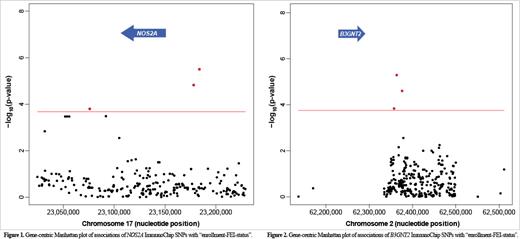
Hemophilia-A (HA) is caused by heterogeneous loss-of-function factor (F) VIII gene ( F8) mutations and deficiencies in plasma FVIII coagulant activity (FVIII:C) that impair intrinsic pathway coagulation amplification. The standard-of-care for severe HA patients is regular infusions of therapeutic FVIII proteins (tFVIIIs) but ~25-30% of all such subjects develop neutralizing anti-tFVIII-antibodies called “FVIII inhibitors (FEIs)” that leave them refractory to treatment and necessitate use of alternative therapies that are less safe and effective, and extremely expensive. We used the ImmunoChip genotyping platform and subjects in the Personalized Alternative Therapies for Hemophilia (PATH) study to scan immune-mediated disease (IMD) genes for novel and/or replicated genomic sequence variations associated with FEI risk while accounting for non-independence of data due to genetic relatedness and F8 mutational heterogeneity. The “enrollment-FEI-status” of 450 North American PATH study subjects-which include 446 that self-identified as being only either black-African (n=204) or white-European (n=242), and four that self-identified as being both white-European and either black-African (n=3) or Asian (n=1)-was the dependent variable. The F8 mutation data and a genetic relatedness matrix were incorporated into a binary linear mixed model of genetic association with the novel FEI outcome of enrollment-FEI-status based on whether or not FEIs of any titer (AT) developed-Yes (AT:FEIs+) versus No (AT:FEI-)-regardless of their “historical-FEI-status', i.e., whether or not they had ever developed FEIs of AT prior to study entry. We adopted a gene-centric association strategy to scan as ”candidates“ a subset of 101 genes-of the ~2,000 total immune system genes that are interrogated by the naturally occurring sequence variations able to be genotyped simultaneously on the ImmunoChip-which comprise what we refer to herein as ”pleiotropic-IMD-genes“ because they have been implicated previously in the development of either (i) two or more autoimmune diseases (n=80) or (ii) at least one autoimmune disease if they were also implicated in FEI risk previously (n=25). (Note that four of the pleiotropic-IMD-genes previously implicated in FEI risk were also in the 80 genes implicated previously in the development of multiple autoimmune diseases such that we scanned 101 distinct pleiotropic-IMD-genes for association with FEI development in the PATH study.) Enrollment-FEI-status was significantly associated with SNPs in NOS2A ( Figure 1) and B3GNT2 ( Figure 2), which are involved directly and indirectly, respectively, in anti-microbial-/-tumoral-immunity. Among the pleiotropic-IMD-genes previously implicated in FEI risk, we identified strong associations with SNPs in CTLA4 (p=2.2E-5). We also quantified the influence of the different F8 mutation types on FEI risk for the first time and demonstrated that the F8-mutation-effect underlies ~15% of the total heritability of FEI development. The additive genetic heritability together with the SNPs in the pleiotropic-IMD-genes were found to account for >50% of the patient-specific variability in FEI risk. Finally, race was shown to be a significant determinant of FEI risk independent of the effects of F8 mutation types and non- F8-genetics.
Disclosures
Chitlur:Takeda: Honoraria; BPL Inc: Honoraria; HRSA/MCHB: Research Funding; Children's Foundation: Research Funding; Novo Nordisk: Consultancy, Honoraria; Genzyme Corp: Honoraria; Genentech Inc: Honoraria, Research Funding; Agios Pharmaceuticals: Honoraria, Research Funding; Novartis Pharmaceuticals: Research Funding. Escobar:CSL Behring: Consultancy; uniQure: Consultancy, Research Funding; NHF: Consultancy; Kedrion: Consultancy; Sanofi: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Pfizer: Consultancy; LFB: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; HEMA Biologics: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Novo Nordisk: Consultancy, Research Funding; BioMarin: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal