Abstract
Posttransplant lymphoproliferative disorder (PTLD) is an important and potentially life-threatening complication of solid organ transplant and hematopoietic stem cell transplant (HSCT). Given the heterogeneity of PTLD and the risk of infectious complications in patients with immunosuppression, the treatment of this disease remains challenging. Monomorphic PTLD and lymphoma of B-cell origin account for the majority of cases. Treatment strategies for PTLD consist of response-adapted, risk-stratified methods using immunosuppression reduction, immunotherapy, and/or chemotherapy. With this approach, ∼25% of the patients do not need chemotherapy. Outcomes for patients with high risk or those who do not respond to frontline therapies remain dismal, and novel treatments are needed in this setting. PTLD is associated with Epstein-Barr virus (EBV) infection in 60% to 80% of cases, making EBV-directed therapy an attractive treatment modality. Recently, the introduction of adoptive immunotherapies has become a promising option for refractory cases; hopefully, these treatment strategies can be used as earlier lines of therapy in the future.
Introduction
Posttransplant lymphoproliferative disorder (PTLD) is a serious complication that can occur after solid organ transplantation (SOT) and allogeneic hematopoietic stem cell transplantation (HSCT). The term PTLD encompasses a heterogeneous group of lymphoproliferative disorders divided according to the International Consensus Classification of Mature Lymphoid Neoplasms into 4 groups: nondestructive PTLDs, polymorphic PTLDs (pPTLDs), monomorphic PTLDs (mPTLDs), and classic Hodgkin lymphoma PTLDs (HL-PTLDs).1-3
Most PTLDs arise from B cells. Latent Epstein-Barr virus (EBV) infection is present in 52% to 80% of cases.4 The incidence ranges between 1% and 20% after SOT, depending on factors including the type of organ transplanted, degree and type of immunosuppression (IS), and EBV status of the donor and recipient.5,6 In SOT recipients, PTLD has a bimodal distribution; the first peak occurs 12 to 24 months after transplantation (early PTLD), and a second peak 5 to 10 years after transplantation (late PTLD). The incidence of PTLD after HSCT is 1% but is more common in transplant cases that use profound T-cell depletion, which increases the risk of EBV reactivation, in which it can occur in >10%.7
The rising number and improved long-term survival of SOT recipients has led to an increase in the prevalence of PTLD.8,9 Although the number of pPTLD has remained stable, there has been an increase in mPTLD over time.10 This underscores the need for close monitoring of transplant recipients and the development of more effective and less toxic treatments for this serious complication.
Case 1: frontline PTLD
A 30-year-old male with primary sclerosing cholangitis and ulcerative colitis underwent a liver transplantation in 2009. In May 2020, he presented with abdominal pain, diarrhea, and weight loss. Imaging showed a new enterocolic fistula between the proximal jejunum and transverse colon. His IS consisted of tacrolimus and low-dose steroids. Colonoscopy and study of a biopsy specimen from the transverse colonic mass were consistent with mPTLD germinal center-type, EBV-negative (EBV−) status, and CD30–. In June 2022, he underwent a partial small bowel and colon resection. Positron emission tomography (PET) after surgery showed hypermetabolic concentric intestinal wall infiltration at the splenic flexure, consistent with that of PTLD. He received 4 weekly doses of rituximab. A repeat PET showed a complete metabolic response (CR). He received 4 additional doses of rituximab every 3 weeks with ongoing CR.
The goal of the initial therapy of PTLD is to achieve CR while minimizing toxicities, given the significant risk of infectious complications due to patients’ immunocompromised status.
Reduction of IS (RIS) is the first-line therapy for PTLD and involves discontinuing the antimetabolites mycophenolate or azathioprine, and reducing the dose of calcineurin inhibitor tacrolimus or cyclosporine. Decisions regarding the strategy, timing, and duration of RIS are highly complex and should be taken in close coordination with the transplant team. Factors that must be considered include the following: organ type, indication for transplant, time from transplant, history of rejection, and center-specific practices.11-14 There are few long-term responses to RIS, and most often, this strategy cannot be used alone. Early lesions and pPTLD tend to respond better than other subtypes. In 1 study, RIS demonstrated a 6% partial response (PR) and no CRs, and the rate of graft rejection was significant at 38%.15 In a larger retrospective study, the CR rate was 37%, but the rejection rate was similar at 32%.16 Furthermore, RIS may increase the risk of graft-versus-host disease in allogeneic HSCT recipients.
In addition to RIS, systemic therapy is indicated for most patients with mPTLD.17,18 In a retrospective review of 80 patients, Evens et al found that the addition of rituximab to RIS in frontline treatment significantly prolonged progression-free survival (PFS) and overall survival (OS).19 Fifty-nine (74%) patients received rituximab, leading to a 3-year PFS and OS of 70% and 73%, respectively, compared with 21% (P < .0001) and 33% (P = .0001), respectively, without rituximab. Patients with bulky disease or high international prognostic index (IPI) scores were more often treated with rituximab and chemotherapy, which is a common practice. In a multivariate analysis, the authors found hypoalbuminemia, central nervous system (CNS) involvement, and bone marrow involvement to be prognostic factors, which have been confirmed in other studies.18,20-25
The PTLD-1 trial was a landmark study that established the role of sequential treatment. This prospective study accrued 70 patients, demonstrating the safety and efficacy of 4 weekly doses of rituximab monotherapy, followed by 4 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) administered every 21 days.26 Twenty percent of the patients achieved CR after rituximab, and this increased to 57% after the completion of CHOP. The treatment-related mortality (TRM) with this approach was 11%, which was much lower than that of the historical data. Furthermore, this study confirmed that the response to rituximab is an important prognostic factor for OS; age and Eastern Cooperative Oncology Group performance status >2 were the most important baseline characteristics predicting the outcome.27 A subsequent study evaluating a risk-stratified sequential approach showed excellent outcomes with rituximab consolidation in patients who achieved CR after rituximab induction (low-risk group). In patients who did not achieve CR (high-risk group), treatment was escalated to rituximab-CHOP (R-CHOP). Approximately 25% of the patients did not require chemotherapy. The overall response rate (ORR) was 88%, the median OS was 6.6 years, and the TRM was 8%.28 In the multivariable analysis, both response to rituximab and baseline IPI (<3 or ≥3) were highly significant prognostic factors for the time to progression and OS.
The PTLD-2 trial tested the safety and efficacy of subcutaneous rituximab. After rituximab monotherapy induction, patients in CR and those in PR with IPI < 3 (low risk) continued with rituximab monotherapy. Most others (high risk) received R-CHOP every 21 days. Thoracic SOT recipients whose condition progressed were considered to be at very high risk and received alternating R-CHOP and modified rituximab, dexamethasone, cytarabine, and oxaliplatin. In the low-risk group, the 2-year event-free survival was 66% vs 52% in the historical comparator that received CHOP (P = .4). The results for the high-risk group were inferior, with a 2-year PFS of 54%. Notably, for the small group of patients at very high risk, the results were disappointing despite intensification, underscoring the need for more effective therapies in these populations.
Alternative approaches have been evaluated. Surgical resection has a limited role in PTLD but may be considered in patients with gastrointestinal involvement at a high risk of local complications, such as perforation, obstruction, and/or bleeding.
A small single-center retrospective study performed at Columbia University evaluated the results of various treatment strategies for PTLD. Of the 122 patients, 72 (59%) received rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH), and 9.8% received R-CHOP. R-EPOCH was chosen because of the similarities between AIDS-associated diffuse large B-cell lymphoma (DLBCL) and PTLD because they are both associated with impaired T-cell function. R-EPOCH was dosed according to the AIDS malignancy protocol, with cyclophosphamide dosing starting at 187 mg/m2.29 The other agents in this regimen were adjusted based on organ function to improve tolerability. The 2-year OS for R-EPOCH-treated patients was 76.1%, whereas that of R-CHOP–treated patients was 41.7%.30 However, it should be noted that baseline characteristics of the treatment groups and safety data, specifically neutropenia and infectious complications, have not been reported in this abstract. This study was not designed to compare these modalities but demonstrated that R-EPOCH is effective, warranting investigation in a larger prospective study. Treatment with R-EPOCH could be considered if an infusional therapy approach is preferred, if the patient has high-grade features or MYC and BCL2 rearrangements.
A single-arm phase 2 study evaluated the activity of ibrutinib in combination with rituximab; chemotherapy was added according to a risk-stratified sequential treatment strategy. This approach did not result in a sufficiently high CR rate to justify further investigation. In addition, the survival outcomes were similar to those reported previously using a risk-stratified sequential treatment approach.31
CD30 represents an ideal target in PTLD because ∼70% to 85% of cases express CD30.32 In a phase 1/2 study, the combination of rituximab plus brentuximab vedotin was tested in 20 patients, with ORR and CR rates of 75% and 60%, respectively. However, despite the high response rate and encouraging long-term outcomes, toxicity was significant in grade 3/4 neutropenia and infections, occurring in 40% and 25% of the patients, respectively.33
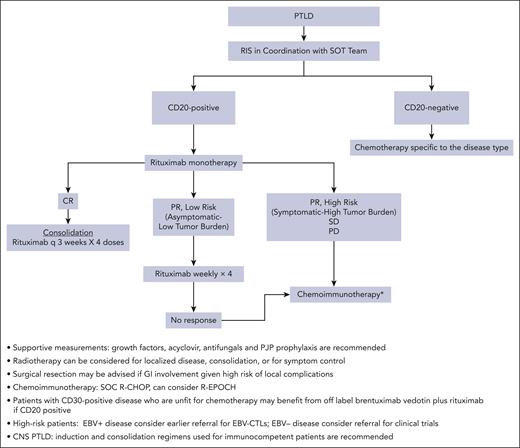
Our standard therapeutic approach for SOT mPTLD DLBCL subtype is RIS combined with monotherapy with rituximab, followed by escalation to R-CHOP or R-EPOCH based on the response (Figure 1). This approach has been shown to be safe and results in excellent long-term outcomes in patients with low-risk disease. After the successful eradication of PTLD and recovery from the immunosuppressive effects of chemotherapy, IS management should be discussed with the transplant team and be based on the risk of rejection and EBV reactivation. Therefore, close monitoring is recommended.
Algorithm for management of PTLD in a frontline setting. GI, gastrointestinal; PJP, Pneumocystis jirovecii pneumonia; q, every; SD, stable disease; SOC, standard of care.
Algorithm for management of PTLD in a frontline setting. GI, gastrointestinal; PJP, Pneumocystis jirovecii pneumonia; q, every; SD, stable disease; SOC, standard of care.
Case 2: relapsed EBV+ PTLD
A 58-year-old EBV-exposed female underwent a double-lung transplant for cystic fibrosis in April 2000. IS included cyclosporine and low-dose prednisone. In October 2017, she underwent an ultrasound to rule out an upper-extremity thrombus; imaging revealed an axillary mass. EBV DNA quantitative level was 1056 IU/mL. Biopsy revealed an EBV-positive (EBV+) pPTLD with light-chain restriction and aggregates of plasmacytoid cells. PET demonstrated right axillary lymphadenopathy measuring 1.8 × 0.8 cm and 1.2 × 0.6 cm and maximum standardized uptake value = 9.1; there were several chest wall subcutaneous nodules up to 3.9 cm (maximum standardized uptake value = 6.1). She was treated with single-agent rituximab from November 2017 to December 2019. EBV was undetectable after treatment; however, it soon reactivated and trended up to 1900 IU/mL by April 2021. At that time, the patient had newly enlarged lymph nodes and biopsy confirmed relapse. EBV peaked at 7400 IU/mL in July 2021. The cyclosporine trough goal was lowered to 100 ng/mL. She enrolled in a clinical trial and received HLA-matched EBV-stimulated T cells for 6 cycles between July 2021 and January 2022. PET at completion demonstrated stable right axillary lymphadenopathy, Deauville 4, with resolution of other lymphadenopathy. The treatment was stopped at that time, and the EBV level was 47 IU/mL. She received radiation to the lesion, with a total of 40Gy as consolidation. Throughout her treatment, she remained symptom-free and continued playing tennis regularly. To this date, she remains in remission.
Treatment of relapsed EBV+ PTLD remains challenging. In a retrospective multicenter review of 86 patients who experienced failure of rituximab plus chemotherapy, the investigators observed a median OS of 4.1 months from the time of relapse.34 With a median follow-up of 12.9 months, 73.3% of the patients died: PTLD was the cause in 65%, TRM in 15.9%, and organ rejection or failure in 3.2%. With this in mind, choosing a treatment plan with a low-risk of toxicity and high chance of long-term remission is ideal.
Antiviral therapy for EBV-related lymphomas has not been effective. Drugs such as acyclovir exert antiviral effects by inhibiting lytic viral replication in epithelial cells by targeting viral DNA polymerase. In the setting of PTLD, EBV replicates using host lymphocyte DNA polymerase during lymphocyte cell division; therefore, conventional antiviral therapy is ineffective.35 However, the EBV virus expresses a number of specific antigens, making it an attractive target.
The idea of leveraging adoptive immunotherapy to treat EBV infection was first introduced in the mid-1990s through donor leukocyte infusions after allogeneic HSCT to aid in a more rapid reconstitution of immunity against EBV.36-39 However, the responses were not durable because of alloreactive cells within the infusion product. This led to the concept of manufacturing ex vivo–expanded, virus-specific T cells by exposing donor lymphoblastoid cells to a laboratory EBV strain.40,41 After infusion, these cells expand upon viral stimulation, achieving long-term persistence. Impressively, in a clinical study conducted at 3 different centers with EBV-specific cytotoxic T cells, led by Heslop, a pioneer in this field, 114 patients with EBV+ HSCT were enrolled: 13 with PTLD, 11 of whom achieved long-term remission. The remainder of the patients had EBV+ status after HSCT and received the infusion as prophylaxis against PTLD; all remained disease-free. Importantly, the treatment was well tolerated and did not result in alloreactivity. A study conducted by Comoli et al explored a similar strategy in 5 patients with PTLD who were unresponsive to RIS. These patients were treated with rituximab plus chemotherapy, followed by surgery, and underwent consolidation with EBV-specific cytotoxic T lymphocytes that successfully eradicated relapsed EBV+ PTLD.42
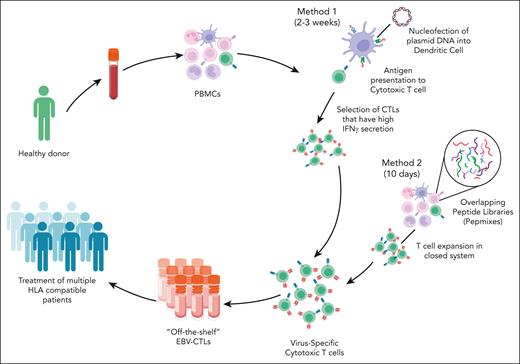
The aforementioned studies were possible in the setting of allogeneic HSCT with use of donor-derived lymphocytes; however, in the SOT setting, this resource is not available. An alternative approach to create banks of HLA-typed, EBV-stimulated T cells from healthy donors has gained traction (Figure 2).43,44 Advantages of banked products include faster access and availability. Barriers and potential risks include high cost, rejection, lack of persistence, and induction of alloreactivity. In the multicenter phase 3 ALLELE study of tabelecleucel for EBV+ PTLD, 43 patients who had experienced rituximab or rituximab plus chemotherapy failure were enrolled.45 The ORR was 51.2%, with CRs in 12 patients. The median duration of response (DOR) was 23 months, and the median OS was 18.4 months, with a 1-year OS of 56% in the SOT recipient population, a significant improvement compared with what has previously been reported in the relapsed setting. Importantly, no TRM was observed, and there was no evidence of allograft rejection (Table 1). Tabelecleucel was approved by the European Medicines Agency in December 2022 and has become the first EBV-targeting cellular therapy to gain regulatory approval.
Methods for manufacturing “off-the-shelf” EBV-specific cytotoxic T cells. There are several methods for manufacturing off-the-shelf EBV-specific CTLs. This figure shows 2 common approaches. The cells are collected from a healthy donor. Peripheral blood mononuclear cells (PBMCs) are selected and stimulated using 1 of several methods. Method 1 uses the nucleofection of plasmid DNA into dendritic cells. These dendritic cells are used to present antigen to cytotoxic T cells. This is followed by the selection of cytotoxic T cells with high levels of interferon gamma (IFN-γ) secretion. This method takes 2 to 3 weeks in total. Method 2 uses overlapping peptide mixes or pepmixes of EBV nuclear antigen to stimulate PBMCs. The T cells are, then, selected and expanded in a closed system. This method takes ∼10 days to manufacture the EBV CTLs. EBV CTLs are banked and can be infused into HLA-matched recipients.
Methods for manufacturing “off-the-shelf” EBV-specific cytotoxic T cells. There are several methods for manufacturing off-the-shelf EBV-specific CTLs. This figure shows 2 common approaches. The cells are collected from a healthy donor. Peripheral blood mononuclear cells (PBMCs) are selected and stimulated using 1 of several methods. Method 1 uses the nucleofection of plasmid DNA into dendritic cells. These dendritic cells are used to present antigen to cytotoxic T cells. This is followed by the selection of cytotoxic T cells with high levels of interferon gamma (IFN-γ) secretion. This method takes 2 to 3 weeks in total. Method 2 uses overlapping peptide mixes or pepmixes of EBV nuclear antigen to stimulate PBMCs. The T cells are, then, selected and expanded in a closed system. This method takes ∼10 days to manufacture the EBV CTLs. EBV CTLs are banked and can be infused into HLA-matched recipients.
Results from EBV CTL trials in SOT recipients with PTLDs
| Study ID author (y) . | CTL type . | Number of patients . | Median age (range) . | Types of SOTs, n . | PTLD subtype, n . | EBV response . | Response rates . | Survival . | Length of follow-up . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Prockop46 (2020) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 13 | 10 y (0.7-75.2) | Kidney, 5 Heart, 3 Liver, 2 Small bowel, 1 Heart + liver, 1 | mPTLD, 8 pPTLD, 4 HL-like, 1 | Undetectable in 7 of 13 patients | ORR, 54.8% CR, 2 of 13 PR, 5 of 13 | Patients with SD 1 y survival = 81.8% Patients with PR/CR 1 y survival = 88.9% | 115 mo |
| Kazi47 (2019) | HLA-matched, third party | 20 | 31 y (1-82) | Kidney, 10 Liver, 3 Heart, 2 Small bowel, 1 Small bowel, 1 Pancreas, 1 | Not specified | Not reported | ORR, 75% CR, 10 of 20 PR, 5 of 20 | Median, 3.87 y | 6 mo | |
| Chiou48 (2018) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 11 | 40 mo (12-144) | Liver, 5 Small bowel + liver, 5 Liver + kidney, 1 | mPTLD, 7 pPTLD, 2 HL-like PTLD, 1 Unspecified, 1 | Undetectable in all patients | ORR, 73.7% CR, 7 of 11 PR, 1 of 11 | Overall, 5 y 85.7% | 14 y |
| Vickers49 (2014) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 4 | 18.5 y (1-53) | Kidney, 1 Heart, 1 Liver, 1 Heart + kidney, 1 | Not specified | Not reported | ORR, 100% CR, 4 of 4 | Not reported | Not reported |
| Gallot50 (2014) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 3 | 45 y (42-60) | Heart, 1 Heart + lung, 1 Kidney, 1 | mPTLD, 3 | Not reported | ORR, 66% CR, 1 of 3 | Not reported | Not reported |
| Haque44 (2007) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 31 | 41 y (1-76) | Kidney, 13 Liver, 10 Liver + small bowel, 3 Heart, 2 Lung, 2 Heart + lung, 1 | mPTLD, 11 pPTLD, 7 Hyperplastic PTLD, 7 HL-like PTLD, 5 Burkitt PTLD, 1 | Decreased in 10 patients | ORR, 48.4% CR, 12 of 31 PR, 3 of 31 | 2 y OS, 30% | 7.5 y |
| Gandhi51 (2007) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 3 | 52 y (18-58) | Kidney, 1 Heart + lung, 1 Lung, 1 | mPTLD, 2 pPTLD, 1 | Undetectable in 2 of 3 | ORR, 66.7% CR, 2 | 106 days | 249 d |
| Savoldo52 (2006) | HLA-matched, third party | Autologous EBV-specific CTL | 6 | 2.5 y (0-35 y) | Liver, 4 Heart, 2 | Unspecified, 4 PTLD, 2 | Decreased in 4 patients | ORR, 100% CR, 1 of 6 PR, 5 of 6 | OS, 100% | 6 mo |
| Comoli53 (2005) | HLA-matched, third party | Autologous EBV-specific CTL | 5 | 11 y (2-14) | Kidney, 5 | mPTLD, 2 pPTLD, 1 Plasmacytoma, 1 Hyperplasia PTLD. 1 | From 0 to 300 copies | ORR, 100% CR, 5 of 5 | OS, 100% | 31 mo |
| Study ID author (y) . | CTL type . | Number of patients . | Median age (range) . | Types of SOTs, n . | PTLD subtype, n . | EBV response . | Response rates . | Survival . | Length of follow-up . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Prockop46 (2020) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 13 | 10 y (0.7-75.2) | Kidney, 5 Heart, 3 Liver, 2 Small bowel, 1 Heart + liver, 1 | mPTLD, 8 pPTLD, 4 HL-like, 1 | Undetectable in 7 of 13 patients | ORR, 54.8% CR, 2 of 13 PR, 5 of 13 | Patients with SD 1 y survival = 81.8% Patients with PR/CR 1 y survival = 88.9% | 115 mo |
| Kazi47 (2019) | HLA-matched, third party | 20 | 31 y (1-82) | Kidney, 10 Liver, 3 Heart, 2 Small bowel, 1 Small bowel, 1 Pancreas, 1 | Not specified | Not reported | ORR, 75% CR, 10 of 20 PR, 5 of 20 | Median, 3.87 y | 6 mo | |
| Chiou48 (2018) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 11 | 40 mo (12-144) | Liver, 5 Small bowel + liver, 5 Liver + kidney, 1 | mPTLD, 7 pPTLD, 2 HL-like PTLD, 1 Unspecified, 1 | Undetectable in all patients | ORR, 73.7% CR, 7 of 11 PR, 1 of 11 | Overall, 5 y 85.7% | 14 y |
| Vickers49 (2014) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 4 | 18.5 y (1-53) | Kidney, 1 Heart, 1 Liver, 1 Heart + kidney, 1 | Not specified | Not reported | ORR, 100% CR, 4 of 4 | Not reported | Not reported |
| Gallot50 (2014) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 3 | 45 y (42-60) | Heart, 1 Heart + lung, 1 Kidney, 1 | mPTLD, 3 | Not reported | ORR, 66% CR, 1 of 3 | Not reported | Not reported |
| Haque44 (2007) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 31 | 41 y (1-76) | Kidney, 13 Liver, 10 Liver + small bowel, 3 Heart, 2 Lung, 2 Heart + lung, 1 | mPTLD, 11 pPTLD, 7 Hyperplastic PTLD, 7 HL-like PTLD, 5 Burkitt PTLD, 1 | Decreased in 10 patients | ORR, 48.4% CR, 12 of 31 PR, 3 of 31 | 2 y OS, 30% | 7.5 y |
| Gandhi51 (2007) | HLA-matched, third party | EBV-BLCL–sensitized EBV CTLs | 3 | 52 y (18-58) | Kidney, 1 Heart + lung, 1 Lung, 1 | mPTLD, 2 pPTLD, 1 | Undetectable in 2 of 3 | ORR, 66.7% CR, 2 | 106 days | 249 d |
| Savoldo52 (2006) | HLA-matched, third party | Autologous EBV-specific CTL | 6 | 2.5 y (0-35 y) | Liver, 4 Heart, 2 | Unspecified, 4 PTLD, 2 | Decreased in 4 patients | ORR, 100% CR, 1 of 6 PR, 5 of 6 | OS, 100% | 6 mo |
| Comoli53 (2005) | HLA-matched, third party | Autologous EBV-specific CTL | 5 | 11 y (2-14) | Kidney, 5 | mPTLD, 2 pPTLD, 1 Plasmacytoma, 1 Hyperplasia PTLD. 1 | From 0 to 300 copies | ORR, 100% CR, 5 of 5 | OS, 100% | 31 mo |
BLCL, B lymphoblastic cell line; SD, stable disease.
Other approaches for targeting EBV have been attempted by drawing the virus into the lytic cycle, in which it may be amenable to antiviral therapy. This can potentially be achieved with epigenetic therapies. In a clinical study of the histone deacetylase inhibitor nanatinostat plus valganciclovir in relapsed/refractory EBV+ lymphomas, 55 patients were enrolled, of whom 4 had PTLD.54 Across all histologies, the ORR was 40%, and CR 19%. For patients with DLCBL (N = 6), the ORR and CR rate were 67% and 33%, respectively. There was 1 CR among the patients with PTLD. The median DOR for all the patients was 10.4 months. The combination was fairly well tolerated, with side effects common to histone deacetylase inhibitors, such as nausea, constipation, and cytopenias. Epigenetic therapy could also be used to potentially increase the immunogenicity of PTLD through the induction of antigen presentation and activation and expansion of T cells, making epigenetic targeting an intriguing strategy to be used in combination with EBV-specific adoptive T-cell infusions.55
Most EBV+ PTLDs are CD30+. Although there are no studies dedicated to the use of brentuximab vedotin for relapsed PTLD, its success in the frontline setting coupled with data on its activity in other relapsed CD30+ lymphomas makes this another viable choice.56-58
Taken together, there has been substantial progress in directing therapies toward EBV targets as a means toward a chemotherapy-free approach for PTLD. Although most of these options are only available through clinical trials, they remain a cornerstone for the treatment of EBV+ relapsed disease and will likely have a future impact as preventive and frontline therapies (Table 2).
Clinical trials focusing on PTLD
| Clinical trial identifier . | Title of the study . | Role in PTLD . | Target population . |
|---|---|---|---|
| NCT03266653 | EBV-specific cytotoxic T-lymphocytes (CTLs) for refractory EBV infection | Preventive | Children and adults |
| NCT05183490 | R-MVST cells for treatment of viral infections | Preventive | Adults |
| NCT04989491 | Evaluation of the efficacy of a treatment by one single dose of rituximab (375mg/m2) in the prevention of the EBV primary infection and posttransplant lymphoproliferative disorder in adult EBV seronegative patients who received an EBV seropositive kidney allograft (REPLY) | Preventive | Adults |
| NCT04507477 | Ex-vivo delivery of rituximab to prevent PTLD in EBV mismatch lung transplant recipients: a pilot trial | Preventive | Adults |
| NCT02580539 | A study of the safety and efficacy of EBV specific T-cell lines (EBV-TCL-01) | Preventive or frontline | Adults |
| NCT02900976 | Rituximab and LMP-specific T-cells in treating pediatric solid organ recipients with EBV-positive, CD20-positive posttransplant lymphoproliferative disorder | Frontline | Children and adults |
| NCT04337827 | Rituximab and acalabrutinib in newly diagnosed B-cell posttransplant lymphoproliferative disorder | Frontline | Adults |
| NCT04554914 | A study to evaluate tabelecleucel in participants with EBV–associated diseases | Frontline | Children and adults |
| NCT05786040 | Tafasitamab and rituximab for front-line treatment of posttransplant lymphoproliferative disorder | Frontline | Adults |
| NCT01192464 | EBV CTLs expressing CD30 chimeric receptors for CD30+ lymphoma (CARCD30) | Frontline or relapsed | Children and adults |
| NCT03131934 | Immunotherapy with tacrolimus resistant EBV CTL for lymphoproliferative disease after solid organ transplant (ITREC) | Frontline or relapsed | Children and adults |
| NCT05011058 | An open-label, phase 2 trial of nanatinostat in combination with valganciclovir in patients with EBV+ relapsed/refractory lymphomas (NAVAL-1) | Relapsed | Adults |
| NCT03394365 | Tabelecleucel for solid organ or allogeneic hematopoietic cell transplant participants with EBV-associated posttransplant lymphoproliferative disease (EBV+ PTLD) after failure of rituximab or rituximab and chemotherapy (ALLELE) | Relapsed | Children and adults |
| NCT04664179 | EBV-specific T-lymphocytes for treatment of EBV+ lymphoma (CILESTE) | Relapsed | Children and adults |
| NCT04925544 | Clinical trial of a novel small molecule EBNA1 inhibitor, VK 2019, in patients with EBV+ nasopharyngeal cancer (NPC) and other EBV-associated cancers, with pharmacokinetic and pharmacodynamic correlative studies | Relapsed | Adults |
| NCT05714748 | Application of mRNA immunotherapy technology in EBV-related refractory malignant tumors | Relapsed | Adults |
| NCT02287311 | Most closely matched 3rd party rapidly generated LMP, BARF1 and EBNA1 specific CTL, EBV+ lymphoma (MABEL) | Relapsed | Children and adults |
| Clinical trial identifier . | Title of the study . | Role in PTLD . | Target population . |
|---|---|---|---|
| NCT03266653 | EBV-specific cytotoxic T-lymphocytes (CTLs) for refractory EBV infection | Preventive | Children and adults |
| NCT05183490 | R-MVST cells for treatment of viral infections | Preventive | Adults |
| NCT04989491 | Evaluation of the efficacy of a treatment by one single dose of rituximab (375mg/m2) in the prevention of the EBV primary infection and posttransplant lymphoproliferative disorder in adult EBV seronegative patients who received an EBV seropositive kidney allograft (REPLY) | Preventive | Adults |
| NCT04507477 | Ex-vivo delivery of rituximab to prevent PTLD in EBV mismatch lung transplant recipients: a pilot trial | Preventive | Adults |
| NCT02580539 | A study of the safety and efficacy of EBV specific T-cell lines (EBV-TCL-01) | Preventive or frontline | Adults |
| NCT02900976 | Rituximab and LMP-specific T-cells in treating pediatric solid organ recipients with EBV-positive, CD20-positive posttransplant lymphoproliferative disorder | Frontline | Children and adults |
| NCT04337827 | Rituximab and acalabrutinib in newly diagnosed B-cell posttransplant lymphoproliferative disorder | Frontline | Adults |
| NCT04554914 | A study to evaluate tabelecleucel in participants with EBV–associated diseases | Frontline | Children and adults |
| NCT05786040 | Tafasitamab and rituximab for front-line treatment of posttransplant lymphoproliferative disorder | Frontline | Adults |
| NCT01192464 | EBV CTLs expressing CD30 chimeric receptors for CD30+ lymphoma (CARCD30) | Frontline or relapsed | Children and adults |
| NCT03131934 | Immunotherapy with tacrolimus resistant EBV CTL for lymphoproliferative disease after solid organ transplant (ITREC) | Frontline or relapsed | Children and adults |
| NCT05011058 | An open-label, phase 2 trial of nanatinostat in combination with valganciclovir in patients with EBV+ relapsed/refractory lymphomas (NAVAL-1) | Relapsed | Adults |
| NCT03394365 | Tabelecleucel for solid organ or allogeneic hematopoietic cell transplant participants with EBV-associated posttransplant lymphoproliferative disease (EBV+ PTLD) after failure of rituximab or rituximab and chemotherapy (ALLELE) | Relapsed | Children and adults |
| NCT04664179 | EBV-specific T-lymphocytes for treatment of EBV+ lymphoma (CILESTE) | Relapsed | Children and adults |
| NCT04925544 | Clinical trial of a novel small molecule EBNA1 inhibitor, VK 2019, in patients with EBV+ nasopharyngeal cancer (NPC) and other EBV-associated cancers, with pharmacokinetic and pharmacodynamic correlative studies | Relapsed | Adults |
| NCT05714748 | Application of mRNA immunotherapy technology in EBV-related refractory malignant tumors | Relapsed | Adults |
| NCT02287311 | Most closely matched 3rd party rapidly generated LMP, BARF1 and EBNA1 specific CTL, EBV+ lymphoma (MABEL) | Relapsed | Children and adults |
R-MSVT, rapidly generated multivirus-specific T cells.
Case 3: relapsed EBV– PTLD
A 23-year-old female was diagnosed with EBV– mPTLD with plasmablastoid features, CD20+, and CD30– in her jejunum after presenting with abdominal pain.59 She underwent a heart transplant at 11 months for congenital restrictive cardiomyopathy. She and the donor had an EBV– status. At the age of 20 years, she underwent a kidney transplantation from a living–related donor with EBV+ status. She was managed with tacrolimus, mycophenolic acid, and low-dose prednisone until the diagnosis of PTLD, the time point at which mycophenolic acid was stopped. The patient was then treated with R-EPOCH. Vincristine was held for cycles 1, 2, and 4, given the full-thickness bowel involvement and symptoms concerning for obstruction. Cyclophosphamide was dosed according to the AIDS malignancy consortium protocol.29 All other components, including doxorubicin, were administered at full dose. An interim scan after cycle 4 revealed persistent disease that was confirmed through a biopsy specimen analysis. The patient received gemcitabine, carboplatin, and obinutuzumab but developed grade 4 thrombocytopenia and Escherichia coli bacteremia and ultimately experienced progressive disease. She received polatuzumab vedotin for 1 cycle, followed by a small bowel resection for residual disease. The patient was maintained on tacrolimus with a goal of 4 to 6 ng/mL and prednisone 5 mg daily, and T cells were collected for chimeric antigen receptor T-cell (CAR-T) manufacturing. She received lisocabtagene maraleucel and remained on low-dose IS. She tolerated the therapy without any negative sequelae and was discharged on day +10. She has remained in CR upon PET >15 months later.
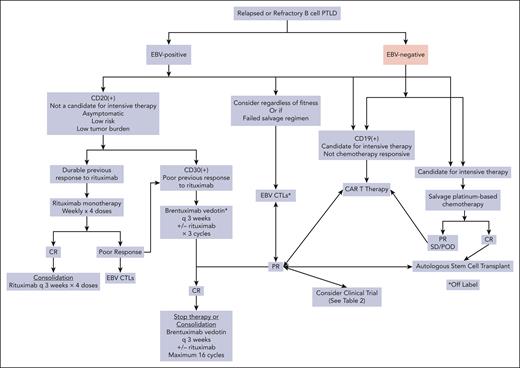
There is little guidance for the treatment of relapsed or refractory EBV– CD30– mPTLD (Figure 3). Depending on the clinical scenario, low-intensity therapy may be preferred. Studies have been performed to evaluate the role of rituximab monotherapy after chemotherapy failure. In a small study evaluating 8 patients (5 of whom had not received rituximab as first-line), the median PFS after rituximab salvage therapy was 9 months, and the ORR was 50%. Only 1 patient achieved long-term disease control.60
Algorithm for management of relapsed/refractory PTLD. POD, progression of disease.
Algorithm for management of relapsed/refractory PTLD. POD, progression of disease.
Considering the more intensive and durable treatment options, the National Comprehensive Cancer Network guidelines suggest following the standard algorithm for relapsed DLBCL, such as salvage therapy followed by autologous SCT (ASCT). There is a paucity of data on the outcome of ASCT in patients with SOT. In a review of 3645 cases of PTLD from 49 health care organizations, only 9.7% had ASCT included as part of their treatment description.61 The European Society for Blood and Marrow Transplantation performed a retrospective review focusing on the outcomes of those with SOT-PTLD who received an ASCT between 2001 and 2017. Twenty-one patients were identified after kidney SOT (N = 10), liver (N = 5), and lung, heart, and kidney-pancreas (N = 2 each). Several patients had more than 1 SOT. The median age at ASCT was 47 years. Only 3% had EBV+ PTLD, and the representative subtypes were mPTLD (N = 14), plasmacytoma-like (N = 3), T-cell (N = 2), Burkitt-like (N = 1), and pPTLD (N = 1). Most patients received carmustine, etoposide, cytarabine, and melphalan or a high-dose melphalan conditioning regimen, and 56% continued receiving IS before ASCT. Engraftment of neutrophils occurred at a median of 10.5 days, and that of platelets occurred at 13 days. With a median follow-up of 64 months, there were 4 TRM events, and an additional 8 patients died. The 1-year nonrelapse mortality was 24%. The 3-year PFS and OS were 62% and 61%, respectively. Three patients required a second SOT after ASCT.62 This retrospective review demonstrates that ASCT is feasible; however, it must be conducted with rigorous patient selection and active participation from the entire multidisciplinary team caring for the patient.
CAR-T has gained acceptance as a second-line therapy for early relapsing DLBCL.63,64 Its use in the setting of PTLD is met with many challenges; however, its potential for positive outcomes has been recognized. Most of the literature regarding this modality in PTLD are limited to case reports, with a total of 41 cases through 2023, demonstrating the limited experience.59,65-70
Portuguese et al performed a systematic review of the literature identifying 17 patients who had clinical data regarding the outcome of CAR-T for PTLD.66 Twelve of these patients had kidney, liver, heart (N = 2 for each), or kidney/pancreas (N = 1) SOT. EBV-mPTLD accounted for 14 cases, and EBV+ Burkitt PTLD accounted for 2 of them. The median age was 46 years, and the median time from transplant to CAR-T was 10 years. The products used included axicabtagene ciloleucel (N = 11), tisagenlecleucel (N = 3), lisocabtagene maraleucel (N = 1), and investigational CAR-T (N = 2). Although 88.2% of patients experienced cytokine release syndrome (CRS), only 5.9% had grade 3 CRS. Likewise, 41.2% of the patients experienced immune effector cell–associated neurotoxicity, with 23.5% having grade 3 and 5.9% having grade 4. The ORR was 82.4%, with a CR rate of 58.8% and progressive disease experienced in 11.8%. The median DOR was short at 6.5 months. In those who achieved CR, the DOR was 7.7 months, with 50% experiencing a recurrence. Allograft rejection occurred in 23.5%, but none of these led to graft failure or rejection-related death.
In a multicenter retrospective study evaluating CAR-T for relapsed/refractory SOT-PTLD, 22 patients were identified.67 SOT included kidney (N = 14), liver (N = 3), heart (N = 2), and intestinal, lung, and kidney followed by pancreas (N = 1 each). The median time from SOT to PTLD was 107 months. All had mPTLD, with 1 having high-grade B-cell lymphoma and 1 having mantle cell lymphoma; 82% had EBV– status. IS was interrupted in 64% before CAR-T infusion. Axicabtagene ciloleucel was used for 17 patients, tisagenlecleucel for 4, and brexucabtagene autoleucel for 1 patient. CRS was experienced in 18 of the 22 patients, with only 1 experiencing grade 3 and 1 grade 4 CRS. Immune effector cell–associated neurotoxicity was experienced in 16 patients, with 6 having grade 3 and 3 grade 4 neurotoxicity. The ORR was 64%, with a CR rate of 55%. There were 2 TRM events. The 2-year PFS and OS were 35% and 58%, respectively. Three patients experienced graft rejection.
Limitations remain in the overall DOR, which may be shortened by the need to continue IS, potentially limiting the durability and persistence of CAR-Ts. Limiting steroids before collection and reducing disease burden before infusion may improve outcomes. Careful consideration of the choice of product is also advised. A prospective, multicenter study that specifically evaluates this patient population is of great importance. In addition, emerging strategies to enhance the effectiveness of CAR-Ts in the setting of PR using bispecific antibodies (NCT05633615) and targeted treatments could potentially further improve outcomes.71
Rare subtypes
The cases discussed represent the majority of PTLD variants; however, less common presentations and pathologic varieties should be approached according to their nonposttransplant counterparts (Table 3).
Management of rare subtypes of PTLD
| PTLD subtype . | Associated pathology . | Treatment options . | Additional considerations . |
|---|---|---|---|
| Hodgkin PTLD | Most are EBV-related Large Reed Sternberg cells may be positive for CD20 and CD79a; however CD15 is frequently negative |
| Worse prognosis than non-SOT–related cHL Use of checkpoint blockade associated with organ rejection and death (use with extreme caution) |
| Primary CNS lymphoma | May present as mPTLD or pPTLD, and these entities do not correlate with prognosis Three subtypes:
| Rituximab plus high-dose methotrexate (>1.5 g/m2) Rituximab plus high-dose cytarabine (1 g/m2) Whole brain radiotherapy | Occurs most frequently after kidney SOT Kidney transplant is not an absolute contraindication for methotrexate |
| Plasmablastoid DLBCL | 100% MUM1/IRF4+ 82% CD138+ 64% CD30+ 55% EBER+ Most have MYC and chromosome 17/TP53 derangements | In addition to treatment paradigm outlined in Figure 1, the addition of these may be considered:
| Occasionally occurs after nonplasmablastoid PTLD |
| T-cell PTLD | Can present as any of the mature T-cell lymphoma subtypes Most common subtypes:
| Treat based on recommendations for each disease entity | Rare, ∼5% Often occurs as late event |
| PTLD subtype . | Associated pathology . | Treatment options . | Additional considerations . |
|---|---|---|---|
| Hodgkin PTLD | Most are EBV-related Large Reed Sternberg cells may be positive for CD20 and CD79a; however CD15 is frequently negative |
| Worse prognosis than non-SOT–related cHL Use of checkpoint blockade associated with organ rejection and death (use with extreme caution) |
| Primary CNS lymphoma | May present as mPTLD or pPTLD, and these entities do not correlate with prognosis Three subtypes:
| Rituximab plus high-dose methotrexate (>1.5 g/m2) Rituximab plus high-dose cytarabine (1 g/m2) Whole brain radiotherapy | Occurs most frequently after kidney SOT Kidney transplant is not an absolute contraindication for methotrexate |
| Plasmablastoid DLBCL | 100% MUM1/IRF4+ 82% CD138+ 64% CD30+ 55% EBER+ Most have MYC and chromosome 17/TP53 derangements | In addition to treatment paradigm outlined in Figure 1, the addition of these may be considered:
| Occasionally occurs after nonplasmablastoid PTLD |
| T-cell PTLD | Can present as any of the mature T-cell lymphoma subtypes Most common subtypes:
| Treat based on recommendations for each disease entity | Rare, ∼5% Often occurs as late event |
ALCL, anaplastic large cell lymphoma; ALK-ALCL, ALK negative anaplastic large cell lymphoma; cHL, classical Hodgkin lymphoma; EBER, EBV-encoded RNA; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified; RAS, rat sarcoma.
Although rare, primary CNS PTLD occurs most frequently after kidney SOT. A study published 1 decade ago demonstrated an ORR of only 60%, with 13 TRM events and a 3-year OS rate of 43%.72 However, less than half of the patients in this study received rituximab therapy. In a recent report of 24 patients, the median time from transplant to PTLD CNS was 8.1 years, and 21 of the 24 patients had CNS-only disease. Interestingly, 6 of these cases were pPTLD, underscoring that morphology alone can be misleading when considering the aggressiveness of the disease. The patients were treated with combinations of steroids, rituximab, and high-dose methotrexate as well as radiotherapy. It is important to note that kidney SOT is not an absolute contraindication for the use of high-dose methotrexate. The 5-year OS was 67.5%. The investigators also performed targeted next-generation DNA sequencing and genome-wide copy number analysis to identify 3 distinct subtypes with associated clinical features.73 Sporadic primary central nervous system (PCNS) LBCL–like type (N = 1) demonstrated MYD88 and CD79B mutations similar to those observed in PCNS lymphoma not related to SOT. The systemic rat sarcoma (RAS)–driven type (N = 3) demonstrated extra-CNS involvement and genetic features similar to systemic PTLD. Finally, the EBV-driven, CNS-limited subtype (N = 20) showed no oncogenic alterations. Across all 3 subtypes, Ki67 levels < 80% showed the best correlation with survival.
Plasmablastoid lymphomas are usually present in the context of immune dysregulation, and although they account for 6% to 7% of PTLDs, many PTLDs have a morphology resembling that of plasma cells.74,75 In a case series at Columbia University from 2002 to 2019, 18 samples were identified from 11 patients and analyzed to characterize the molecular features and immunophenotype.76 All samples were MUM1/IRF4+, and 82% were CD138+; variable CD30 expression was noted in 64%, and EBV-encoded RNA (EBER+) in 55%. Three cases had prior nonplasmablastoid PTLD. Most cases had derangements in MYC and chromosome 17/TP53. Mutations were common in epigenetic modifier, DNA damage response, and repair pathway genes. The efficacy of bortezomib has been trialed for this disease, given the similarities to plasma cell disorders, with some success.77,78 At Columbia University, we have also observed success with daratumumab targeting CD38 when combined with chemotherapy.79
T-cell PTLDs represent 5% of all PTLDs and tend to present as a late event after SOT.80,81 They can present as any of the mature T-cell lymphoma subtypes; however, in a meta-analysis, the most frequent subtypes included hepatosplenic T-cell lymphoma, primary cutaneous anaplastic large-cell lymphoma (ALCL), peripheral T-cell lymphoma-not otherwise specified, and ALK negative anaplastic large cell lymphoma. EBV can infect T cells or B cells, which then induce a brisk T-cell immune response. In general, these lymphomas have inferior outcomes, and the use of targeted therapies such as brentuximab vedotin and clinical trials should be considered.
HL-PTLD has a worse prognosis than HL in the general population. In a study that compared HL-PTLD with HL in the surveillance epidemiology and end results program (SEER) registry, the 5-year OS for patients with HL-PTLD was 57% compared with 80% for patients with HL. Outcomes were worse in patients who did not receive chemotherapy (ie, RIS alone) or nontraditional HL regimens.82 The incorporation of brentuximab vedotin into frontline HL has improved outcomes, and it is likely that this will translate into improved survival in HL-PTLD. Checkpoint blockade has also been very successful in treating classical Hodgkin lymphoma; however, in a review of 119 reported cases of immune checkpoint inhibitor use for post-SOT malignancies, rejection occurred in 41.2%, graft failure in 23.5%, and immune-related adverse events in 18.5%. Graft failure was the cause of death in 24%.83 With this in mind, caution must be taken when considering this treatment modality in the posttransplant setting.
Conclusions
As organ transplantations increase, so do PTLDs, and consequently there is a pressing need for large, multi-institution prospective clinical trials for this disease entity. Traditionally, PTLD has been a diagnosis excluded from clinical trials because of concerns regarding poor performance status and organ function, drug-drug interactions, potential of toxicity due to IS, and disease heterogeneity. There are exciting advances, however, as we see deepened experiences of the incorporation of novel therapies that are beginning to shape our practice. In addition, ground-breaking work is underway using advanced technologies such as cell-free DNA hybrid capture to characterize the tumor microenvironment and virome in an effort to better understand biology.84 This type of work will undoubtedly lead to better, biologically based risk analysis and treatment strategies for this rare yet challenging disease.
Acknowledgments
The authors thank Ran Reshef for his dedication and contributions to PTLD research, collaboration in the care of these patients, and critical review and editing of this manuscript. In addition, the authors acknowledge Govind Bhagat for his dedication to and research in PTLD, and Hua-Jay Cherng for his critical review and editing of this manuscript.
This work was supported by the Herbert Irving Scholar Program UL1 TR001873 (J.E.A.) and The Esther and Oded Aboodi Lymphoma Research Fund (J.E.A.).
Authorship
Contribution: B.P. and J.E.A. wrote the manuscript.
Conflict-of-interest disclosure: B.P. receives honoraria from Takeda and Seagen. J.E.A. is a member of the advisory board of AstraZeneca.
Correspondence: Jennifer E. Amengual, Division of Hematology and Oncology, Herbert Irving Comprehensive Cancer Center, Columbia University, 161 Fort Washington Ave, Garden Level, New York, NY 10032; e-mail: jea2149@columbia.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal