R-loops are three-stranded structures composed of an RNA-DNA hybrid and a single strand of DNA. R-loops play critical roles in gene regulation and pathologic roles in DNA damage response. Current methods to map R-loops require at least 500,000 cells. Consequently, R-loop characterization in primary cells is limited. Furthermore, there are no genomic tools to identify R-loops in single cells. We therefore developed here methods to map genome-wide R-loops at single cell resolution. R-loop mapping in primary stem and mature hematopoietic cell populations, and individual single cells revealed striking cell-type specific R-loops.
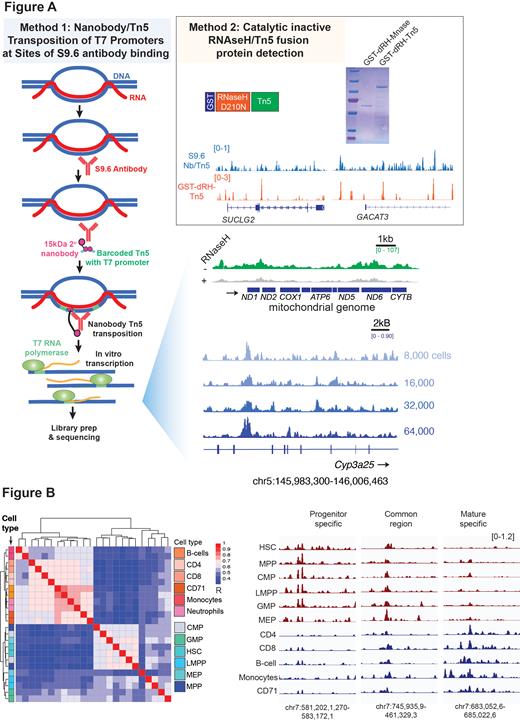
Current methods to map R-loops utilize immunoprecipitation with a monoclonal antibody (clone “S9.6”) or catalytically inactive, but R-loop binding competent, RNAseH1. Motivated by success mapping chromatin modifications in single cells through fusion of Tn5 transposase to a nanobody as well as insertion of T7 promoters to amplify DNA at sites of transposition, we combined both techniques to identify R-loops in limited cell numbers, down to the single cell level. We used a nanobody-Tn5 fusion to identify S9.6 and developed an orthogonal recombinant fusion protein of enzymatic dead RNAseH1 fused to Tn5 (Figure A).
We first applied our nanobody-Tn5 method to identify R-loops bound by S9.6 in mouse lineage-negative c-Kit + bone marrow cells in biological duplicates of increasingly reduced cell numbers (64,000, 32,000, 16,000, and 8,000 cells). This revealed remarkable reproducibility of R-loop detection and characteristics. R-loops identified were sensitive to RNAseH1 and enriched at sites with G/C skew and mitochondrial DNA (a site with abundant physiologic R-loops), suggesting authentic, bona fide R-loops mapped in as few 8,000 cells.
We next evaluated R-loops across FACS-isolated hematopoietic stem and mature cells from mouse and human bone marrow. Mapping of R-loops in 10,000 cells in duplicate revealed reproducible R-loops which were characteristic of each cell population. Hierarchical clustering of R-loop profiles delineated mature from stem and progenitor cells and discovered increased abundance of genic R-loops in hematopoietic stem and progenitors relative to mature cells (Figure B). Application of our enzymatic dead RNaseH1/Tn5 fusion protein to CD34 + cells revealed a similar genome-wide distribution of R-loops as with the S9.6/nanobody-Tn5 method.
There was high correlation of long- and short-term HSC-specific R-loops with sites characteristic of active transcription (based on H3K4me1/2/3 as well as H3K27Ac). Transcription factor motifs activated in unique hematopoietic cell types were enriched at R-loops within the same cell types. For example, GATA motif usage was enriched at R-loops in CMP, MEP, and erythroid precursors. We also detected massive increases in R-loop abundance at the immunoglobulin heavy chain locus in primary B-cells stimulated to undergo class switch recombination. This provides further proof of our low input method to identify R-loops, as physiologic R-loops are known to occur at this locus to promote class switch recombination.
Despite recent advances in single cell genomics there are no single cell maps of R-loops. We therefore profiled R-loops using our nanobody-Tn5, low-input S9.6 CUT&Tag approach in individual human CD34 + and mouse c-Kit + cells. We obtained 1,579 single-cell profiles from human and 1,716 from mouse. Uniform manifold approximation and projection (UMAP) identified 12 clusters in human and 9 mouse hematopoietic clusters. Aggregated single-cell R-loop profiles closely resembled bulk profiles indicating that single-cell data captured features of multi-cell maps. Pseudotime analyses of R-loops inferred LT-HSCs as the shared root of hematopoiesis in both mouse and human and identified seven terminal states without prior knowledge of the number of branches in the hematopoietic development.
These data provide the first maps of R-loops in primary normal hematopoietic cells and identify scheduled, physiologic cell-type specific R-loops which have not previously been uncovered. The atlas of R-loops across hematopoiesis generated here will motivate future studies to understand the roles of R-loops in hematopoietic differentiation. Moreover, the methods presented map R-loop abundance at nucleotide resolution in primary and limited cell types which can be used for wide-reaching applications.
Disclosures
Abdel-Wahab:Nurix Therapeutics: Research Funding; Minovia Therapeutics: Research Funding; Amphista Therapeutics: Consultancy; AbbVie, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Consultancy; Loxo/Lilly: Consultancy; Harmonic Discovery: Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal