Introduction: Fludarabine and cyclophosphamide (Flu/Cy) lymphodepletion (LD) prior to chimeric antigen T-cell (CAR T) infusion favorably impacts CAR T expansion and efficacy. A national shortage of Flu prompted our center to explore substitute Flu with Cladribine (Cla) as part of Cla/Cy LD. Here, we report our experience with this regimen prior to brexucabtagene autoleucel (brexu-cel) for patients with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) and mantle cell lymphoma (MCL).
Methods: We performed a retrospective, single-center analysis of consecutive patients who received brexu-cel for RR B-ALL and MCL at Moffitt Cancer Center (1/2022 and 4/2023). The Cla/Cy LD regimen utilized Cy at 500 mg/m 2 x 3 days for MCL or 900 mg/m 2 x 1 day for B-ALL (two patients received MCL dosing) and substituted Flu 30 mg/m 2 with Clad 5 mg/m 2 x 3 days. We compared best overall response (BOR), including measurable residual disease (MRD) status by the clonoSEQ® Assay (Adaptive Biotechnologies Corporation, Seattle, USA) ± PCR for B-ALL, event-free survival (EFS, event = progression or death) and overall survival (OS) in those receiving standard Flu/Cy vs Clad/Cy LD. CRS and ICANS were graded as per ASTCT criteria. For patients with a sustained response ≥6 months and who did not undergo stem cell transplant, we assessed serial absolute neutrophil counts (ANC), CD4, CD8, CD19 counts and IgG levels post-CAR T. The HEMATOTOX score (HS, Rejeski et al, 2021) was used to stratify patients for risk of post-CAR T cytopenias.
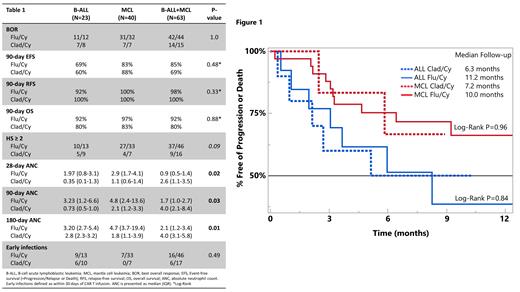
Results: We analyzed 63 patients who received brexu-cel: 23 with ALL (10 Cla/Cy) and 40 with MCL (7 Cla/Cy). Rates of CRS grade ≥ 3 (12% vs Flu/Cy 4%; p=0.29) and ICANS grade ≥ 3 (24% vs Flu/Cy 40%; p = 0.25) did not differ with LD. Two ALL (1 Flu/Cy, 1 Clad/Cy) and one MCL (Flu/Cy) patients died prior to day 28 reassessment. BOR did not differ by LD and histology (Figure 1). MRD negativity in ALL was also comparable (Cla/Cy 5/7, Flu/Cy 10/11, p=0.52).
Among ALL patients, 5 received alloSCT consolidation (1 Cla/Cy). Progression was noted in 3/8 Cla/Cy and 6/11 Flu/Cy patients (p=1.0). Fifteen ALL patients experienced early infections (6/10 Cla/Cy, 9/13 FluCy, p=0.69), including bacterial (5), viral (4), and fungal (3). Three deaths (2 Cla/Cy) occurred before day 100, all due to refractory fungemia post anakinra. Two later deaths were attributed to relapse (both Flu/Cy).
Among MCL patients, progression was noted in 16 (1 Cla/Cy). Seven MCL patients experienced early infection (all Flu/Cy), including bacterial (6), fungal (2), and viral (1). Two deaths occurred before day 100, 1 related to CRS and fungemia post anakinra (Flu/Cy), and another from stroke (Cla/Cy). Four (all Flu/Cy) have developed a second cancer (2 MDS/AML, 1 prostate, 1 liver), and 1 each died in remission from late infection (sepsis) and intracranial hemorrhage (both Flu/Cy).
Cla/Cy patients had higher median ANCs at 28, 90, and 180 days (Table 1). When only patients with HS ≥ 2 were considered, 28-day ANC remained higher after Cla/Cy LD (1.57 vs Flu/Cy 0.67; p=0.04), numerically higher at 90 days (2.36 vs Flu/Cy 1.82; p=0.38) and again significantly higher at 180 days (3.96 vs Flu/Cy 1.2; p=0.05). Median CD4 was higher for Cla/Cy patients at day 28 (0.232 vs Flu/Cy 0.121; p=0.04), and numerically higher at day 90 (0.244 vs Flu/Cy 0.152; p=0.31) and 180 (0.254 vs Flu/Cy 0.161; p=0.21). Median CD8 was similar at day 28 (0.197 vs 0.155; p=0.46), 90 (0.435 vs Flu/Cy 0.167; p=0.65), and 180 (0.557 vs 0.220; p=0.14).
There was no difference in IgG levels at day 28 (502 vs Flu/Cy 466; p=0.87), 90 (385 vs Flu/Cy 393; p=0.76), or 180 (424 vs Flu/Cy 426; p=0.94). Median CD19 was greater for Cla/Cy at day 90 (median 0.095 vs 0.000; p<0.01) and numerically higher at day 180 (0.213 vs Flu/Cy 0.000; p=0.29).
Median duration of followup was 245d for ALL (FluCy 361d, ClaCy 194d) and 746d for MCL (FluCy 812d, ClaCy 215d). Median DOR, EFS, and OS did not differ by LD (Figure 1).
Conclusions: Cla/Cy LD prior to brexu-cel is feasible in ALL and MCL patients with comparable efficacy. Toxicity rates including CRS, ICANS and early infections were similar to those seen with Flu/Cy, but there was a trend towards earlier recovery of neutrophils, B-, and T-cells with Cla/Cy. The trend was demonstrable even in patients at high risk of prolonged post-CAR T neutropenia as identified by the HS. Longer follow-up and correlative analyses are warranted to further explore differences between Cla/Cy and Flu/Cy.
Disclosures
Faramand:Kite: Research Funding; Gilead: Research Funding. Bachmeier:Kite Pharma: Consultancy. Locke:GammaDelta Therapeutics: Consultancy; Wugen: Consultancy; Umoja: Consultancy; Clinical Care Options Oncology: Other; Daiichi Sankyo: Consultancy; ASH: Other; CERo Therapeutics: Other: Institutional; Cowen: Consultancy; Gerson Lehrman Group (GLG): Consultancy; Legend Biotech: Consultancy; Emerging Therapy Solutions: Consultancy; Individual Patents: Patents & Royalties: Several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy; Takeda: Consultancy; BioPharma Communications CARE Education: Other; Iovance: Consultancy; EcoR1: Consultancy; Kite, a Gilead Company: Consultancy, Other: Institutional ; Novartis: Consultancy, Other: Institutional ; Aptitude Health: Other; Sana: Consultancy; Janssen: Consultancy; Cellular Biomedicine Group: Consultancy; Caribou: Consultancy; Calibr: Consultancy; BMS/Celgene: Consultancy, Other: Institutional ; bluebird bio: Consultancy, Other: Institutional ; Amgen: Consultancy; Allogene: Consultancy, Other: Institutional ; A2: Consultancy, Other: Travel support; Imedex: Other; National Cancer Institute: Other: Institutional ; Leukemia and Lymphoma Society: Other: Institutional ; Society for Immunotherapy of Cancer: Other: Institutional . Jain:Myeloid Therapeutics: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria, Research Funding; Loxo@Lilly: Research Funding; Incyte: Research Funding. Shah:Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, Kite/Gilead: Other: Travel, Accommodations, Expenses; Takeda, AstraZeneca, Adaptive Biotechnologies, BMS/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, Kite/Gilead, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus Therapeutics, Lilly, Pepromene: Consultancy; Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, Gilead Sciences: Honoraria; Incyte, Jazz Pharmaceuticals, Kite/Gilead, SERVIER: Research Funding; DSMC, Pepromene Bio: Membership on an entity's Board of Directors or advisory committees; Moffitt Cancer Center: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal