Introduction Central Nervous System (CNS) involvement in Diffuse Large B Cell Lymphoma (DLBCL) is a rare complication with bad prognosis. The CNS Prognostic Index is employed to categorize patients at a high risk of CNS relapse; however, its effectiveness is not accurate. The discovery of precise biomarkers for predicting CNS involvement in DLBCL patients remains unmet. Our objective was to characterize the immune, transcriptomic, genomic and epigenomic hallmarks of systemic tumors at diagnosis of patients who later experienced relapse in the CNS. By exploring these biological profiles, we sought to gain valuable insights into the underlying mechanisms and potential biomarkers associated with CNS relapse in DLBCL.
Methods We conducted a retrospective study including 38 patients diagnosed with nodal DLBCL who experienced relapse within the first two years after diagnosis. Among these patients, 12 individuals relapsed specifically in the CNS, while 26 relapsed in systemic sites. As a control group, we included 21 patients who did not relapse. DNA and RNA were extracted from FFPE tumor samples collected at diagnosis. To evaluate immune cell infiltration, we utilized the nCounter PanCancer Immune Profiling Panel (Nanostring); cell-of-origin (COO) was determined using the Lymph2Cx assay. Transcriptomic profiles were generated by bulk RNA sequencing and mutational analysis was performed using a custom targeted NGS panel, which included the most frequently mutated genes observed in lymphoid neoplasms. Methylation profiles were obtained using the Illumina Infinium MethylationEPIC Array 850K.
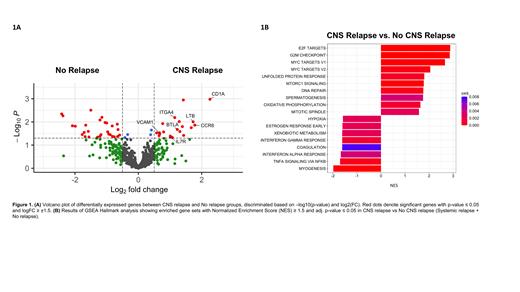
Results Immune profiling analysis showed cell composition was not significantly different between groups; however, we observed distinct immune gene expression patterns at diagnosis in patients who experienced CNS relapse. Multiple genes were overexpressed in the CNS-relapsed group compared to non-relapsed group, which encompassed various functional categories including chemokine signaling such as IL7R (FC=2; P=0.05) and CCR6 (FC=3.4; P=0.01); adhesion like VCAM1 (FC=1.6; P=0.03) and ITGA4 (FC=2.2; P=0.006); and immune regulation such as CD1A (FC=4.8; P=0.001), LTB (FC=3.3; P=0.009) and BTLA (FC=2.3; P=0.02) (Fig.1A). The ITGA4 gene also presented higher expression levels in the CNS-relapsed group when compared to both systemic-relapsed and non-relapsed groups combined (FC=1.7; P=0.03). No significant differences were observed in the COO classification between groups. Whole-genome transcriptomic data revealed enrichment in gene sets associated with proliferation and survival in the CNS-relapsed group (Fig.1B). Analysis of genetic alterations indicated that patients with CNS relapse exhibited an increased mutation load and frequency in PIM1, IGLL5, and KMT2D genes. Finally, analysis of the CNS relapse-related epigenome showed 343 differentially hypomethylated CpG sites compared to both systemic and non-relapsed groups together, affecting a total of 211 genes. Pathway enrichment analysis of these genes revealed a link between CNS infiltration and cell migration and locomotion.
Conclusions Tumors from DLBCL patients that will relapse in the CNS present a higher chemokine, adhesion and proliferation signatures at diagnosis. Of notice, BTLA, CCR6 and IL7R proteins have been previously reported to be implicated in B cell entry into the CNS in B-cell malignancies. Interestingly, patients with CNS relapse demonstrated significant overexpression of the ITGA4 gene, which encodes the integrin α4 subunit (CD49d) of the Very Late Antigen-4. Engagement between CD49d on B cells and its endothelial ligand VCAM-1 is required for migration of these cells across the blood-brain barrier. In fact, Natalizumab (anti-CD49d mAb) is administered in multiple sclerosis to impede entrance and accumulation of B cells in the brain. Initial experiments in mice have shown increased expression of CD49d in lymphoma B cells that have infiltrated the brain, compared to those in the spleen. Herein, we have demonstrated that DLBCL cells at diagnosis already own specific transcriptomic and (epi)genetic hallmarks that could confer a higher capacity to infiltrate the CNS. Validation of these findings in larger cohorts of patients could improve selection of patients with an increased risk of CNS involvement in need of prophylactic CNS-directed therapies at diagnosis.
Disclosures
Bobillo:AstraZeneca, Abbvie: Honoraria; Vall d'Hebron University Hospital: Current Employment. Abrisqueta:Abbvie: Consultancy, Honoraria, Speakers Bureau; Astrazeneca: Consultancy, Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau; Beigene: Consultancy; BMS: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau. Esteller:Ferrer International: Consultancy; Quimatrix: Consultancy. Bosch:Roche: Honoraria; BeiGene: Consultancy; Lilly: Consultancy; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Karyospharm: Other; Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal