Introduction: Prognostically adverse mutations in patients with primary myelofibrosis (MF) include ASXL1, EZH2 IDH1, IDH2, and two types of splicing mutations U2AF1 Q157 and SRSF2 (as in MIPSS70.v2 score). The other splicing mutations (SPM), such as SF3B1 or ZRSR2, do not appear to have impact on prognosis. In patients with MF secondary to polycythemia vera or essential thrombocytopenia (PPV-MF, PET-MF), the role of SPM is less known, and they are not included in the MYSEC-PM score.
Objective: We aimed to evaluate the role of SPM on the outcome of patients with PPV/PET-MF, and their interplay with other HMR on prognosis of all MF patients from our center.
Methods: We retrospectively reviewed medical charts of 114 patients with MF from our institution who had SPM on next generation sequencing (51+ gene myeloid panel). We assessed impact of high-risk SPM (HR_spl: U2AF1 Q157; SRSF2) and remaining SPM (low risk, LR_spl: SF3B1, PRPF40B, U2AF2, ZRSR2) on the outcome of MF patients. Separate analysis was carried per disease phenotype (MF vs PET/PPV-MF), and presence of the other high-risk molecular mutations (HMR: ASXL1, IDH1/2, EZH2). Descriptive statistics was used for demographic variables; Kaplan-Meier curve with log-rank test was used for overall survival (OS), calculated from the time of presentation.
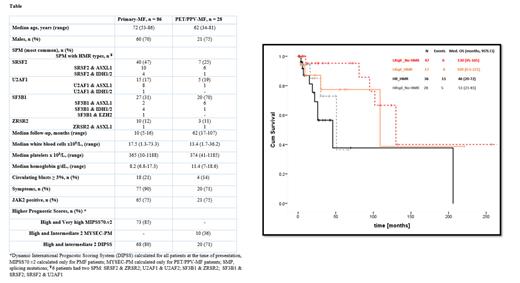
Results: Table summarizes clinical characteristics of all patients. The most common SPM were SRSF2 and SF3B1 (41% each), followed by U2AF1 (17.5%) and ZRSR2 (11%). 6 patients had more than one SPM. The distribution of SPM types did not statistically differ between PMF and PET/PPV-MF patients, albeit numerically, more patients with PMF had SRSF2 and PET/PPV-MF had SF3B1 (table). 77% of all patients had higher DIPSS score; 85% of PMF patients had higher MIPSS70.v2 score and 36% of PET/PPV-MF had higher MYSEC-PM. HMR mutations were detected in 77 (68%) patients: ASXL1 (65%), EZH2 (19%), IDH1/2 (16%). ASXL1 co-occurred more frequently with SF3B1 in PET/PPV-MF patients (75% vs 25%), and with SRSF2 (62% vs 38%) and U2AF1 (89% vs 11%) in PMF patients.
The median OS (months [95% CI]) according to SPM: SRSF2, U2AF1, SF3B1 and others (U2AF2 in 2 patients, PRPF40B in 1 patient and ZRSR2 in 13 patients) was as follows: 46 [23-70], not reached, 130 [104-156], unreached, respectively. The median OS (months [95% CI]) per HR_spl (n = 61) and LR_spl (n = 67) was 51 [26-76] vs 130 [101-159], p < 0.01, HR 0.31, 95% CI 0.13-0.74. Sub-analysis for PMF (n = 86) and PET/PPV-MF (n = 28) showed comparable results, median OS for HR_spl and LR_spl in PMF of 51 vs 109 months and for PET/PPV-MF of 46 and 130 months, respectively. The median OS (months [95% CI] of HR_spl with and without HMR and LR_spl with and without HMR as shown in Figure was 46 [20-72] and 51 [21-81] vs 109 [0.5-221] and 130 [95-165], respectively. Two years survival for patients with HR_spl and LR_spl with and without HMR was at 69% vs 85% and 87% vs 95%, respectively. Median OS (months; 95% CI]) of each individual SPM without and with HMR was 51 [not estimated] and 46 [11-82] for SRSF2; unreached for all groups in U2AF1 and ZRSR2, respectively; 130 [98-161] and 109 [10-220] for SF3B1.
Conclusions: Various SPM seem to have similar effect on survival of PET/PPV-MF and PMF patients. PET/PPV-MF patients have more low risk SPM (SF3B1) and the co-occurrence of HMR does not appear to impact the outcome. Among the high-risk SPM, SRSF2 has the worst prognostic role irrespective of concurrent HMR. Further validation of our data, including association with received therapy, is ongoing and will be presented at the conference.
Disclosures
Bose:Incyte, BMS, CTI, Morphosys, Blueprint, Cogent, Sumitomo: Honoraria, Research Funding; Kartos, Telios, Ionis, Disc, Janssen, Geron: Research Funding; GSK, Novartis, Karyopharm, AbbVie, Pharma Essentia, Jubilant, Morphic: Honoraria. Pemmaraju:CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karger Publishers: Other: Licenses; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASCO Cancer.Net Editorial Board: Other: Leadership; United States Department of Defense (DOD): Research Funding; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kantarjian:Abbvie: Consultancy, Honoraria; Ascentage Pharma Group: Honoraria; Novartis (Inst): Research Funding; Bristol-Myers Squibb (Inst): Research Funding; Novartis: Honoraria; Taiho Pharmaceutical: Honoraria; Pfizer: Honoraria; Precision Biosciences: Honoraria; Amgen: Honoraria; Daiichih-Sankyo (Inst): Honoraria, Research Funding; Immunogen (Inst): Honoraria, Research Funding; Ipsen: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; Shenzhen Target Rx: Honoraria; Ascentage Pharma (Inst): Research Funding; AstraZeneca/MedImmune: Honoraria; Astellas Pharma: Honoraria; Amgen (Inst): Research Funding; Abbvie (Inst): Research Funding; KAHR Medical: Honoraria. Masarova:MorphoSys US: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal