiated risk with hydroxycarbamide (HC).21,23,27 Concerning mutation status, JAK2 V617F variant allele frequency of >35%, CALR type 1/1-like, and MPL mutations have been identified as additional adverse factors for myelofibrotic transformation.21,28 In comprehensive genomic profiling studies, more than half of patients with ET showed only a JAK2, CALR, or MPL mutation, with the minority having additional recurrent genomic aberrations.4 However, a group of these additional mutations (SH2B3, IDH2, SF3B1, U2AF1, EZH2, TP53) confer an adverse risk, being associated with less favorable myelofibrosis-free, leukemia-free, or overall survival.29 In other studies, spliceosome mutations were associated with less favorable overall and myelofibrosis-free survival, and TP53 mutations predicted leukemic transformation; the Mutation-Enhanced International Prognostic Systems for ET risk model incorporates these molecular variables with additional clinical factors (Table 3).31 An alternative approach to prognostic scoring employs the use of multivariable, multistate modeling through which comprehensive genomic and clinical information is used to generate individually tailored predictions of transformation and survival risk for a particular patient with MPN4 (Table 3). This work highlights the potential drivers of transformation and the benign nature of triple-negative ET.
Current risk stratification systems in ET
| IPSET (revised)30 . | MIPSS-ET31 . | MPN personalized risk calculator4 . | ||
|---|---|---|---|---|
| Factor . | Points∗ . | |||
| Very low | No prior thrombosis + age ≤60 y + JAK2-unmutated | Adverse mutation† | 2 | Available genomic data plus clinical factors (age, Hb, WBC, plts, sex, prior thrombosis, splenomegaly) |
| Low | No prior thrombosis + age ≤ 60 y + JAK2-mutated | Age > 60 y | 3 | |
| Intermediate | No thrombosis + age >60 y + JAK2-unmutated | Male sex | 1 | |
| High | Thrombosis history (any age / genotype), or age >60 y + JAK2-mutated | WBC ≥11 × 109/L | 1 | |
| IPSET (revised)30 . | MIPSS-ET31 . | MPN personalized risk calculator4 . | ||
|---|---|---|---|---|
| Factor . | Points∗ . | |||
| Very low | No prior thrombosis + age ≤60 y + JAK2-unmutated | Adverse mutation† | 2 | Available genomic data plus clinical factors (age, Hb, WBC, plts, sex, prior thrombosis, splenomegaly) |
| Low | No prior thrombosis + age ≤ 60 y + JAK2-mutated | Age > 60 y | 3 | |
| Intermediate | No thrombosis + age >60 y + JAK2-unmutated | Male sex | 1 | |
| High | Thrombosis history (any age / genotype), or age >60 y + JAK2-mutated | WBC ≥11 × 109/L | 1 | |
Hb, hemoglobin; MIPSS-ET, Mutation-Enhanced International Prognostic Systems for ET; Plts, platelets; WBC, white blood cell count.
Total score: 0 to 1, low risk; 2 to 5, intermediate risk; ≥6, high risk.
SRSF2/SF3B1/U2AF1/TP53.
Thrombotic and hemorrhagic risk stratification
Patients aged >60 years or with a previous history of thromboembolic events have consistently been shown to have an increased risk of future thrombosis and have been conventionally considered to be at high risk and are offered cytoreductive therapy to reduce platelet count.32,33 In addition, the JAK2 V617F mutation has been shown to independently increase the risk of vascular events and has now been incorporated into the revised IPSET score (Table 3) for risk stratification.10,30,34 Other risk factors for thrombosis reported in ET include cardiovascular risk factors (diabetes, hypertension, and cigarette smoking), leucocytosis, and bone marrow reticulin levels at diagnosis, although these are not usually used in isolation for individual treatment decisions.26,34,35 The risk of major hemorrhage in ET has been associated with leucocytosis and, more significantly, a high platelet count, which may reflect an acquired von Willebrand syndrome.35 Testing for the latter is not routinely recommended but should be considered in patients with atypical bleeding or when planning major surgery or epidural anesthesia in patients with an uncontrolled thrombocytosis; some experts also recommend testing before initiating aspirin in the context of marked thrombocytosis.36
Management
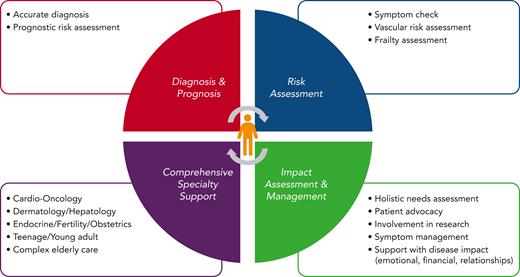
A diagnosis of ET has important implications including a reduction in life expectancy and, for some patients, the ability to work, raise a family, and have sufficient energy to match lifestyle aspirations. A comprehensive approach to care would include full cardiovascular risk assessment and monitoring, which could include telemedicine, as well as access to specialist services, for example, fertility support, pregnancy, management of menopause, and services for patients that are frail or who are medically complex, in addition to dermatology, endocrinology, nutrition, health psychology, and management of challenging symptoms such as fatigue, which should be objectively assessed using standardized scores37 (Figure 2).
Components of comprehensive care for patients with ET. CV, cardiovascular; IFN, interferon; yrs, years.
Components of comprehensive care for patients with ET. CV, cardiovascular; IFN, interferon; yrs, years.
Risk-stratified antiplatelet and cytoreductive therapy
Patients are stratified according to their risk of vascular complications, with those aged >60 years or with a previous history of vascular events being consistently considered at high risk of thrombosis, together with some further risk stratification offered by the IPSET score (previous section).7,38 Patients with a platelet count of >1500 × 109/L also warrant cytoreductive therapy in view of hemorrhagic risk. Regarding acquired von Willebrand disease, if the patient is asymptomatic then cytoreduction is likely only required in preparation for surgery. A recent consensus statement for the management of CALR-positive ET still recommends cytoreduction once the platelet count is >1500 × 109/L38. Risks of bleeding for triple-negative disease linked with extreme thrombocytosis are not quantified and, at present, these patients are not differentiated because further data are required. Interestingly, data from Spain recently suggested that patients with CALR-mutated ET were less likely to respond to treatment, and more likely to develop drug-resistance.39
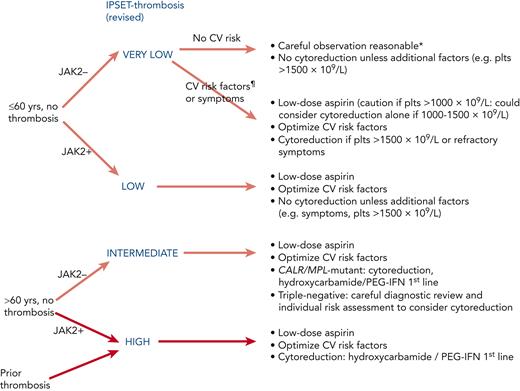
Low risk
Patients lacking the high-risk factors of age or previous vascular events might fall into the IPSET very–low- or low-risk groups (Figure 3), according to JAK2 mutation status. Recent retrospective data in low-risk disease suggested that whereas use of antiplatelet agents was associated with a lower rate of thrombotic events in patients with mutated JAK2, a higher rate of bleeding was seen in CALR-mutant disease.41 Current recommendations to use aspirin in JAK2-mutant disease remain but to exercise caution in those with a marked thrombocytosis (>1000 × 109/L), and aspirin is now typically omitted in young patients with mutated CALR without cardiovascular risk factors; similar recommendations would likely be appropriate for patients with MPL-mutant or triple-negative disease. Aspirin can also be useful for microvascular symptoms such as erythromelalgia and migraine.
Antiplatelet and cytoreductive therapy stratified according to revised IPSET score.7,30,38,40 ∗Aspirin is recommended in pregnancy. ¶CV risk factors comprise active smoking, diabetes, hypertension, hypercholesterolemia, and obesity. CV, cardiovascular.
The “intermediate-risk” Medical Research Council (MRC) Primary Thrombocythaemia 1 (PT-1) trial randomized patients aged 40 to 59 years and without high-risk features to HC plus aspirin or aspirin alone and found no difference in either the incidence of vascular events or the rate of progression to myelofibrosis or leukemia (note that the definition of intermediate risk disease in the trial was not that of IPSET as described in previous sections).42 International consensus concludes that the routine use of cytoreduction in patients lacking high-risk factors is therefore not recommended,38,43 although it might occasionally be considered for severe symptoms, such as headaches, that have not responded to antiplatelet therapy. In the latter situation it is reasonable to adopt a target-of-symptom control rather than a particular platelet count, although standardized symptom scores should preferably be used to objectively assess success.
High risk
Patients conventionally classified as being at high risk according to age and/or vascular history may be further stratified into IPSET intermediate and high-risk groups (Table 3). It is recommended that all such patients receive low-dose aspirin, based largely on extrapolation of data from the ECLAP study in PV.44 Concerning the dose of aspirin, some studies are underway assessing the benefit of twice-daily aspirin dosing, especially in high-risk or JAK2-mutant disease but this is largely experimental.45 In older patients (>70 years of age), it is recommended that aspirin is combined with a proton pump inhibitor for gastric protection.46 The role of clopidogrel or equivalent agents is unclear but they are often used interchangeably.
In patients at high risk, HC was shown to reduce thrombotic events compared with no cytoreductive therapy in a randomized trial and this has formed the basis for reducing the platelet count in high-risk disease.47 However, the optimal platelet count target is not known, particularly because platelet count does not correlate with risk of thrombotic events35 nor does it have achievement of response according to European LeukemiaNet criteria48 correlated with improved outcome.49 In patients with high-risk factors the aim is typically to achieve a platelet count in the normal range, although a more moderate target (eg, <600 × 109/L) might be appropriate in patients who do not have evidence of very high vascular risk and in whom first-line therapy is not fully tolerated or effective. Taking the IPSET intermediate-risk group, specifically, consensus guidelines continue to recommend cytoreduction for patients with CALR-mutated ET aged >60 years, because there is a lack of randomized trial data gathered before molecular subclassification and there are no data suggesting it would be safe or beneficial to avoid cytoreduction in this group.40 The authors would also use cytoreduction in those aged >60 years with MPL-mutated ET; although, for patients with triple-negative disease, in whom a low thrombotic rate has been reported,50 decisions around cytoreduction require careful consideration of diagnostic certainty and individual risk factors.
The MRC high-risk PT-1 trial compared treatment with HC plus low-dose aspirin with anagrelide plus low-dose aspirin.51 Patients receiving anagrelide plus aspirin were more likely to reach the composite primary end point (arterial thrombosis, venous thrombosis, or major hemorrhage) and to discontinue allocated treatment. Compared with HC plus aspirin, treatment with anagrelide plus aspirin was associated with a significantly increased rate of arterial thrombosis, major hemorrhage, and myelofibrotic transformation but a decreased rate of venous thromboembolism. These results have established HC plus aspirin as first-line therapy for most patient with ET that are at high risk. In a subsequent analysis, differences between these agents for arterial thrombotic events were limited to JAK2-mutated ET.10
It is rare not to achieve some control of the blood count with HC but, over time, patients can develop toxicity or become resistant to this agent. Consensus criteria for HC resistance and/or intolerance have been shown to have utility in selecting patients with higher risk disease.49,52 In particular, anemia was associated with a high incidence of myelofibrosis and death from any cause. These criteria also summarize key features of drug intolerance that suggest adjusting the therapeutic approach, such as skin ulceration, skin cancers, fever, or pneumonitis.
Concerning anagrelide, the phase IV EXELS study with >3700 patients with ET confirmed the findings of the high-risk PT-1 trial but demonstrated that alternative cytoreductive therapies, more frequently used in older patients, were more associated with acute leukemia (0.28 vs 0.07 events per 100 patient-years) and other malignancies (1.29 vs 0.44 events per 100 patient-years).53 A second study (the ANAHYDRET trial) reported noninferiority between HC and anagrelide in patients with ET, as strictly defined by the World Health Organization diagnostic criteria, but the study was underpowered to see the differences observed in the PT-1 study in which patients were also taking aspirin.54 In these studies, the most common side effects were headache, palpitations, and diarrhea. Anagrelide is a useful second-line agent; it has important cardiac toxicity and has been linked to higher rates of myelofibrotic transformation but, unlike HC, has no potential risk of leukemia, skin cancer, or leg ulceration. Anagrelide is often added to HC as therapy is initiated and sometimes patients are successfully maintained on lower doses of both agents; for example, combination therapy was used in 9.5% of the patients in the EXELS study and other case series also documented the benefit of this combination.55-57
Pegylated interferon alfa (PEG) can achieve good control of the platelet count in ET and is frequently used in younger patients, although side effects may be more problematic than with HC. It has not been studied as widely in ET as in PV and only 1 phase 3 comparative trial has been completed (MPD-RC 112) to date, which included 168 patients with ET or PV treated for a median of 81.0 weeks with PEG or HC.58 Response rates did not differ although PEG led to a greater reduction in JAK2 V617F allele burden at 24 months. Thrombotic events and disease progression were infrequent in both arms, whereas grade 3/4 adverse events were more frequent with PEG (46% vs 28%). Other studies also support the efficacy of PEG in ET.59,60 Important toxicities for interferon include flu-like symptoms, which are usually transient and easily managed, but also autoimmune disease and psychological concerns such as anxiety and depression, all of which can have a long latency. The importance of molecular response is unclear but there does appear to be less likelihood of response in CALR-mutated ET and in patients with ET with additional mutations.61-63 Ropeginterferon alfa-2b (Besremi) has been approved for the treatment of PV64 and is being assessed in clinical trials for patients with ET.65
Busulfan can achieve good control of the platelet count66-69 but is only used in patients with limited life expectancy because of concerns over its long-term leukemogenic potential, a factor which also needs to be considered for other alkylating agents and for radioactive phosphorus (32P).27 For example, in a cohort of 36 patients with ET or PV receiving busulfan after HC resistance, 3 developed MDS or acute myeloid leukemia.66
The JAK 1 and 2 inhibitor ruxolitinib has been assessed in patients with ET resistant to, or intolerant of, HC.70 In a randomized study (MAJIC-ET), ruxolitinib treatment did not deliver better rates of hematological response than best-available therapy, although symptoms did improve, indicating that it could be an option in refractory symptomatic patients.71 Another study, RUXO-BEAT, is ongoing.
Unmet needs in treatment, and novel agents
No new treatment for ET has been approved in over a decade but there are clearly areas for improvement in our therapeutic algorithm. A challenge for those seeking to deliver a novel therapy is how success should be defined. A trial seeking to reduce the risk of disease transformation as a primary end point would likely require several thousand years of patient observation yet would have high value to patients, whereas aiming to control the platelet count could be questioned as a sufficiently relevant target. These problems could be addressed with a surrogate end point such as reduction of mutant allele burden, but the benefit of this to clinical outcomes remains unproven. Provisional data suggest that changes in allele burden driven by agents such as PEG in JAK2-mutated ET might be different to that in CALR-mutated, MPL-mutated, or triple-negative disease61-63 and this novel end point might need to be evaluated separately in these subgroups. A further important unmet need is the improvement of symptoms, particularly fatigue.5,6
Currently, there are 3 trials evaluating novel pharmacological therapies in ET. No results are available thus far from SURPASS-ET comparing ropeginterferon alfa-2b with anagrelide,65 or MANIFEST evaluating the bromodomain inhibitor pelabresib72 in ET. Interestingly, interim results with bomedemstat, a LSD1 inhibitor, indicate that platelet and leucocyte control are obtained in the majority of patients with maintenance of hemoglobin levels; early results also suggest benefits for fatigue reduction and molecular data are also being collected.73 Other strategies currently in early stages of assessment include vaccination, and recombinant antibodies.74,75
Challenging scenarios
The PV/ET interface
A small proportion of patients with JAK2-mutated ET can progress to PV and it can be difficult to distinguish these entities in some cases.76 A relevant question for patients with JAK2-mutated ET is whether the hematocrit should be targeted to <0.45 if it rises above this level but without reaching diagnostic criteria for PV. In practical terms, this scenario should prompt evaluation for other secondary causes of erythrocytosis, which might include additional investigations such as an erythropoietin level, and perhaps red cell mass studies or a repeat bone marrow biopsy. Although some teams venesect such patients, this is not routinely recommended in the absence of data from ET cohorts showing a benefit of hematocrit control. It is uncertain whether patients with JAK2-mutated ET at high risk of vascular events might be better protected by a cytoreductive regimen that includes a target hematocrit of <0.45.
Care at the extremes of life
Patients with ET are typically >50 years of age but the condition has been described in children and it has a peak in young women. Childhood cases should be carefully evaluated; the available literature suggests that children may not always have conventional mutational profiles and some are diagnosed without bone marrow histology.77 In fact, all childhood MPN require careful expert evaluation and a prospective collection of data to best inform practice; international management guidelines are required in this area because childhood disease could behave very differently to that in adulthood.22 In contrast, many patients with ET are in, or beyond, their eighth decade of life, bringing additional dimensions to care such as maximizing fitness with access to services for management of complex medical comorbidities, polypharmacy, and frailty, bringing the need to utilize occupational and physical therapy services in hospital and community settings.78
Contraception, fertility, pregnancy, and menopause
As a disease affecting patients of childbearing potential, these issues merit specific discussion with patient with ET. Estrogen-containing contraception is generally contraindicated, although for the purpose of managing the menopause, lower doses are used and, for most patients, hormonal replacement therapy including estrogen can be used if indicated79; those patients with prior thrombosis or high thrombotic risk should be individually discussed. Fertility treatments typically involve higher doses of estrogen and the routine use of low molecular weight heparin is recommended during fertility therapy and for pregnancy thereafter in patients with MPNs.80 Concerning pregnancy outcomes, there are prospective data,81 large registry analyses82 and a meta-analysis83 suggesting good outcomes. Specific guidance for pregnancy management is reviewed elsewhere.84
Thrombosis management beyond antiplatelet and cytoreductive therapy
Prevention and management of both arterial and venous thrombosis is a mainstay of ET therapy and practitioners should have a process for evaluating these and conventional cardiovascular risks; for example, a patient who continues to smoke is not optimally managed. Some specific issues merit additional consideration here, including use of thrombophilia testing (not recommended without other indication to test), novel oral anticoagulants, combined therapies, perioperative management, and complex thrombotic scenarios, in particular splanchnic vein thrombosis. The latter is almost exclusively a feature of JAK2-mutated disease85 and is best managed in collaboration with gastroenterology and/or hepatology teams. Although thrombosis is an indication for cytoreduction in ET, splanchnic vein thrombosis is often seen in patients with normal blood counts in whom cytoreductive management becomes empirical and there is less evidence of benefit.86 Anticoagulation is indicated but needs to be balanced against risk of bleeding.86,87 Although vitamin K antagonists are a mainstay of anticoagulation, they can sometimes be combined with aspirin in patients at highest risk, for example, in the event of stent thrombosis.88
Data are emerging concerning the use of direct oral anticoagulants (DOACs) in patients with MPN and in general suggest that these can be used safely,89-92 including a study of patients with splanchnic thrombosis that excluded Budd-Chiari syndrome.93 Studies to date have generally been observational and retrospective, however, and do not allow for comparison between different DOACs or with vitamin K antagonists, with potential confounding from cytoreductive therapy in some reports. Some patients will receive these drugs for management of atrial fibrillation and the benefit of continuing aspirin in the scenario where a patient is receiving a DOAC is likely to be outweighed by the bleeding risk.
Future priorities and conclusions
To improve quality of life for patients with ET we must adopt models of care that address their needs comprehensively, paying particular attention to fatigue and other symptoms that cause daily morbidity. With fatigue, management and identifying whether it is because of comorbidity, psychology, disease, or therapy side effects are challenging and often require a multidisciplinary approach. In addition, we should continue to focus research efforts on identified patient concerns such as disease progression, and ensuring that consistent standards of care are provided across health care settings.94 For clinical trials, important issues remain in the choice of clinically meaningful endpoints that should be chosen for cytoreductive and disease-modifying agents, as discussed in previous sections. Available risk stratification models could be utilized to target novel agents to those at higher risk of progression. As we better understand the heterogeneity of vascular risk within ET, there are additional questions in the areas of antiplatelet and anticoagulant therapy, including optimal administration and dosing of aspirin and whether DOACs might provide superior prophylaxis over antiplatelets in some patient groups. These areas are also difficult to study in randomized prospective studies in view of the low frequency of events, and often require international collaboration to collect large, generalizable data sets. Lastly, a better understanding of the biology, natural history, and therapeutic needs of patients with triple-negative thrombocytosis remains an important diagnostic priority, many of whom are currently managed with cytoreductive therapies in the absence of molecular evidence of a clonal disorder.
Authorship
Contribution: C.N.H. wrote the original draft of the manuscript; A.C.G. and A.L.G. contributed new sections and edited the manuscript; and all authors approved the final version.
Conflict-of-interest disclosure: A.L.G. has received speaker honoraria from Novartis and participated in advisory boards for Novartis, AOP Orphan, and Celgene. A.C.G. has received speaker fees from Novartis and Bristol Myers Squibb/Celgene. C.N.H. has received institutional research funding from Novartis, Celgene, and Constellation Pharmaceuticals Inc, (a MorphoSys Company); consulting fees from Keros, Galecto, AOP, and Roche; advisory role and speaker funding from Novartis, Celgene, CTI BioPharma, AbbVie, Jansen, Constellation Pharmaceuticals Inc (a MorphoSys Company), Galecto, CTI BioPharma, Roche, Geron, Promedior, AbbVie, and AOP Pharma; and support from Novartis for attending meetings.
Correspondence: Claire N. Harrison, Guy’s and St Thomas’ NHS Foundation Trust, Great Maze Pond, London SE1 9RT, United Kingdom; e-mail: claire.harrison@gstt.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal