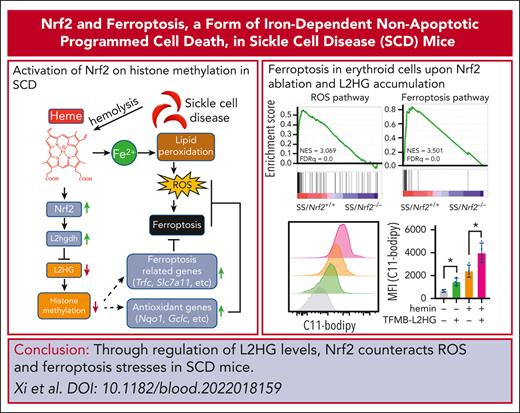
Key Points
Nrf2 regulates L2HG for histone methylation modification in SCD.
Nrf2 ablation and L2HG accumulation sensitize erythroid cells to ferroptosis.
Abstract
Sickle cell disease (SCD) is a chronic hemolytic and systemic hypoxia condition with constant oxidative stress and significant metabolic alterations. However, little is known about the correlation between metabolic alterations and the pathophysiological symptoms. Here, we report that Nrf2, a master regulator of cellular antioxidant responses, regulates the production of the metabolite l-2-hydroxyglutarate (L2HG) to mediate epigenetic histone hypermethylation for gene expression involved in metabolic, oxidative, and ferroptotic stress responses in SCD. Mechanistically, Nrf2 was found to regulate the expression of L2HG dehydrogenase (L2hgdh) to mediate L2HG production under hypoxia. Gene expression profile analysis indicated that reactive oxygen species (ROS) and ferroptosis responses were the most significantly affected signaling pathways after Nrf2 ablation in SCD. Nrf2 silencing and L2HG supplementation sensitize human sickle erythroid cells to ROS and ferroptosis stress. The absence of Nrf2 and accumulation of L2HG significantly affect histone methylation for chromatin structure modification and reduce the assembly of transcription complexes on downstream target genes to regulate ROS and ferroptosis responses. Furthermore, pharmacological activation of Nrf2 was found to have protective effects against ROS and ferroptosis stress in SCD mice. Our data suggest a novel mechanism by which Nrf2 regulates L2HG levels to mediate SCD severity through ROS and ferroptosis stress responses, suggesting that targeting Nrf2 is a viable therapeutic strategy for ameliorating SCD symptoms.
Introduction
Sickle cell disease (SCD) is a complicated pathophysiological condition involving chronic inflammation originating from hemolysis, elevated oxidative stress, and systemic hypoxia. Oxidative stress is generated by multiple mechanisms, including inflammation, hypoxia/reperfusion injury, and chronic hemolysis.1,2 Ferroptosis, which is characterized by the accumulation of lipid peroxides, is a recently defined form of iron-dependent nonapoptotic programmed cell death.3 Recent studies have demonstrated that ferroptosis is involved in various pathophysiological disorders such as cancer.4 In SCD and thalassemia, there are strong correlations among ferroptosis, iron overload, and heme dysregulation after chronic transfusion.5,6 However, the underlying mechanisms are not yet fully understood.
The transcription factor Nrf2 is a master regulator of cellular oxidative stress response and controls the expression of antioxidant genes by binding to the antioxidant response element (ARE) for gene regulation.7,8 Previous studies have shown that Nrf2 modulates the susceptibility of cells to chemical toxicants and is associated with disease conditions.9,10 We recently established an Nrf2 knockout SCD mouse model, in which an exacerbated SCD phenotype was observed.11 Notably, Nrf2 has been shown to recruit both DNA and histone modifiers to affect DNA and histone modifications around ARE motifs to permit transcription.8,12-16 Nrf2 also participates in the assembly of mediator complexes for transcription preinitiation.17,18 Thus, the binding of Nrf2 to ARE motifs may affect epigenetic modifications to trigger a cascade of events ultimately inducing the expression of antioxidant genes. However, such an Nrf2-mediated chromatin modification mechanism is largely unknown.
Importantly, oxidative stress, hypoxia, and metabolic programming are usually associated with epigenetic modifications.19-21 Cells exposed to oxidative stress exhibit histone hypermethylation, whereas hypoxia causes the hypermethylation of both DNA and histones in Hif1a-dependent and independent manners.22,23 Although SCD bears systemic hypoxia, whether hypoxia affects histone methylation remains unclear. In addition, the metabolic demand of patients with SCD are increased because of chronic hemolysis.24-26 Recent findings indicate that altered metabolic programming for 2-oxoglutarate (2OG), fumarate, and succinate is accommodated in hematopoietic stem cell development,27,28 erythroid commitment,28 and oxidative stress in SCD.29,30 The metabolite 2OG is an essential cofactor for 2OG-dependent dioxygenases, such as the ten-eleven translocation family of DNA hydroxylases and JmjC domain–containing histone lysine demethylases (KDMs),31 whereas fumarate and succinate compete for 2OG regulatory signaling.32 Thus, a connection presumably exists between aberrant metabolic programming and epigenetic DNA/histone modifications in SCD; however, the underlying molecular mechanism remains unknown. Recently, Nrf2 and its competitor Bach1 have been shown to rewire the metabolic programming of 2OG under malignant conditions.33,34 However, whether Nrf2 regulates gene expression through 2OG reprogramming and epigenetic histone methylation modifications under conditions such as SCD has not yet been demonstrated. In this study, we determined that Nrf2 regulates the generation of l-2-hydroxyglutarate (L2HG) to affect histone methylation and regulate genes involved in reactive oxygen species (ROS) and the ferroptosis stress response. Our data support that Nrf2 activation is a viable therapeutic strategy for the treatment of SCD.
Materials and methods
Animals
Mouse spleen and bone marrow erythroid cells
Splenic and bone marrow erythroid cells from SCD mice were examined for Ter119 and CD71 expression and analyzed via flow cytometry, as previously reported.36
Human erythroid progenitors
Erythroid progenitors (EPs) from patients with SCD were generated using peripheral blood mononuclear cells (PBMNs), followed by CD34+ cell selection. The anonymous collection of discarded blood samples from children with SCD undergoing chronic transfusion was classified as exempted by the institutional review board at Augusta University and did not require informed consent. Human EPs from healthy donors were obtained from AllCells (Alameda, CA). EPs from both patients with SCD and healthy donors were cultured in a modified Fibach 2-phase liquid culture system.37 Briefly, during phase 1, cells were grown in Iscove modified Dulbecco medium with 15% fetal bovine serum, 15% human AB serum, 10 ng/mL interleukin-3, 50 ng/mL stem cell factor and 2 IU/mL erythropoietin (Sigma-Aldrich, St Louis, MO). Phase 2 was initiated on day 7 using a similar medium without stem cell factor or interleukin-3. On day 12 of culture, EPs from patients with SCD and healthy individuals were collected for erythropoiesis based on the expression of CD71, CD235a, Band3, and CD49d using a previously established strategy.38
NRF2 silencing in EPs from patients with SCD
On day 4 of culture, EPs from patients with SCD were transduced with lentiviral shNRF2 particles and selected with puromycin until analysis.37 The short hairpin RNA (shRNA) silencing effect was determined via RNA and immunoblotting analyses.
Gene expression analyses
Whole-genome RNA sequencing (RNA-seq) using spleen Ter119+ cell total RNA from SS/Nrf2+/+ and SS/Nrf2–/– mice at 2-3-month of age was performed and differential expression for gene set enrichment analysis was performed. Differentially expressed genes using DESeq2 with a q value < 0.05 were considered to be significantly differentially expressed.
Quantitative real time-PCR and western blotting
RNA isolation, complementary DNA synthesis, and quantitative polymerase chain reaction (PCR) were performed as previously described,11 using gene-specific primers (supplemental Table 1, available on the Blood website). Whole cell or histone extracts were prepared and analyzed using the indicated antibodies (supplemental Table 2).
ChIP-seq and ChIP-PCR
Bone marrow Ter119+ cells of SS/Nrf2+/+ and SS/Nrf2–/– mice were collected with Ter119+ microbeads (Miltenyi) for the chromatin immunoprecipitation sequencing (ChIP-seq) experiment using a ChIP-IT High sensitivity Kit (Active Motif) with modifications. Chromatin was sonicated using Bioruptor (Diagenode Diagnostics) from 200 to 1000 bp and immunoprecipitated with H3K27Me3 antibody (Millipore). The ChIP-seq DNA library was prepared using the TruSeq ChIP Sample Preparation kit (Illumina) and sequenced at 75bp single-reads with an average depth of 35 million reads on a NextSeq 500 Illumina machine. ChIP sequencing reads were mapped to the reference mouse genome (mm9) using Bowtie2 and analyzed using MACS2 for peak calling.
To determine the effect of L2HG on NRF2 binding, 2 different sources of EPs from patients with SCD were cultured on day 10 and treated with cell-permeable L2HG or phosphate-buffered saline under hypoxia (1% O2) and oxidative stress (200 μM H2O2) for 2 days, and on day 12, the genome-wide association of NRF2 were determined via ChIP-seq. ChIP-seq reads were mapped to the reference human genome (GRCh37/hg19) using Bowtie2 and analyzed using MACS2 for peak calling.
ChIP-PCR was performed39 using the indicated antibodies (supplemental Table 2) and gene-specific primers (supplemental Table 1). Normal rabbit immunoglobulin G was used as a nonspecific antibody control.
Quantification of metabolites and 2HG enantiomers
Statistical analysis
Data from at least 3 biological replicates are reported as mean ± SD. Statistical differences were determined using an unpaired Student t test or two-way analysis of variance with corresponding 2-tailed significance (P value). Statistical analysis was performed using GraphPad Prism 9 software (GraphPad Software Inc, San Diego, CA), with normality test performed using Shapiro-Wilk test, and multiple comparison performed using Bonferroni multiple comparison test. Differences were considered significant when P < .05 (∗P < .05; ∗∗P < .01; and ∗∗∗P < .001).
See supplemental Materials for additional methods.
Results
Nrf2 modulates histone methylation in SCD
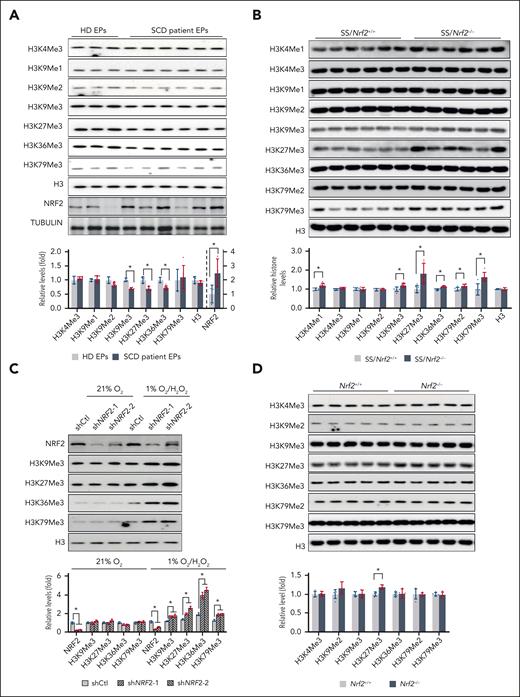
Previously, the expression of Nrf2, a master regulator of the ROS stress response, and its downstream targets was found to be activated in SCD (supplemental Figure 1).42,43 We also recently demonstrated that ablation of Nrf2 exacerbated symptoms in SCD mice.11 Notably, histone methylation is mediated by different enzymes and is affected by specific physiological and pathophysiological conditions such as oxidative stress. To determine whether epigenetic modifications, especially histone methylation, might be involved in the mechanism of NRF2-mediated gene expression and disease severity in SCD, we assessed the levels of histone methylation and NRF2 expression in EPs of healthy people and patients with SCD. In 12-day cultured EPs of healthy people and patients with SCD (supplemental Figure 2), we observed reduced histone methylation modifications, whereas NRF2 expression was significantly increased in EPs from patients with SCD compared with those of healthy people (Figure 1A). Therefore, Nrf2 may participate in the regulation of histone methylation in SCD for disease severity. To evaluate this, we first examined whether Nrf2 deletion affects erythropoiesis in SCD mice using the criteria described previously (supplemental Figure 3A-B).36 Compared with SS/Nrf2+/+ mice, SS/Nrf2–/– mice showed evidence of increased early basophilic erythroblast populations (EryA, CD71highTer119highFSChigh) in both the spleen and bone marrow (supplemental Figure 3C), suggesting that ineffective erythropoiesis in SCD mice was more severe in the absence of Nrf2.
Nrf2 expression affects histone methylation in SCD. (A) Histone methylation and NRF2 levels in EPs from HD and patients with SCD. EPs from HDs and patients with SCD were cultured in a modified Fibach 2-phase culture system, and on day 12 of culture, they were determined using the indicated histone and NRF2 antibodies. (B) Histone methylation in the histone extracts of spleen Ter119+ cells from SS/Nrf2+/+ and SS/Nrf2–/– mice. (C) Histone methylation in 12-day EPs from patients with SCD cultured under normoxia (21% O2), treated with H2O2 (200 μM) and hypoxia (1% O2), H2O2. (D) Histone methylations in non-SCD C57BL/6 mice were determined using the indicated antibodies in the spleen Ter119+ cell histone extracts of adult Nrf2+/+ and Nrf2–/– mice. Total histone H3 was used as a loading control. Data represent mean ± SD; n = 3 to 6. ∗P < .05. HD, healthy donor.
Nrf2 expression affects histone methylation in SCD. (A) Histone methylation and NRF2 levels in EPs from HD and patients with SCD. EPs from HDs and patients with SCD were cultured in a modified Fibach 2-phase culture system, and on day 12 of culture, they were determined using the indicated histone and NRF2 antibodies. (B) Histone methylation in the histone extracts of spleen Ter119+ cells from SS/Nrf2+/+ and SS/Nrf2–/– mice. (C) Histone methylation in 12-day EPs from patients with SCD cultured under normoxia (21% O2), treated with H2O2 (200 μM) and hypoxia (1% O2), H2O2. (D) Histone methylations in non-SCD C57BL/6 mice were determined using the indicated antibodies in the spleen Ter119+ cell histone extracts of adult Nrf2+/+ and Nrf2–/– mice. Total histone H3 was used as a loading control. Data represent mean ± SD; n = 3 to 6. ∗P < .05. HD, healthy donor.
Next, we determined histone methylation modifications in the adult spleen and bone marrow erythroid cells of SS/Nrf2+/+ and SS/Nrf2–/– mice. Interestingly, the levels of H3K4Me1, H3K9Me3, H3K27Me3, and H3K79Me3 were significantly higher in the spleen Ter119+ cells of SS/Nrf2–/– mice than in the spleen Ter119+ cells of SS/Nrf2+/+ mice, whereas H3K4Me3, H3K9Me1, H3K9Me2, H3K36Me3, and H3K79Me2 remained at comparable levels in both genotypes (Figure 1B). Similarly, in SCD mouse bone marrow Ter119+ erythroid cells, Nrf2 ablation increased the histone methylation of H3K4Me1, H3K9Me3, H3K27Me3, and H3K79Me3 (supplemental Figure 4).
To further validate that NRF2 regulates histone methylation, we determined the levels of H3K9Me3 and H3K27me3 in NRF2-silenced EPs from patients with SCD on day 12 of culture. Surprisingly, we did not detect any changes in histone methylation under normal air culture conditions (Figure 1C). Notably, SCD bore oxidative stress and some levels of hypoxia,44,45 and recent studies have shown that hypoxia is critical for histone hypermethylation.22 To assess whether Nrf2-mediated histone methylation is oxygen content–dependent, EPs from patients with SCD were cultured in hypoxia (1% O2) with H2O2 (200 μM) treatment. Compared with control shRNA, shNRF2 increased H3K9Me3 and H3K27Me3 levels under hypoxic and oxidative stress conditions (Figure 1C) and affected the expression of erythropoiesis surface markers CD71, CD235a, and CD49d (supplemental Figure 5), indicating a role for NRF2 in erythropoiesis under hypoxia and oxidative stress. In contrast, compared with wild-type mice, Nrf2 knockout nonsickled C57BL/6 mice showed increased levels of H3K27Me3, but no other histone methylation modifications, in spleen Ter119+ cells (Figure 1D). Together, these data suggest that Nrf2-mediated histone methylation requires a condition of hypoxia and oxidative stress in SCD.
Nrf2 ablation affects L2HG production to induce histone hypermethylation
To reveal the mechanism by which Nrf2 ablation affects histone methylation, we assessed the factor(s) involved in methylation modifications, including the methyl donor S-adenosyl-methionine, and the expression of methyltransferases or demethylases. No difference was observed in S-adenosyl-methionine levels (Figure 2A) or the expression of methyltransferases/demethylases, except for Kdm6a, between SS/Nrf2+/+ and SS/Nrf2–/– mouse spleen Ter119+ cells (Figure 2B; supplemental Figure 6). Kdm6a specifically demethylates the methyl group from H3K27Me3 but not from other histone methylations.46 Therefore, other unappreciated factors might contribute to the aforementioned global histone methylation changes after Nrf2 ablation.
Nrf2 regulates L2HG production to affect histone methylation. (A) S-adenosyl methionine levels in spleen Ter119+ cells from SS/Nrf2+/+ and SS/Nrf2–/– mice. (B) Quantitative PCR determined the expression of selected core histone methylases and demethylases in spleen Ter119+ cells from SS/Nrf2+/+ and SS/Nrf2–/– mice. (C) GC-MS analysis of metabolite levels in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens (top 25 metabolites with differences are presented). (D) Relative levels of 2HG and 2OG in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens. (E-F) Levels of L2HG and D2HG (E) and their ratios with respect to 2OG (F) in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens after normalization to protein content. (G) Effect of cell-permeable 2OG (DMOG), D2HG (octyl-D-2HG) and L2HG (TFMB-L2HG) on H3K9Me3 and H3K27Me3 levels in EPs from patients with SCD. EPs were cultured for 12 days with H2O2 (200 μM)/hypoxia (1% O2) and treated with the indicated concentrations of DMOG, octyl-D-2HG, or TFMB-L2HG, and histone methylation levels were determined in the histone extracts. (H) Total 2HG, D2HG, and L2HG levels in PBMNs from HD and patients with SCD. Data represent mean ± SD (n = 6) (A-F,H) or 3 biological replicates (G). ∗P < .05. DMOG, dimethyl 2OG; TFMB-L2HG, trifluoromethylbenzyl ester derivative of L2HG.
Nrf2 regulates L2HG production to affect histone methylation. (A) S-adenosyl methionine levels in spleen Ter119+ cells from SS/Nrf2+/+ and SS/Nrf2–/– mice. (B) Quantitative PCR determined the expression of selected core histone methylases and demethylases in spleen Ter119+ cells from SS/Nrf2+/+ and SS/Nrf2–/– mice. (C) GC-MS analysis of metabolite levels in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens (top 25 metabolites with differences are presented). (D) Relative levels of 2HG and 2OG in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens. (E-F) Levels of L2HG and D2HG (E) and their ratios with respect to 2OG (F) in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens after normalization to protein content. (G) Effect of cell-permeable 2OG (DMOG), D2HG (octyl-D-2HG) and L2HG (TFMB-L2HG) on H3K9Me3 and H3K27Me3 levels in EPs from patients with SCD. EPs were cultured for 12 days with H2O2 (200 μM)/hypoxia (1% O2) and treated with the indicated concentrations of DMOG, octyl-D-2HG, or TFMB-L2HG, and histone methylation levels were determined in the histone extracts. (H) Total 2HG, D2HG, and L2HG levels in PBMNs from HD and patients with SCD. Data represent mean ± SD (n = 6) (A-F,H) or 3 biological replicates (G). ∗P < .05. DMOG, dimethyl 2OG; TFMB-L2HG, trifluoromethylbenzyl ester derivative of L2HG.
Importantly, most histone demethylases belong to the 2OG-dependent dioxygenases,31 whose enzymatic activities are regulated by metabolites such as succinate, fumarate, and 2HG.23,32 To assess whether Nrf2 regulates histone methylation through these metabolites, we determined their levels in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens using GC-MS. Thirty-eight metabolites exhibited significant changes between SS/Nrf2+/+ and SS/Nrf2–/– mouse spleen (Figure 2C; supplemental Table 3). Interestingly, the most increased metabolite was 2HG, whereas 2OG remained at similar levels after Nrf2 ablation (Figure 2D). Thus, Nrf2 ablation causes an increased 2HG/2OG ratio, which may exert a regulatory effect on histone methylation in SCD.
There are 2 enantiomers of 2HG, D-2-hydroxyglutarate (D2HG) and L2HG, which are indistinguishable in standard GC-MS assays.47 Therefore, chiral derivation followed by liquid chromatography MS analysis was used to determine their levels in SCD mice (supplemental Figure 7A). We observed that Nrf2 ablation preferentially induces the levels of L2HG in the spleen and bone marrow Ter119+ cells of SCD mice, causing a significantly increased ratio of L2HG/2OG (Figure 2E-F; supplemental Figure 7B). To affirm that the elevated levels of L2HG will alter histone methylation, we treated EPs from patients with SCD with cell membrane-permeable D2HG, L2HG, or 2OG under oxidative stress (200 μM H2O2) and hypoxic (1% O2) conditions. L2HG and D2HG treatment significantly increased whereas 2OG decreased H3K9Me3 and H3K27Me3 levels (Figure 2G), and L2HG had more profound effects on histone methylation than D2HG.
To confirm the correlation between NRF2 expression and L2HG levels in patients with SCD, we determined L2HG levels in PBMNs of healthy donor and patients with SCD and detected that the levels of L2HG, but not D2HG, were significantly higher in PBMNs from patients with SCD than in healthy donors (Figure 2H). Together, these data suggest that Nrf2 expression correlates with the levels of L2HG but not D2HG to modulate histone methylation in SCD.
Nrf2 modulates L2HG levels through L2hgdh
Next, we determined the metabolic route by which NRF2 regulates L2HG levels by performing 13C-labeling experiments with uniformly labeled U-13C6-glucose and U-13C5-glutamine in 12-day cultured EPs from patients with SCD under hypoxia/oxidative stress (1% O2/200 μM H2O2). By examining the isotopomer distribution, we found significantly higher levels of U-13C5 glutamine-derived trichloroacetic acid cycle intermediates and 2HG compared with U-13C6-glucose (Figure 3A; supplemental Figure 8A-B), in agreement with previous findings.48,49 Further 2HG derivation confirmed L2HG as the main 2HG isotype, whereas NRF2 silencing accumulated L2HG levels but showed neglectable effect on D2HG levels (Figure 3B), consistent with the aforementioned in vivo finding that Nrf2 ablation increased L2HG levels in SCD mouse spleen.
Nrf2 regulates L2hgdh expression to modulate the production of glutamine-derived L2HG. (A) Control or NRF2 silenced EPs from patients with SCD were cultured for 12 days in glutamine-free Dulbecco’s modified Eagle medium media supplemented with 15% dialyzed fetal bovine serum, 15% dialyzed human AB serum and 2 IU/mL erythropoietin under hypoxia and oxidative stress (1% O2/200 μM H2O2), and traced with 2mM U-13C5 glutamine for 12 hours. The ion intensity of m + 0, m + 1, m + 2, m + 3, m + 4, m + 5, and m + 6 and the relative ion intensity of selected trichloroacetic acid cycle intermediates (α-ketoglutarate [2OG], succinate, fumarate, malate, and citrate) and 2HG are shown. (B) Metabolites from (A) were treated with TSPC and determined the derivatized TSPC-2HG (total), TSPC-D2HG, and TSPC-L2HG. (C) Quantitative real-time PCR showing the relative messenger RNA levels of L2HG/D2HG generation/utilization enzyme genes Mdh1, Mdh2, lactate dehydrogenase A (Ldha), L2hgdh, and D2hgdh in spleen Ter119+ cells of SS/Nrf2+/+ and SS/Nrf2–/– mice. (D) Mouse L2hgdh gene promoter activities were measured via GFP reporter expression in the presence of DMF or after NRF2 silencing (shNRF2) in KU812 cells. (E) Transcriptional activities of the mouse L2hgdh promoter and ARE motif mutants (motifs I-IV, located −980, −810, −800, and −452 base pairs upstream of the TSS were measured via GFP reporter expression. The putative ARE motifs are underlined. Data from 3 different wells (A-B), 6 mice per group (C), or 3 biological replicates (C-E) are presented as the mean ± SD. ∗P < .05; ∗∗P < .01. GFP, green fluorescent protein; TSPC, N-(p-toluenesulfonyl)-l-phenylalanyl chloride.
Nrf2 regulates L2hgdh expression to modulate the production of glutamine-derived L2HG. (A) Control or NRF2 silenced EPs from patients with SCD were cultured for 12 days in glutamine-free Dulbecco’s modified Eagle medium media supplemented with 15% dialyzed fetal bovine serum, 15% dialyzed human AB serum and 2 IU/mL erythropoietin under hypoxia and oxidative stress (1% O2/200 μM H2O2), and traced with 2mM U-13C5 glutamine for 12 hours. The ion intensity of m + 0, m + 1, m + 2, m + 3, m + 4, m + 5, and m + 6 and the relative ion intensity of selected trichloroacetic acid cycle intermediates (α-ketoglutarate [2OG], succinate, fumarate, malate, and citrate) and 2HG are shown. (B) Metabolites from (A) were treated with TSPC and determined the derivatized TSPC-2HG (total), TSPC-D2HG, and TSPC-L2HG. (C) Quantitative real-time PCR showing the relative messenger RNA levels of L2HG/D2HG generation/utilization enzyme genes Mdh1, Mdh2, lactate dehydrogenase A (Ldha), L2hgdh, and D2hgdh in spleen Ter119+ cells of SS/Nrf2+/+ and SS/Nrf2–/– mice. (D) Mouse L2hgdh gene promoter activities were measured via GFP reporter expression in the presence of DMF or after NRF2 silencing (shNRF2) in KU812 cells. (E) Transcriptional activities of the mouse L2hgdh promoter and ARE motif mutants (motifs I-IV, located −980, −810, −800, and −452 base pairs upstream of the TSS were measured via GFP reporter expression. The putative ARE motifs are underlined. Data from 3 different wells (A-B), 6 mice per group (C), or 3 biological replicates (C-E) are presented as the mean ± SD. ∗P < .05; ∗∗P < .01. GFP, green fluorescent protein; TSPC, N-(p-toluenesulfonyl)-l-phenylalanyl chloride.
The generation of L2HG is mediated by at least 3 different enzymatic pathways, including malate dehydrogenase 1/2 (Mdh1/Mdh2) and lactate dehydrogenase A, whereas the irreversible conversion of L2HG to 2OG is mediated by L2HG dehydrogenase (L2hgdh).50 To determine the factor(s) that contributes to the accumulation of L2HG after Nrf2 ablation, we measured their expression and enzymatic activity. Although we did not detect changes in the expression or enzymatic activities of Mdh1/Mdh2 or lactate dehydrogenase A (Figure 3C; supplemental Figure 9), a significant reduction in L2hgdh was observed after Nrf2 ablation (Figure 3C), suggesting that reduced L2hgdh expression contributed to L2HG accumulation.
Whether Nrf2 regulates L2hgdh expression was investigated using a plasmid reporter assay. We determined L2hgdh promoter activity in erythroleukemia KU812 cells that expressed both γ- and β-globin genes.51 KU812 cells were cultured under hypoxia (1% O2) and oxidative stress for 2 reasons: firstly, SCD undergo systemic hypoxia and constant oxidative stress1,2 and secondly, the production of L2HG is preferentially induced under hypoxia.48,49 We observed that treatment with dimethyl fumarate (DMF), an Nrf2 inducer, increased L2hgdh promoter activity by 2.2-fold, whereas shNRF2 decreased promoter activity by 44% (Figure 3D).
In silico analysis of the L2hgdh promoter predicted 4 putative ARE motifs located at −980, −810, −800, and −452 base pairs upstream of the transcription start site (TSS) (I-IV; Figure 3E). Individual ARE motif mutations reduced promoter activity by ∼23% to 47%, whereas mutations of consecutive ARE motifs (II and III) reduced promoter activity by 63.5%, and mutations of all 4 ARE motifs reduced promoter activity by 73% (Figure 3E). To determine whether Nrf2 binds directly to the L2hgdh promoter, we performed ChIP assays using SCD mouse spleen Ter119+ cells. We detected an abundant level of Nrf2 associated with the gene promoter but not with the downstream gene body (supplemental Figure 10). Together, these data suggest that Nrf2 regulates L2hgdh expression and the generation of L2HG in SCD.
Nrf2 ablation and L2HG accumulation impair ferroptosis signaling in SCD
Next, we performed RNA-seq to determine the effect of Nrf2 ablation on gene expression in SCD mouse spleen Ter119+ erythroid cells. The transcription and protein expression levels of Nqo1, Gclc, Gclm, Cbr1, Cbr3, Gstm1-5, Txnrd1, and Abcc2/3/4, which are involved in the oxidative stress response, were significantly reduced (Figure 4A-B; supplemental Figure 11), confirming the role of Nrf2 in regulating the ROS stress response. Interestingly, enrichment of gene signatures corresponding to ferroptosis signaling for the regulation of heme and iron metabolism were among the highest-ranking regulated pathways (Figure 4A), with the expression levels of genes involved in heme transport and degradation (Slc48a1 and Hmox1), iron storage and transport (Ftl1, Fth1, Slc40a1, and Slc11a2), and cysteine transport (Slc7a11) significantly decreased after Nrf2 ablation (Figure 4B; supplemental Figure 11). In agreement, SS/Nrf2–/– mice showed elevated levels of plasma heme and nonheme iron levels in the liver and spleen, and serum levels of bilirubin, a heme degradation product, compared with SS/Nrf2+/+ mice (Figure 4C-D).
Nrf2 and L2HG regulate ferroptosis in SCD. (A) RNA-seq determined that ROS and ferroptosis significantly affect signaling pathways in Nrf2 ablated SCD mouse bone marrow Ter119+ cells (n = 3). (B) Immunoblotting showing the expression of Nrf2 targeted proteins that were involved in antioxidant (Nqo1, Cat, Gclc, and Gstt1) and ferroptosis stress responses (Hmox1, Fech, Tfrc, Ftl1, Fth1, and Slc7a11) in SS/Nrf2+/+ and SS/Nrf2–/– spleens. The transcription factor Tbp and β-actin are loading controls. (C) Distinct levels of heme, iron, and bilirubin in the serum, spleen, and liver of SS/Nrf2+/+ and SS/Nrf2–/– mice. (D) Iron levels in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens and liver tissues by Prussian Blue staining (relative staining intensity) (right). (E-F) NADPH:NADP+ ratio (E), GSH and GSSG levels, and GSH:GSSG ratio (F) in the spleens of SS/Nrf2+/+ and SS/Nrf2–/– mice. (G) Flow cytometry analysis of lipid peroxidation levels in spleen Ter119+ cells of SS/Nrf2+/+ and SS/Nrf2–/– mice after stained with C11-Bodipy. (H) Immunoblotting showing the lipid peroxidation levels of 4HNE production in the spleen of SS/Nrf2+/+ and SS/Nrf2–/– mice. Data represent mean ± SD of 3 biological replicates. ∗P < .05; ∗∗P < .01. In panels B-C, a one-way analysis of variance (ANOVA) with Bonferroni multiple comparison test was used for statistical analysis. 4HNE, 4-hydroxynonenal; NADPH, reduced NADP; Tbp, TATA-box binding protein.
Nrf2 and L2HG regulate ferroptosis in SCD. (A) RNA-seq determined that ROS and ferroptosis significantly affect signaling pathways in Nrf2 ablated SCD mouse bone marrow Ter119+ cells (n = 3). (B) Immunoblotting showing the expression of Nrf2 targeted proteins that were involved in antioxidant (Nqo1, Cat, Gclc, and Gstt1) and ferroptosis stress responses (Hmox1, Fech, Tfrc, Ftl1, Fth1, and Slc7a11) in SS/Nrf2+/+ and SS/Nrf2–/– spleens. The transcription factor Tbp and β-actin are loading controls. (C) Distinct levels of heme, iron, and bilirubin in the serum, spleen, and liver of SS/Nrf2+/+ and SS/Nrf2–/– mice. (D) Iron levels in SS/Nrf2+/+ and SS/Nrf2–/– mouse spleens and liver tissues by Prussian Blue staining (relative staining intensity) (right). (E-F) NADPH:NADP+ ratio (E), GSH and GSSG levels, and GSH:GSSG ratio (F) in the spleens of SS/Nrf2+/+ and SS/Nrf2–/– mice. (G) Flow cytometry analysis of lipid peroxidation levels in spleen Ter119+ cells of SS/Nrf2+/+ and SS/Nrf2–/– mice after stained with C11-Bodipy. (H) Immunoblotting showing the lipid peroxidation levels of 4HNE production in the spleen of SS/Nrf2+/+ and SS/Nrf2–/– mice. Data represent mean ± SD of 3 biological replicates. ∗P < .05; ∗∗P < .01. In panels B-C, a one-way analysis of variance (ANOVA) with Bonferroni multiple comparison test was used for statistical analysis. 4HNE, 4-hydroxynonenal; NADPH, reduced NADP; Tbp, TATA-box binding protein.
The accumulation of iron and heme has been demonstrated to generate excessive ROSs through the Fenton reaction, thereby increasing oxidative stress and inducing ferroptosis.52 Indeed, there were significantly reduced levels of NADPH and GSH with a reduced ratio of NADPH:NADP+ and GSH:GSSG in SCD mouse spleen after Nrf2 ablation (Figure 4E-F). Meanwhile, Nrf2 ablation also significantly increased lipid peroxidation, as determined via C11-Bodipy staining and 4-hydroxynonenal production (Figure 4G-H), with ferroptosis mainly detected in early basophilic erythroblasts (supplemental Figure 12). Together, these data suggest that Nrf2 ablation promotes ferroptosis and exacerbates the severity of SCD.
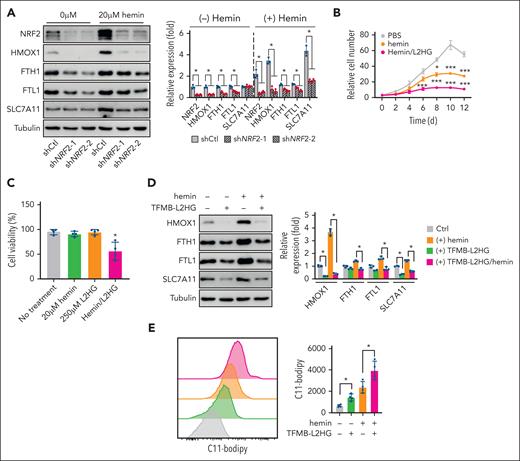
Excessive accumulation of heme and iron is a critical cause of ferroptosis in SCD.5 To determine whether Nrf2 silencing promotes ferroptosis induced by hemin, we measured lipid ROS levels in EPs from patients shNRF2 SCD cultured with H2O2 under hypoxia. shNRF2 suppressed the expression of genes involved in iron and heme metabolism in SCD EPs (Figure 5A).
Nrf2 silencing enhances the ferroptosis induced by hemin in EPs from patients with SCD. (A) Immunoblotting showing the effect of shNRF2 and hemin treatment on the expression of NRF2 and ferroptosis stress response proteins in 12-day cultured EPs from patients with SCD. (B-C) Cell proliferation (B) and viability (C) of EPs from patients with SCD cultured with TFMB-L2HG, hemin, or both at the indicated concentrations. (D-E) Effect of TFMB-L2HG (250 μM) and hemin (20 μM) treatment on ferroptosis stress response proteins in EPs from patients with SCD via immunoblotting (D), and flow cytometry analysis of lipid peroxidation levels stained with C11-Bodipy (E). Data represent the mean ± SD of 3 biological replicates. In panel A, a one-way ANOVA with Bonferroni multiple comparison test was used for statistical analysis. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Ctrl, control.
Nrf2 silencing enhances the ferroptosis induced by hemin in EPs from patients with SCD. (A) Immunoblotting showing the effect of shNRF2 and hemin treatment on the expression of NRF2 and ferroptosis stress response proteins in 12-day cultured EPs from patients with SCD. (B-C) Cell proliferation (B) and viability (C) of EPs from patients with SCD cultured with TFMB-L2HG, hemin, or both at the indicated concentrations. (D-E) Effect of TFMB-L2HG (250 μM) and hemin (20 μM) treatment on ferroptosis stress response proteins in EPs from patients with SCD via immunoblotting (D), and flow cytometry analysis of lipid peroxidation levels stained with C11-Bodipy (E). Data represent the mean ± SD of 3 biological replicates. In panel A, a one-way ANOVA with Bonferroni multiple comparison test was used for statistical analysis. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Ctrl, control.
The trichloroacetic acid cycle metabolites α-ketogluterate, succinate, fumarate, malate, and D2HG have been previously found to regulate ferroptosis in malignant conditions.53,54 To determine whether L2HG is involved in the regulation of hemin-induced ferroptosis, we treated EPs from patients with SCD with cell-permeable L2HG. L2HG supplementation presented a dose-dependent suppression of cell proliferation (Figure 5B). When hemin was added, the cell growth rate was suppressed further with significantly reduced cellular viability (Figure 5B-C). Notably, HMOX1 expression was induced by hemin treatment; however, this induction was suppressed by L2HG (Figure 5D). L2HG also suppressed the expression of several other iron and cysteine metabolism–related proteins including FTH1, FTL1, and SLC7A11 (Figure 5D). In alignment, we detected significantly increased cellular levels of lipid peroxidation after hemin and L2HG treatment (Figure 5E). Together, these data indicate that Nrf2 silencing and L2HG accumulation sensitize erythroid cells to hemin-induced ferroptosis.
Nrf2 ablation and L2HG accumulation regulate target gene expression through histone methylation
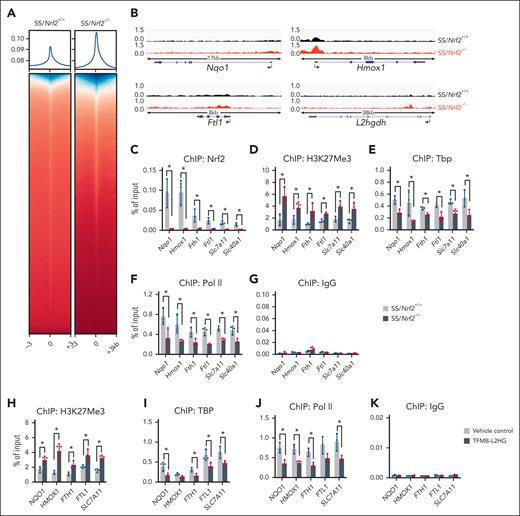
To gain insights into the role of Nrf2 ablation and L2HG accumulation in gene expression through histone methylation modifications, we conducted ChIP-seq to assess the genome-wide association of H3K27Me3 in SCD mouse bone marrow Ter119+ erythroid progenitor cells. Analysis of H3K27Me3 enrichment around TSSs (defined as TSS ± 3 kb) revealed an overall increase in H3K27Me3 levels after Nrf2 ablation (Figure 6A). In Nrf2 classic downstream target antioxidant gene loci Nqo1, Cat, Gclc, and Gclm, we observed a significantly increased association of H3K27Me3, whereas, in iron and heme metabolism genes Hmox1, Ftl1, Fech, Fth1, and Slc40A1 and the ferroptosis-related gene Slc7A11, we observed an increased association of H3K27Me3 (Figure 6B; supplemental Figure 13), supporting the important role of Nrf2 in regulating antioxidative stress and ferroptosis stress response in SCD. Notably, in agreement with the decreased expression of L2hgdh and accumulation of L2HG after Nrf2 ablation, we detected an increased association of H3K27Me3 in the L2hgdh gene locus (Figure 6B). Therefore, Nrf2, by regulating the expression of L2hgdh and L2HG levels modulates target gene expression through histone methylation modification.
Nrf2 ablation and L2HG accumulation affect the chromatin structure of target genes. (A) ChIP-seq analysis of the enrichment of H3K27Me3 around gene promoters (± 3kb) in bone marrow Ter119+ erythroid cells of SS/Nrf2+/+ and SS/Nrf2–/– mice. (B) ChIP-seq data tracks for H3K27Me3 enrichment at representative antioxidant (Nqo1), iron/heme metabolism (Hmox1 and Ftl1) and L2hgdh gene loci in the bone marrow Ter119+ erythroid cells of SS/Nrf2+/+ and SS/Nrf2–/– mice. A schematic representation of genes is shown below the panels, with the blue arrow indicating the TSS and gene orientation. The data are representative of biological triplicate samples. (C-G) ChIP-PCR analysis of the association of Nrf2 (C), H3K27Me3 (D), Tbp (E), and Pol II (F) with the promoter regions of antioxidant (Nqo1, Cat, Gclc, and Gstt1) and iron/heme metabolism (Hmox1, Fth1, Ftl1, Slc7a11, and Slc40a1) related genes in SCD mouse bone marrow Ter119+ cells after normalization to the input. Normal rabbit immunoglobulin G was used as an antibody control (G). (H-J) ChIP-PCR analysis of the association of H3K27Me3 (H), TBP (I), and RNA Pol II (J) with antioxidant (NQO1) and iron/heme metabolism (HMOX1, FTH1, FTL1, and SLC7A11) gene loci in erythroid progenitor from patients with SCD cells after TFMB-L2HG (250 μM) treatment. (K) Immunoglobulin G was used as an antibody control. Data represent mean ± SD of 3 biological replicates (n = 3). ∗P < .05. For panels C-F and H-J, a one-way ANOVA with Bonferroni multiple comparison test was used for statistical analyses.
Nrf2 ablation and L2HG accumulation affect the chromatin structure of target genes. (A) ChIP-seq analysis of the enrichment of H3K27Me3 around gene promoters (± 3kb) in bone marrow Ter119+ erythroid cells of SS/Nrf2+/+ and SS/Nrf2–/– mice. (B) ChIP-seq data tracks for H3K27Me3 enrichment at representative antioxidant (Nqo1), iron/heme metabolism (Hmox1 and Ftl1) and L2hgdh gene loci in the bone marrow Ter119+ erythroid cells of SS/Nrf2+/+ and SS/Nrf2–/– mice. A schematic representation of genes is shown below the panels, with the blue arrow indicating the TSS and gene orientation. The data are representative of biological triplicate samples. (C-G) ChIP-PCR analysis of the association of Nrf2 (C), H3K27Me3 (D), Tbp (E), and Pol II (F) with the promoter regions of antioxidant (Nqo1, Cat, Gclc, and Gstt1) and iron/heme metabolism (Hmox1, Fth1, Ftl1, Slc7a11, and Slc40a1) related genes in SCD mouse bone marrow Ter119+ cells after normalization to the input. Normal rabbit immunoglobulin G was used as an antibody control (G). (H-J) ChIP-PCR analysis of the association of H3K27Me3 (H), TBP (I), and RNA Pol II (J) with antioxidant (NQO1) and iron/heme metabolism (HMOX1, FTH1, FTL1, and SLC7A11) gene loci in erythroid progenitor from patients with SCD cells after TFMB-L2HG (250 μM) treatment. (K) Immunoglobulin G was used as an antibody control. Data represent mean ± SD of 3 biological replicates (n = 3). ∗P < .05. For panels C-F and H-J, a one-way ANOVA with Bonferroni multiple comparison test was used for statistical analyses.
Next, we determined whether altered H3K27Me3 levels affected chromatin structure and gene expression in SCD mice. In the antioxidant and iron/heme metabolism–related genes, we observed a significantly increased association of H3K27Me3, along with an insignificant association of Nrf2 in bone marrow Ter119+ erythroid cells of SS/Nrf2–/– mice compared with those of SS/Nrf2+/+ mice (Figure 6C-D). In alignment with the increased H3K27Me3 association, significant decreases in the association of general transcription factor TATA-box binding protein (Tbp) and RNA polymerase II (Pol II) with the antioxidant and iron/heme-related genes were observed after Nrf2 ablation (Figure 6E-G), suggesting that Nrf2 modulates chromatin structures in these gene loci to regulate gene expression through histone methylation modification.
Whether L2HG affects histone methylation for target gene expression in regulating ROS and ferroptosis responses was subsequently investigated in EPs from patients with SCD cultured under hypoxia and oxidative stress. We observed that L2HG treatment significantly decreased the expression of CD71 but had a minimal effect on CD235a (supplemental Figure 14A-B). Meanwhile, L2HG treatment significantly reduced the association of NRF2 with antioxidants, heme/iron metabolism, and ferroptosis-related gene loci (supplemental Figure 15), along with increased H3K27Me3 association, whereas the association of Tbp and Pol II was reduced (Figure 6H-K). Indeed, L2HG treatment caused significant sickling of the EPs (supplemental Figure 14C). Together, these results suggest that by mediating histone methylation modification, Nrf2 ablation and L2HG accumulation regulate the expression of target genes that affect oxidative and ferroptosis stress responses in SCD.
Nrf2 activation alleviates ferroptosis stress in SCD disease
To determine whether Nrf2 activation ameliorates disease severity through L2HG, SCD mice were treated for 4 weeks with the Nrf2 activator DMF, which we previously demonstrated to activate human γ-globin genes37 and alleviate mouse SCD symptoms.55 Compared with the vehicle control, DMF significantly reduced the levels of plasma heme, serum iron, and bilirubin (Figure 7A), indicating improvements in the disease phenotype. In alignment with the improved metabolism of iron and heme, the expression of typical antioxidant and ferroptosis-related proteins Nqo1, Gclc, Hmox1, Fth1, Ftl1, and Slc7a11 increased after DMF treatment (Figure 7B). To determine whether DMF improves SCD phenotype through Nrf2, we treated SS/Nrf2–/– mice in a similar way. However, DMF treatment did not affect the metabolism of iron/heme or the expression of antioxidant or ferroptosis-related proteins in SS/Nrf2–/– mice (supplemental Figure 16), suggesting an-Nrf2 dependent effect of DMF on SCD.
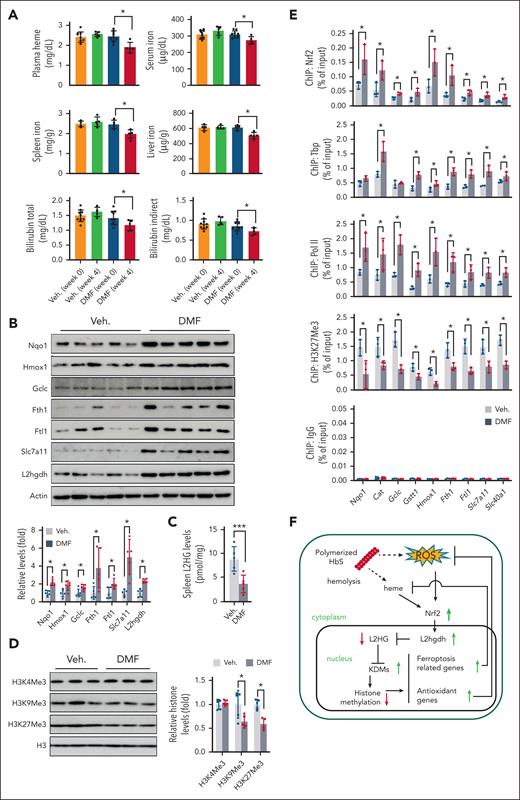
Nrf2 activation reverses SCD ferroptosis stress. (A) Levels of heme, nonheme iron, and bilirubin (total and indirect) in SCD mice before and after chronic DMF or vehicle 0.08% hydroxyethyl cellulose (Veh) treatment. (B) Immunoblotting showing the expression of antioxidants (Nqo1 and Gclc), ferroptosis stress response proteins (Hmox1, Fth1, Ftl1, and Slc7a11), and L2hgdh in the spleen Ter119+ cells of SCD mice. (C) L2HG levels in spleen Ter119+ cells from SCD mice. (D) Immunoblotting showing histone methylation in the histone extracts of spleen Ter119+ cells of SCD mice. (E) ChIP-PCR analysis of the association of Nrf2, Tbp, RNA Pol II, and H3K27Me3 with the promoter regions of antioxidant (Nqo1, Cat, Gclc, and Gstt1), and ferroptosis stress response genes (Hmox1, Fth1, Ftl1, Slc7a11, and Slc40a1) in the SCD mouse spleen Ter119+ cells. Data represent mean ± SD (n = 3-10). ∗P < .05. (F) Schematic depicting the Nrf2 function in SCD EPs. ROS stress and heme accumulation from chronic hemolysis activate Nrf2 and subsequently L2hgdh expression to reduce the levels of L2HG. Reduced L2HG increases the enzymatic activities of α-ketoglutarate–dependent hydroxylases such as KDM and thus decreases global histone methylation, which further leads to the upregulated expression of antioxidant and ferroptosis-related genes to protect against heme and ROS stress. For panels B and E, a one-way ANOVA with Bonferroni multiple comparison test was used for the statistical analyses. Veh., vehicle.
Nrf2 activation reverses SCD ferroptosis stress. (A) Levels of heme, nonheme iron, and bilirubin (total and indirect) in SCD mice before and after chronic DMF or vehicle 0.08% hydroxyethyl cellulose (Veh) treatment. (B) Immunoblotting showing the expression of antioxidants (Nqo1 and Gclc), ferroptosis stress response proteins (Hmox1, Fth1, Ftl1, and Slc7a11), and L2hgdh in the spleen Ter119+ cells of SCD mice. (C) L2HG levels in spleen Ter119+ cells from SCD mice. (D) Immunoblotting showing histone methylation in the histone extracts of spleen Ter119+ cells of SCD mice. (E) ChIP-PCR analysis of the association of Nrf2, Tbp, RNA Pol II, and H3K27Me3 with the promoter regions of antioxidant (Nqo1, Cat, Gclc, and Gstt1), and ferroptosis stress response genes (Hmox1, Fth1, Ftl1, Slc7a11, and Slc40a1) in the SCD mouse spleen Ter119+ cells. Data represent mean ± SD (n = 3-10). ∗P < .05. (F) Schematic depicting the Nrf2 function in SCD EPs. ROS stress and heme accumulation from chronic hemolysis activate Nrf2 and subsequently L2hgdh expression to reduce the levels of L2HG. Reduced L2HG increases the enzymatic activities of α-ketoglutarate–dependent hydroxylases such as KDM and thus decreases global histone methylation, which further leads to the upregulated expression of antioxidant and ferroptosis-related genes to protect against heme and ROS stress. For panels B and E, a one-way ANOVA with Bonferroni multiple comparison test was used for the statistical analyses. Veh., vehicle.
Notably, elevated expression of L2hgdh after DMF treatment was also detected (Figure 7B) along with significantly reduced L2HG levels in SCD mice (Figure 7C). To further demonstrate that changes in L2HG levels affect histone methylation, we determined global and gene-specific histone methylation in SCD mouse spleen Ter119+ erythroid cells. Significantly reduced levels of histone methylation were observed for H3K9Me3 and H3K27Me3 after DMF treatment compared with the vehicle control (Figure 7D). Furthermore, chromatin structure analysis showed that DMF treatment increased the association between Tbp and Pol II and reduced H3K9Me3 and H3K27Me3 signals in antioxidant- and ferroptosis-responsive genes (Figure 7E). Similarly, in EPs from patients with SCD, DMF treatment decreased histone methylation modifications and induced the expression of NRF2 and its downstream antioxidant and ferroptosis response proteins (supplemental Figure 17). Together, these data suggest that Nrf2, by regulating L2hgdh expression, modulates L2HG levels to affect chromatin structure for gene regulation and that Nrf2 activation produces protective effects against ferroptosis stress in SCD (Figure 7F).
Discussion
Compared with healthy people, patients with SCD have aberrant metabolic regulation of many metabolites, including fumarate, glutamate, glycine, malate, and others.1,2,30,56,57 In addition, patients with SCD at steady state and those under vaso-occlusive crisis show significant differences in their metabolome profiles.58 Alterations in metabolites have been demonstrated to contribute to the hypoxic response,59 red blood cell sickling,30 inflammation,60 and disease progression61; however, an important question of whether metabolite changes affect gene expression and the severity of SCD was still unanswered.
Interestingly, we found that Nrf2 absence increased global histone hypermethylation in SCD mouse spleen and bone marrow erythroid cells. Although we identified KDM6a as a downstream target of Nrf2 that contributes to histone H3K27Me3 modification,62 Kdm6a expression is less likely to correlate with the alterations in global histone hypermethylation modifications in SCD. Other factors contributing to the alteration of histone methylation include the availability of the methyl donor S-adenosyl methionine,63 histone methylation demethylase cofactor 2OG, and its competitors, such as succinate, fumarate, and 2HG.32,64 Via metabolic profiling analysis, we did not detect level changes for S-adenosyl methionine, 2OG, succinate, fumarate, or D2HG; however, we detected a significant increase in L2HG. Notably, 2HG was considered a cul-de-sac metabolite without physiological functions65 and has remained largely unnoticed for decades. Recent metabolome studies have shown that these metabolites are at abnormal levels in SCD 229,30; however, their functions other than energy support have not been well studied. Recently, L2HG was demonstrated to modulate the enzymatic activities of 2OG-dependent dioxygenases, such as the ten-eleven translocation family of DNA hydroxylases and JmjC domain–containing histone lysine demethylases.62,66 Consistent with previous observations, we detected an increase in L2HG-mediated global and gene-specific loci histone hypermethylation in SCD. In contrast, in a non-SCD mouse model, we did not observe any effect of Nrf2 ablation on histone methylation or L2HG level changes, suggesting a cellular environment-specific effect of Nrf2 on L2HG and histone methylation in SCD. Notably, SCD endures significant levels of systemic hypoxia and elevated oxidative stress, which are critical contributors to DNA and histone methylation modifications. Subsequent mechanistic studies in SCD EPs demonstrated that both hypoxia and oxidative stress participate in Nrf2-mediated histone methylation modifications. Therefore, Nrf2-regulated L2HG generation and histone methylation modification requires a premise of hypoxia and oxidative stress environment in SCD.
Previously, it was found that Nrf2 executes its function through ARE-dependent9 and -independent18 mechanisms to recruit epigenetic modifiers that affect DNA and histone modifications and permit transcription. We also demonstrated that the direct DNA-binding activity of Nrf2 contributes to the regulation of fetal hemoglobin globin and antioxidant gene expression to influence SCD severity.11,37,55 Our findings in this study showed that Nrf2 affects the levels of L2HG to modulate histone methylation globally and gene loci-specific for chromatin structure modifications to mediate gene expression regulation. Therefore, Nrf2 is not only a traditional transcription factor that regulates gene expression through direct DNA-binding activity but is also an orchestrator of gene regulation through multiple aspects.
Our observations suggest that Nrf2/L2HG signaling affects the expression of genes involved in oxidative and ferroptosis stress responses. We previously demonstrated that Nrf2 ablation significantly elevated the levels of ROS11 and nonheme iron content compared with those in wild-type SCD mice. Notably, 1 downstream target of Nrf2 is iron. Nrf2 regulates genes involved in iron and heme metabolism, including AMBP, ABCB6, BMP6, FECH, HRG1, FTL, FTH1, and hepcidin,67-70 and iron accumulation often activates the Nrf2 signaling pathway.71 In patients with SCD, iron deficiency is uncommon; however, chronic blood transfusion for the complications of SCD leads to iron overload and the need for chelation therapy to counteract the harmful effects of iron.72 Therefore, Nrf2 activation is an important option for treating heme excess and iron overload in SCD under blood transfusion therapy.
Notably, Nrf2 has been shown to regulate erythropoiesis through an antioxidative stress response and Nrf2 ablation induces ineffective erythropoiesis in aged mice, and treatment with antioxidants not only ameliorated age-dependent anemia but also decreased ineffective erythropoiesis.73 SCD faces constant systemic hypoxia stress,44 which exacerbates the pathology in multiple organs,74 such as induced vaso-occlusion with Hif1a activation to protect against organ damage and human fetal globin gene expression to suppress the sickling of SCD erythrocytes.75 Nrf2 has been shown to have Hif1a-dependent76 and independent77 effects in connecting hypoxia and oxidative stress response. These findings support an essential role for Nrf2 in SCD for hypoxia response through Hif1a. Importantly, hypoxia itself has been shown to affect gene expression in SCD,78 and its effects are identified to attribute to Hif1a. In this study, we found that hypoxia also induced the generation of L2HG as a competitor of 2OG-dependent dioxygenases, resulting in histone hypermethylation in SCD gene regulation, whereas Nrf2, by modulating the expression of L2HGDH, converts L2HG to 2OG. Therefore, Nrf2 mediated multiple aspects to regulate pathophysiological conditions in thalassemia and SCD.
Importantly, Nrf2, as the master for the regulation of ROS stress, has recently been demonstrated to regulate the ferroptosis response for the expression of multiple signals in iron/heme metabolism and lipid peroxidation under different physiologic and pathophysiologic conditions.79 Although we did not detect any effect of Nrf2 on the expression of Gpx4, a critical regulator of lipid peroxidation, we observed that Nrf2 ablation reduced the expression of heme and iron metabolism proteins and increased heme, bilirubin, and iron levels in both spleen and liver tissues of SCD mice, which consequently increased ferroptosis levels. In SCD, excess heme drives the progression of inflammation and cardiomyopathy through ferroptosis for iron-dependent nonapoptotic cell death.5 Therefore, our findings suggest an essential role for Nrf2 in regulating the ferroptosis stress response through metabolite L2HG mediated epigenetic histone methylation programs for gene regulation in SCD. In this regard, Nrf2 affects virtually all clinical hallmarks of SCD, and targeting Nrf2 presents a pivotal strategy in disease management.
Acknowledgments
The authors thank Rhea-Beth Markowitz for critically reviewing the manuscript; Tianxiang Hu, Bobby Thomas, the late Dorothy Tuan, and Hongyan Xu for their insights and helpful discussions of data. The authors also thank Xiuli An (New York Blood Center) for providing the Band3 antibody. Metabolomics analysis of the mouse spleen was performed with the help of Tyler Van Ry and James Eric Cox at the Metabolomics Core Facility at the University of Utah. This research was conducted, in part, at the Georgia Cancer Center Shared Resources (Cores).
Mass spectrometry equipment was obtained through NCRR Shared Instrumentation grants 1S10OD016232-01, 1S10OD018210-01A1, and 1S10OD021505-01. This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK119762 (X.Z.).
Authorship
Contribution: C.X., J.P., W.Z., C.-S.S.C., and U.S. performed the experiments and analyzed the data; H.S., A.H., and B.S.P. analyzed the data and edited the manuscript; and X.Z. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xingguo Zhu, Department of Pediatrics, Division of Hematology/Oncology, Georgia Cancer Center, Augusta University, Augusta, GA 30912; e-mail: xzhu@augusta.edu.
References
Author notes
RNA-seq and ChIP-seq data are deposited in the NCBI Gene Expression Omnibus database (accession numbers GSE188695, GSE223911, and GSE225966).
Data are available on request from the corresponding author, Xingguo Zhu (xzhu@augusta.edu)
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Nrf2 regulates L2hgdh expression to modulate the production of glutamine-derived L2HG. (A) Control or NRF2 silenced EPs from patients with SCD were cultured for 12 days in glutamine-free Dulbecco’s modified Eagle medium media supplemented with 15% dialyzed fetal bovine serum, 15% dialyzed human AB serum and 2 IU/mL erythropoietin under hypoxia and oxidative stress (1% O2/200 μM H2O2), and traced with 2mM U-13C5 glutamine for 12 hours. The ion intensity of m + 0, m + 1, m + 2, m + 3, m + 4, m + 5, and m + 6 and the relative ion intensity of selected trichloroacetic acid cycle intermediates (α-ketoglutarate [2OG], succinate, fumarate, malate, and citrate) and 2HG are shown. (B) Metabolites from (A) were treated with TSPC and determined the derivatized TSPC-2HG (total), TSPC-D2HG, and TSPC-L2HG. (C) Quantitative real-time PCR showing the relative messenger RNA levels of L2HG/D2HG generation/utilization enzyme genes Mdh1, Mdh2, lactate dehydrogenase A (Ldha), L2hgdh, and D2hgdh in spleen Ter119+ cells of SS/Nrf2+/+ and SS/Nrf2–/– mice. (D) Mouse L2hgdh gene promoter activities were measured via GFP reporter expression in the presence of DMF or after NRF2 silencing (shNRF2) in KU812 cells. (E) Transcriptional activities of the mouse L2hgdh promoter and ARE motif mutants (motifs I-IV, located −980, −810, −800, and −452 base pairs upstream of the TSS were measured via GFP reporter expression. The putative ARE motifs are underlined. Data from 3 different wells (A-B), 6 mice per group (C), or 3 biological replicates (C-E) are presented as the mean ± SD. ∗P < .05; ∗∗P < .01. GFP, green fluorescent protein; TSPC, N-(p-toluenesulfonyl)-l-phenylalanyl chloride.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/4/10.1182_blood.2022018159/2/m_blood_bld-2022-018159-gr3.jpeg?Expires=1765888383&Signature=4iQEl5OjcYbqkwG0pbPScZznM~1T7RJHOZh58v2Zz-AdMDrEjXXEvW3RJyPYdWzsX0yZHd~LM00eALeDFpqPpWmZvR-TZfa0dN6ixKPdAGQecZ3uR6EG79ckUjvBI04dSLljJ3z686D7uNT4rFQJK0-1jmHAtxop3q3Y3zg2j6ZRrq4skS4kGylohYE0LVRcehyP8ai6TaOkJu5N6u3f6HS1LDoXQzV2nzxnHLL831ilVve-ngNEXSnhzq-o9K2F6iz2900IOQHotGBxpgU1Aw3wWTNiw8awPWYGmhw9rBsBiv4Ram1fMVWr0VnIQ6gI3wrbH5HGRLP5I8RcVyvHOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal