Visual Abstract
Bispecific antibodies (BsAb) that target CD3 and CD20 represent a new milestone in the treatment of patients with B-cell non-Hodgkin lymphoma. These drugs have demonstrated remarkable single-agent activity in patients with heavily pretreated disease, and 3 drugs have so far received regulatory approvals in various countries. However, BsAbs can potentially lead to severe toxicity associated with T-cell activation, particularly cytokine release syndrome (CRS). The anticipated widespread use of these off-the-shelf products poses challenges for implementation and highlights the need for guidance in anticipating, mitigating, and managing adverse events. In clinical trials, guidance for the evaluation and treatment of CRS and neurotoxicity associated with BsAb therapy has been modeled after algorithms originally created for chimeric antigen receptor (CAR) T-cell therapies and other immune effector therapies, yet notable differences in timing, quality, and severity exist between the toxicities of BsAbs and CAR T-cell therapies. We therefore convened an international panel of academic and community practice physicians, advanced practitioners, registered nurses, and pharmacists with experience using CD3×CD20 BsAbs in clinical trial and off-trial settings to provide comprehensive, consensus-based recommendations specific to the assessment and management of CD3×CD20 BsAb–related toxicities.
Background
Bispecific antibodies (BsAbs) are synthetic proteins that can bind 2 antigens simultaneously.1 Most BsAbs in clinical development work by redirecting T or natural killer cells to tumor-associated antigens in an Fcγ receptor– and major histocompatibility complex–independent manner, thereby activating an endogenous antitumor immune response.2 Although active in many hematologic and solid malignancies, these agents have demonstrated especially promising results in the treatment of B-cell non-Hodgkin lymphomas (B-NHLs). Recently, several CD3×CD20 BsAbs have been approved by the US Food and Drug Administration, European Medicines Agency (EMA), and Health Canada for patients with relapsed/refractory follicular lymphoma or relapsed/refractory diffuse large B-cell lymphoma.3-7 BsAbs alone or in combinations are currently being studied in a variety of B-NHL subtypes and settings.
While remarkably active, BsAbs can be associated with toxicities related to immune activation, notably cytokine release syndrome (CRS; Table 1). The product labels for the 3 US Food and Drug Administration– and EMA-approved CD3×CD20 BsAbs, mosunetuzumab, epcoritamab, and glofitamab, refer to practice guidelines for the management of CRS. However, there are currently no BsAb-specific consensus guidelines, and guidance is typically modeled after recommendations developed for chimeric antigen receptor (CAR) T-cell therapy without taking into account key differences between the toxicity profiles of these classes of drugs.8 Specifically, BsAbs appear to be associated with a lower incidence and severity of key toxicities such as CRS and neurotoxicity than CAR T-cell therapy. As such, BsAbs have the potential to be administered to a broader patient population, in both academic and community settings. Therefore, there is an acute need for focused, consensus-based guidelines to help clinicians safely manage BsAb-related immune activation toxicities.
Comparison of structure, administration, CRS, and neurotoxicity associated with CD3×CD20 BsAbs in NHL
| Drug . | Mosunetuzumab3 . | Epcoritamab4 . | Glofitamab5 . | Odronextamab6,7 . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure | Fully humanized IgG1 CD3×CD20 BsAb with 1:1 CD3:CD20 ratio of Fab arms | IgG-like anti-CD3×CD20 BsAb. Proprietary format, with point mutations in the Fab portion of the Fc of the antibody and heterodimerization. | Humanized mouse-derived BsAb with 1:2 CD3:CD20 ratio of Fab arms | Fully humanized IgG4 anti-CD3×CD20 BsAb developed using an Fc domain with a mutation in the protein A of the Fc portion | ||||||||||||||||
| Route of administration | IV | SC | IV | IV | ||||||||||||||||
| Dosing schedule | C1: days 1, 8, 15; C2+: day 1, every 21 d, for up to 8 cycles in CR or up to 17 cycles for PR or SD | C1-3: days 1, 8 ,15, and 22; C4-9: days 1 and 15; C10+: day 1, every 28 d until progression | C1: obin, day 1; glofit, days 8 and 15; C2-12: day 1, every 21 d | C1: days 1, 2, 8, 9, 15, 16 of a 21-d cycle; C2-4: days 1, 8, 15 of a 21-d cycle; C5+: day 1, every 14 d; If CR for at least 9 mo: day 1, every 28 d | ||||||||||||||||
| CRS mitigation | ||||||||||||||||||||
| Step-up dosing | C1D1: 1 mg C1D8: 2 mg C1D15: 60 mg C2D1: 60 mg C3+D1: 30 mg | C1D1: 0.16 mg C1D8: 0.8 mg C1D15: 48 mg C1D22: 48 mg C2D1+: 48mg | C1D1: obin 1000 mg C1D8: 2.5 mg C1D15: 10 mg C2D1+: 30 mg | C1D1: 0.2 mg C1D2: 0.5 mg C1D8: 2 mg C1D9: 2 mg C1D15: 10 mg C1D16: 10 mg C2-C4: 80 mg (FL) or 160 mg (DLBCL) C5+: 160 mg (FL) or 320 mg (DLBCL) | ||||||||||||||||
| Premedications | (1) A/P 500-1000 mg, 30 min prior, for C1 and C2 (2) Diphenhydramine 50-100 mg, 30 min prior, for C1 and C2 (3) Dexamethasone 20 mg or methylprednisolone 80 mg, 1 h prior, for C1 and C2. Continue all premedications if CRS with prior dose. | (1) A/P 650-1000 mg, 30-120 min before C1 treatments (2) Diphenhydramine 50 mg, 30-120 min before C1 treatments (3) Dexamethasone 15 mg, 30-120 min before C1 treatments and for 3 consecutive days after. Continue dexamethasone thereafter if G2 or G3 CRS with prior dose. | (1) A/P 500-1000 mg, 30 min before all treatments (2) Diphenhydramine 50 mg, 30 min before all infusions (3) Dexamethasone 20 mg, 1 h before treatment on C1D8, C1D15, C2D1, and C3D1. Continue if CRS with prior dose. | (1) A/P 650 mg, 30-60 min prior, during step-up dosing, continue if IRR or CRS with prior dose (2) Diphenhydramine 25 mg, 30-60 min prior during step-up dosing, continue if IRR or CRS with prior dose (3) Dexamethasone 10 mg orally, 12-24 h before split dose, 20 mg IV on day of dosing, 10 mg orally on the day after step-up dosing. Following first full dose, dexamethasone 10 mg before dosing; continue if CRS with prior dose. | ||||||||||||||||
| Hospitalization | Optional | C1D15: 24-h admission | C1D8: 24-h admission | Performed during step-up dosing | ||||||||||||||||
| CRS occurrence | G1 | G2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 |
| 26% | 17% | 1% | 1% | 0% | 34% | 15% | 3% | 0% | 0% | 47% | 12% | 3% | 1% | 0% | 35%-39% | 13% (DLBCL) | 0% | 0% | 0% | |
| Time course for CRS onset | Median time (h) to CRS onset | Time course for CRS onset | Median time (h) to CRS onset | Time course for CRS onset | Median time (h) to CRS onset | Time course for CRS onset | Median time (h) to CRS onset | |||||||||||||
| C1D1: 23.3% C1D8: 5.6% C1D15: 36.4% C2D1: 10.3% C3+D1: 2.4% | C1D1: 5 C1D8: 20 C1D15: 27 C2D1: 38 | C1D1: 5.8% C1D8: 11.8% C1D15: 42.8% C1D22: 4.9% C3+ 3% | All doses: 24 C1D15: 20 | C1D8: 42.8% C1D15: 25.2% C2: 26% C3+: 0.9% | C1D8: 13.5 (range: 6-52) | C1D1/2: 22%-24% C1D8/9: 27%-32% C1D15/16: 21%-35% C2D1: 14%-17% C2D8+: 9%-14% | All doses: 18-20 | |||||||||||||
| Median duration of CRS | 3 d (1-29 d) | 2 d (range: 1-27 d) | 30.5 h (range, 0.5-317 h) | 8-10 h (range, 0.1-190 h) | ||||||||||||||||
| Neurotoxicity | G 1-2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 | G 1-2 | G 3-4 | G5 | G 1-2 | G 3-4 | G5 | |||||
| 3% | 0% | 0% | 0% | 4.5% | 1.3% | 0% | 0% | 0.6% | 5% | 3% | 0% | 4% (DLBCL) | 0% | 0% | ||||||
| Drug . | Mosunetuzumab3 . | Epcoritamab4 . | Glofitamab5 . | Odronextamab6,7 . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure | Fully humanized IgG1 CD3×CD20 BsAb with 1:1 CD3:CD20 ratio of Fab arms | IgG-like anti-CD3×CD20 BsAb. Proprietary format, with point mutations in the Fab portion of the Fc of the antibody and heterodimerization. | Humanized mouse-derived BsAb with 1:2 CD3:CD20 ratio of Fab arms | Fully humanized IgG4 anti-CD3×CD20 BsAb developed using an Fc domain with a mutation in the protein A of the Fc portion | ||||||||||||||||
| Route of administration | IV | SC | IV | IV | ||||||||||||||||
| Dosing schedule | C1: days 1, 8, 15; C2+: day 1, every 21 d, for up to 8 cycles in CR or up to 17 cycles for PR or SD | C1-3: days 1, 8 ,15, and 22; C4-9: days 1 and 15; C10+: day 1, every 28 d until progression | C1: obin, day 1; glofit, days 8 and 15; C2-12: day 1, every 21 d | C1: days 1, 2, 8, 9, 15, 16 of a 21-d cycle; C2-4: days 1, 8, 15 of a 21-d cycle; C5+: day 1, every 14 d; If CR for at least 9 mo: day 1, every 28 d | ||||||||||||||||
| CRS mitigation | ||||||||||||||||||||
| Step-up dosing | C1D1: 1 mg C1D8: 2 mg C1D15: 60 mg C2D1: 60 mg C3+D1: 30 mg | C1D1: 0.16 mg C1D8: 0.8 mg C1D15: 48 mg C1D22: 48 mg C2D1+: 48mg | C1D1: obin 1000 mg C1D8: 2.5 mg C1D15: 10 mg C2D1+: 30 mg | C1D1: 0.2 mg C1D2: 0.5 mg C1D8: 2 mg C1D9: 2 mg C1D15: 10 mg C1D16: 10 mg C2-C4: 80 mg (FL) or 160 mg (DLBCL) C5+: 160 mg (FL) or 320 mg (DLBCL) | ||||||||||||||||
| Premedications | (1) A/P 500-1000 mg, 30 min prior, for C1 and C2 (2) Diphenhydramine 50-100 mg, 30 min prior, for C1 and C2 (3) Dexamethasone 20 mg or methylprednisolone 80 mg, 1 h prior, for C1 and C2. Continue all premedications if CRS with prior dose. | (1) A/P 650-1000 mg, 30-120 min before C1 treatments (2) Diphenhydramine 50 mg, 30-120 min before C1 treatments (3) Dexamethasone 15 mg, 30-120 min before C1 treatments and for 3 consecutive days after. Continue dexamethasone thereafter if G2 or G3 CRS with prior dose. | (1) A/P 500-1000 mg, 30 min before all treatments (2) Diphenhydramine 50 mg, 30 min before all infusions (3) Dexamethasone 20 mg, 1 h before treatment on C1D8, C1D15, C2D1, and C3D1. Continue if CRS with prior dose. | (1) A/P 650 mg, 30-60 min prior, during step-up dosing, continue if IRR or CRS with prior dose (2) Diphenhydramine 25 mg, 30-60 min prior during step-up dosing, continue if IRR or CRS with prior dose (3) Dexamethasone 10 mg orally, 12-24 h before split dose, 20 mg IV on day of dosing, 10 mg orally on the day after step-up dosing. Following first full dose, dexamethasone 10 mg before dosing; continue if CRS with prior dose. | ||||||||||||||||
| Hospitalization | Optional | C1D15: 24-h admission | C1D8: 24-h admission | Performed during step-up dosing | ||||||||||||||||
| CRS occurrence | G1 | G2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 |
| 26% | 17% | 1% | 1% | 0% | 34% | 15% | 3% | 0% | 0% | 47% | 12% | 3% | 1% | 0% | 35%-39% | 13% (DLBCL) | 0% | 0% | 0% | |
| Time course for CRS onset | Median time (h) to CRS onset | Time course for CRS onset | Median time (h) to CRS onset | Time course for CRS onset | Median time (h) to CRS onset | Time course for CRS onset | Median time (h) to CRS onset | |||||||||||||
| C1D1: 23.3% C1D8: 5.6% C1D15: 36.4% C2D1: 10.3% C3+D1: 2.4% | C1D1: 5 C1D8: 20 C1D15: 27 C2D1: 38 | C1D1: 5.8% C1D8: 11.8% C1D15: 42.8% C1D22: 4.9% C3+ 3% | All doses: 24 C1D15: 20 | C1D8: 42.8% C1D15: 25.2% C2: 26% C3+: 0.9% | C1D8: 13.5 (range: 6-52) | C1D1/2: 22%-24% C1D8/9: 27%-32% C1D15/16: 21%-35% C2D1: 14%-17% C2D8+: 9%-14% | All doses: 18-20 | |||||||||||||
| Median duration of CRS | 3 d (1-29 d) | 2 d (range: 1-27 d) | 30.5 h (range, 0.5-317 h) | 8-10 h (range, 0.1-190 h) | ||||||||||||||||
| Neurotoxicity | G 1-2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 | G 1-2 | G 3-4 | G5 | G 1-2 | G 3-4 | G5 | |||||
| 3% | 0% | 0% | 0% | 4.5% | 1.3% | 0% | 0% | 0.6% | 5% | 3% | 0% | 4% (DLBCL) | 0% | 0% | ||||||
A/P, acetaminophen (paracetamol); C, cycle; CR, complete response; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; G, grade; glofit, glofitamab; IgG, immunoglobulin G; IRR, infusion-related reaction; obin, obinutuzumab; PR, partial response; SC, subcutaneous; SD, stable disease.
With support from the Lymphoma Research Foundation, a panel of investigators with experience in the use of BsAbs for the treatment of lymphoma was created. The panel also included community-based practitioners including physicians, nurses, and pharmacists to ensure that the document would reflect the needs of community-based practices. The overarching goal of this project was to develop expert consensus recommendations specific to the assessment and management of CD3×CD20 BsAb–related adverse events (AEs). Specifically, our aims were to provide: (1) background on the toxicities seen with BsAbs; (2) expert consensus recommendations for pretreatment testing and planning; and (3) recommendations regarding optimal management of CRS, neurologic toxicity, and other toxicities seen with BsAbs. At this stage in the development of CD3×CD20 BsAbs, there are insufficient data to enable high-level evidence-based guidelines. Thus, this work aims at translating the collective knowledge of experienced providers into the development of consensus recommendations. At the same time, the BsAb field is in rapid evolution, with new agents being developed and combinations explored. As this research matures, and on- and off-trial experience grows, the understanding and optimal management of BsAbs toxicity will evolve and require updates and intravenous (IV) refinement to recommendations.
Methods
The Delphi method was adapted to achieve consensus in recommendations.9,10 An initial group reviewed and provided input on key questions related to the evaluation and management of toxicity associated with BsAbs using an ad hoc questionnaire. Thereafter, the panel met virtually over a series of 3 workshops in the spring of 2023. In the first meeting, relevant clinical trial data were reviewed, whereas in the second and third, answers to the aforementioned questionnaires were reviewed and discussed with focus on areas of disagreement until consensus was reached. The group was extended to include broader representation, at which time recommendations were recirculated for input and discussion, and consensus obtained from the entire group by a series of exchanges on remaining points of controversy. Nonbinding feedback was also solicited from key representatives from Genmab A/S, AbbVie Inc, Genentech Inc, Roche AG, and Regeneron Inc, the pharmaceutical companies who sponsored relevant BsAbs clinical trials for additional input on trial data, including key AEs and their management, and the prescribing labels. Authors met with representatives from each company to obtain feedback once. The companies did not provide any funding or other forms of support for these recommendations and were not provided opportunities for iterative comments.
Summary of toxicities observed with CD3×CD20 BsAbs
The most common AE after administration of CD3×CD20 BsAbs is CRS, an acute, systemic inflammatory syndrome characterized by fever, which can be complicated by hypotension, hypoxia, organ dysfunction, or hyperinflammatory syndromes.8 Prophylactic strategies, like step-up dosing during treatment initiation, have been used to mitigate CRS risk across BsAb clinical trials (Table 1). The incidence, timing, and onset of CRS vary by disease subtype, BsAb product, route of administration (IV vs subcutaneous), and dosing schedule (Table 1). In clinical trials, grading of CRS was adjudicated per the 2019 American Society of Transplantation and Cellular Therapy criteria, once available.8 The majority of CRS was grade 1 (20%-50%) to 2 (5%-29%), although higher grade CRS (1%-7%) has been reported.3-7
Neurologic toxicities have also been seen in clinical trials with BsAbs. These were recorded as per the Common Terminology Criteria for Adverse Events as well as the 2019 American Society of Transplantation and Cellular Therapy criteria, both of which were developed to describe immune effector cell–associated neurologic syndrome (ICANS).8 To date, evidence of circulating BsAb drug molecules, activated T cells, or increased proinflammatory cytokines in the cerebrospinal fluid of patients experiencing neurotoxicity while receiving BsAb is lacking. Additionally, mural cells in the brain are known to express CD19 (the target of commercially available CAR T cells) but not CD20 (the target of CD3×CD20 BsAbs).11 In line with these observations, neurotoxicity associated with BsAb therapy is less common and, generally, of lower grade than CAR T-cell–induced ICANS. Additionally, neurologic AEs seen in BsAb trials were often clinically different from ICANS and most frequently consisted of headache and dizziness. Overall, ICANS-like toxicity, including delirium, dysgraphia, tremor, lethargy, difficulty concentrating, etc, was rare (1%-8%) across studies.3-7
Other toxicities discussed in greater detail hereafter include tumor flare reaction, cytopenias, and infectious complications. Tumor flare rarely occurred in clinical trials, with <3% of patients experiencing grade ≥3 symptoms.3-7 Cytopenias are frequently seen after treatment with BsAbs and, although the pathogenesis is poorly understood, it is likely that these AEs are multifactorial in nature (eg, related to disease involvement, prior therapies, and direct BsAb effect). Grade ≥3 anemia and thrombocytopenia occurred in 8% to 19% of patients, whereas neutropenia occurred in 26% to 38% of patients.3-7 Infections are also a concern with BsAbs, because of the potential for B-cell and T-cell impairment and lymphopenia. Infectious complications seen in clinical trials included febrile neutropenia (<5% of patients), urinary tract infections, pneumonia, and COVID-19.3-7
Recommendations on the management of CRS
Pretreatment testing
The panel recommends performing a comprehensive physical examination and routine baseline laboratory testing, including a complete blood count with differential, comprehensive metabolic panel, and lactate dehydrogenase before treatment initiation. The group agreed that baseline testing of cytokine levels, ferritin, C-reactive protein, or other markers of inflammation, is currently of unclear value in predicting CRS occurrence and severity, and should be considered optional in clinical practice.
Baseline cardiac ultrasound or multigated acquisition scan are not required and should be performed as clinically indicated. Although BsAbs are not known to cause direct cardiotoxicity, baseline cardiac evaluation may be helpful to understand a patient’s baseline cardiac reserve and ability to withstand CRS-related hemodynamic changes. In this sense, development of grade 1 CRS in patients with known cardiac comorbidities may prompt more aggressive management to avoid development of hemodynamic instability seen with higher grade CRS. Finally, knowledge of underlying heart disease may affect decisions on treatment location because fluid resuscitation in the outpatient setting may be challenging.
Although additional evidence is required to further identify individuals at highest risk of toxicity related to CD3×CD20 BsAbs, this risk may be higher in both patients who are unable to tolerate toxicity (eg, patients with comorbidities or advanced age) and patients with high-risk disease biology (eg, patients with high disease burden or circulating disease). Closer monitoring may be beneficial in these patient populations until risk is more clearly defined.
Recommendation for evaluation, identification, and management of CRS
Pretreatment phase
A plan for early diagnosis and management of CRS should be discussed before starting CD3×CD20 BsAb therapy. Patients may not need to remain within a specific distance from the treating facility but a nearby hospital with intensive care unit capabilities and at least 2 tocilizumab doses available at all times should be identified. Some members of the panel recommend that, if possible, patients remain within 2 hours of the treating facility during step-up dosing. Developing a “BsAb team” consisting of physicians, advanced practitioners, registered nurses, and pharmacists, may improve care and decision making. A relationship between the outpatient oncology group and hospital system should be in place to ensure prompt communication and appropriate medication and inpatient support if needed (Table 2).
Logistical considerations before the start of CD3×CD20 BsAbs
| Facility |
|
| Personnel |
|
| Patient resources |
|
| Patient and caregiver education |
|
| Facility |
|
| Personnel |
|
| Patient resources |
|
| Patient and caregiver education |
|
Patient education and monitoring
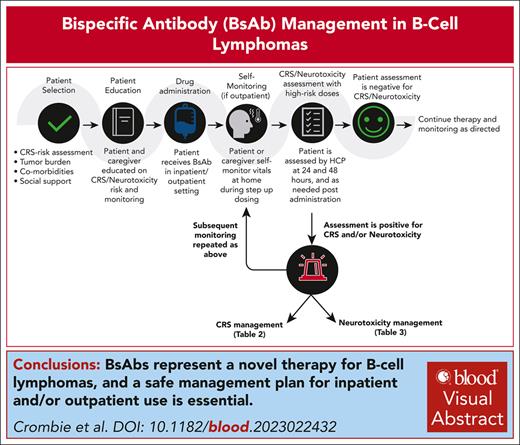
Patients and their caregivers should be provided resources that educate on monitoring, and management of toxicities. These include, but are not limited to, educational materials regarding signs and symptoms of CRS and neurotoxicity, health care team contact information, and instructions for vital sign self-monitoring (eg, measuring temperature). Instructions should include detailed information on when and whom to call, and when to present directly to the preidentified emergency department (ED; Figure 1). In particular, we stress monitoring for specific vital sign changes (eg, fever of >100.4°F) and clinical symptoms of hypoxia or hypotension even if a pulse oximeter and blood pressure cuff is unavailable for home testing. Patients should possess contact numbers to use if symptoms arise at home to allow for effective triaging. Call center staff should also be educated to triage symptoms promptly and appropriately. Patient baseline vital parameters (temperature, pulse oximetry, and blood pressure) should be recorded and may be provided to the patient or caregiver before therapy initiation to serve as baseline values. Patients and their caregivers, when able, should be educated to monitor and record vital signs and symptoms of CRS when outside the health care setting. It is recommended by the panel that patients have thermometers for home temperature monitoring. If available, blood pressure cuffs and pulse oximeters can be used for additional home monitoring, although home use was not universally recommended by the panel. Most members recommend that patients be instructed to monitor their temperature at home 3 times per day for 48 hours after each step-up dose in the outpatient setting. In patients at higher risk and for those with less social support, the panel suggest consideration of a 24-hour and possibly a 48-hour postdose phone call during step-up dosing to follow-up on vital signs and inquire about additional symptoms. All members of the BsAb team can, and should, assist with monitoring and management based on practice-specific training protocols, comfort, and staffing. Carrying a wallet card, which highlights potential AEs of BsAbs and appropriate contact information, is also strongly recommended.
Treatment location
Recognizing that available clinical resources and infrastructure vary geographically, monitoring for CRS may occur in the outpatient or inpatient settings during the period of highest CRS risk. The site of monitoring should generally follow the prescribing label and should be tailored specifically to each clinical site’s capabilities. If a patient lives far from the treating facility, hospitalization for 24 hours after each step-up dose can also be considered. Regardless, it is important to delineate the workflow to escalate CRS care if needed. Although not required per prescribing labels, when step-up dosing occurs in the outpatient setting, clinicians should consider monitoring patients for 1 to 2 hours after dosing and consider admission or transfer to the ED, as necessary, if CRS occurs. Institutional electronic health record systems, if available, can be used to alert providers entering the patient’s chart that the patient is receiving BsAb and is at risk for CRS/neurotoxicity. Additionally, institutions can work closely on education with neighboring ED staff on recognition and acute management of CRS and neurological AEs.
Premedications
Premedications, including the use of prophylactic corticosteroids, should be administered per the prescribing label for each BsAb as outlined in Table 1. Dexamethasone is generally preferred, based on observations suggesting a lower incidence of CRS than with other corticosteroids.5 Corticosteroids before and after administration can be discontinued after cycle 2 is complete if no CRS occurred.
Management of CRS
Fever that occurs in the context of BsAb IV infusion can be difficult to distinguish from infusion-related reactions. In these cases, given the potential for more severe CRS, the panel recommends withholding further infusion on that treatment day.
Defining features of CRS by grade are listed in Table 3. The panel agreed that the management of grade 1 CRS may be initiated in the outpatient setting, and referral to the clinic or ED should be considered on a case-by-case basis. Patients should be instructed to take acetaminophen (paracetamol) (eg, 650-1000 mg) or another antipyretic (eg, nonsteroidal anti-inflammatory drugs) at the time of fever onset. For recurrent fever (eg, ≥6-8 hours after the first episode), without any additional concerning symptoms, antipyretics may be repeated.
Proposed management of CRS for CD3×CD20 BsAbs according to severity
| |
| Grade and definition | Management |
| Grade 1: Fever∗ of ≥100.4°F with/without constitutional symptoms requiring symptomatic treatment, no hypotension or hypoxia | Home:
|
| Grade 2: Fever of ≥100.4°F with either hypotension not requiring pressors and/or hypoxia managed with low-flow nasal canula or blow-by. |
|
| Grade 3: Fever of ≥100.4°F with either hypotension (BP <90/60 or <10 mmHg below, not responsive to fluids and/or hypoxia requiring high-flow nasal canula, face mask, or venturi mask) |
|
| Grade 4: Fever of ≥100.4°F with any of the following: Life-threatening consequences, urgent intervention required; requiring multiple pressors and/or positive pressure respiratory support or mechanical intubation. |
|
| |
| Grade and definition | Management |
| Grade 1: Fever∗ of ≥100.4°F with/without constitutional symptoms requiring symptomatic treatment, no hypotension or hypoxia | Home:
|
| Grade 2: Fever of ≥100.4°F with either hypotension not requiring pressors and/or hypoxia managed with low-flow nasal canula or blow-by. |
|
| Grade 3: Fever of ≥100.4°F with either hypotension (BP <90/60 or <10 mmHg below, not responsive to fluids and/or hypoxia requiring high-flow nasal canula, face mask, or venturi mask) |
|
| Grade 4: Fever of ≥100.4°F with any of the following: Life-threatening consequences, urgent intervention required; requiring multiple pressors and/or positive pressure respiratory support or mechanical intubation. |
|
A/P, acetaminophen (paracetamol); BP, blood pressure; ICU, intensive care unit; MAS, macrophage activation syndrome.
Patients treated with antipyretics or corticosteroids in rare instances may not experience fever as a presenting symptom of CRS.
Tocilizumab dosing: 8 mg/kg IV. Tocilizumab should not be administered more than twice per CRS event (at least 8 hours apart) or 3 times within a 6-week period.
Some members recommend that patients with fevers refractory to standard antipyretic or recurrent (eg, within 6-8 hours), receive dexamethasone (eg, at a dose of 10 mg), although continued observation can also be considered. Many members of the panel recommend providing patients with a home prescription for dexamethasone (eg, 10 mg) before start of therapy to be taken in case of CRS. In this context, patients should only take dexamethasone after discussion with the treating care team. If the patient is able to monitor vital signs (including blood pressure or oxygen saturation) and those remain normal (other than temperature), home administration of dexamethasone can be considered, although subsequent outpatient evaluation is typically recommended. Other members of the panel recommended dexamethasone only after in-person evaluation. For patients unable to monitor vitals at home, prompt evaluation at a prespecified medical center is recommended. Patients whose fever persists or recurs despite dexamethasone should be evaluated in a health care facility. Dexamethasone can be continued every 24 hours until resolution of symptoms with continued monitoring and guidance from the health care team. For patients at higher risk of CRS complications (as defined earlier), the panel recommends urgent in-person evaluation at a dedicated clinic or ED. The panel suggests considering anticytokine therapy for patients with grade 1 CRS and persistent symptoms (>48 hours) despite antipyretics and dexamethasone. Tocilizumab, a monoclonal antibody that binds interleukin-6 receptor, was the most used anticytokine agent in clinical trials of BsAbs and is the agent recommended by the panel. For all patients with fever, alternative etiologies including infection, should always be considered.
Management of patients with grade 2 CRS should occur urgently and in a health care facility, typically an ED. In addition to continuing acetaminophen (paracetamol), patients should receive dexamethasone at the dose of at least 10 mg, at least every 12 hours, while symptoms persist. Hypotension, one of the defining features of grade 2 CRS, should be corrected with IV fluid boluses. The amount and rate of IV fluids should be modulated depending on the patient’s cardiac and renal reserve. Although the site of care for grade 2 CRS may vary, if patients are evaluated in the outpatient setting, they should be closely monitored and admitted if CRS worsens. Generally, patients should be referred to the inpatient setting for ongoing management.
In patients potentially at high risk of severe CRS, such as those with persistent grade 2 CRS after administration of dexamethasone, or those at risk of poor outcome with CRS (eg, patients with comorbidities), the panel recommends early addition of anticytokine therapy (eg, tocilizumab) with the aim of preventing progression to higher-grade CRS. Tociluzumab may also be considered for patients who develop grade 2 CRS while receiving corticosteroids as primary CRS prophylaxis after BsAb dosing, as is recommended for epcoritamab and odronextamab. The EMA labels for glofitamab and mosunetuzumab suggest that tocilizumab should not be administered more than twice per CRS event (at least 8 hours apart) or 3 times within a 6-week period. If >3 doses are required during a 6-week period, alternative anticytokine therapy (eg, anakinra, an interleukin-1 receptor antagonist) should be considered. While acknowledging the widespread use of anticytokine therapy for patients with refractory CRS, the panel stressed the absence of data at present on the outcomes of patients with grade 2 CRS treated without anticytokines. Additionally, the panel noted that, at the time of writing this report, no anticytokine therapy had received regulatory approval for the treatment of BsAb-related CRS.
Grade 3 CRS is a medical emergency and should be managed promptly and aggressively. Patients should be transferred to the intensive care unit if not already admitted to such unit. Antipyretics and corticosteroids should be initiated or continued (Table 3). The panel acknowledged that in this context the optimal type, dose, and schedule of corticosteroids are not established, and different centers have successfully used different regimens. A commonly used schedule includes dexamethasone 10 mg, administered IV every 6 hours, in addition to anticytokine therapy. There is no conclusive evidence supporting changing the anticytokine agent during CRS. Tociluzumab dosing may be repeated as outlined earlier, and alternative anticytokine therapy can be considered after maximal tocilizumab dosing. Hemodynamic and respiratory support with vasopressor agents and advanced oxygen therapy should be implemented according to local institutional practice. Aggressive treatment should continue until resolution of CRS to grade ≤1. The panel recommends tapering, rather than abruptly discontinuing, corticosteroids.
In cases of atypical CRS presentations (eg, persistent CRS-like symptoms for ≥1 week despite appropriate supportive measures; febrile illness outside of the normal CRS timeframes, or with accompanying significant organ dysfunction), the panel recommends implementing a diagnostic workup to rule out alternative diagnoses, such as infections or hemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome.12 Given lack of experience with the latter, the panel recommends that evaluation and management of BsAb-related HLH/macrophage activation syndrome follow that of immune effector cell–associated HLH-like syndrome.12
Grade 4 CRS is a rare but life-threatening complication. It should be managed aggressively and consistently with the patient’s goals of care. All supportive measures outlined for the management of grade 3 CRS should be continued. Corticosteroid therapy may be increased, and some authors identified dexamethasone at the dose of 20 mg every 6 hours as an effective regimen, in combination with anticytokine therapy. Vasopressor support and oxygen therapy should be intensified to support the cardiopulmonary function, as previously described.8 Although some investigators have used repeated high doses of IV methylprednisolone (ie, 1000 mg daily for 2-3 days) in cases of steroid-refractory CRS, this practice is not supported by comparative studies. Aggressive treatment should continue until resolution of CRS to grade ≤1. Corticosteroid therapy should not be discontinued abruptly but tapered at the treating physician’s discretion.
Dose modifications and retreatment
For guidance regarding dose modifications, dose interruptions, or delays, need for repriming (eg, repeating the step-up dosing schedule), and precautionary measures to be implemented after an episode of CRS, the panel recommends that clinicians refer to each BsAb’s prescribing information. Similarly, the appropriateness of retreating a patient with a BsAb after high-grade toxicity, such as CRS, should be determined following the manufacturers’ instructions.
Community oncology perspective
Advancements in lymphoma treatment will promote practice change and use of novel therapies. For example, off-the-shelf products, such as CD3×CD20 BsAbs, have the potential to benefit patients with lymphoma treated in the community where available. Community oncology practices may consider consultation with a nearby community or academic center, to assist with step-up dosing when the CRS risk is highest, particularly if resources for urgent evaluation and management of CRS are not available at the local facility. Use of these therapies may foster increased collaboration between community and academic centers and allow patients access to novel therapies closer to their residence.
Identification, evaluation, and management of neurological toxicity
Neurological AEs have been uncommonly observed across BsAb clinical trials. Therefore, routine neurologic testing for patients who are asymptomatic with normal neurological examination at baseline is not required. Similarly, the panel does not recommend driving restrictions for individuals who are asymptomatic. Patients and caregivers, however, should be educated on potential manifestations of neurologic toxicity and monitor for any changes in neurologic status from baseline. In the case of neurologic signs and symptoms that are deemed consistent with BsAb-related neurotoxicity, the panel recommends following guidelines for the grading and management of ICANS occurring after CAR T-cell therapy (Table 4).13
Neurotoxicity grading and proposed management for CD3×CD20 BsAbs
| Definition: neurological AEs after BsAb therapy most frequently consist of headache and dizziness; occasionally, ICANS-like symptoms occur; these may or may not accompany CRS Symptoms: delirium, dysgraphia, tremor, lethargy, difficulty concentrating, agitation, confusion, expressive aphasia, apraxia, depressed level of consciousness, encephalopathy, and seizures Recommendations: patients and caregivers need to be educated on symptoms and patients cannot drive or operate heavy machinery if symptomatic Workup and evaluation:
| |
| ICE scoring system | |
| Orientation to year, month, city, hospital | 4 points |
| Naming 3 objects | 3 points |
| Following simple commands | 1 point |
| Writing standard sentence | 1 point |
| Attention to count backward from 100 by 10 | 1 point |
| ICANS grading | Management |
| Grade 1: ICE 7-9 or depressed level of consciousness but awakens spontaneously |
|
| Grade 2: ICE 3-6 or depressed level of consciousness but awakens to voice |
|
| Grade 3: ICE 0-2 or depressed level of consciousness but awakens to tactile stimulus or any clinical seizure that resolves rapidly or focal/local edema on neuroimaging |
|
| Grade 4: ICE is 0 or patient is unarousable or requires vigorous or repetitive tactile stimuli, or life-threatening prolonged seizure (>5 min) or repetitive seizures without return to baseline or deep focal motor weakness or diffuse cerebral edema on neuroimaging |
|
| Definition: neurological AEs after BsAb therapy most frequently consist of headache and dizziness; occasionally, ICANS-like symptoms occur; these may or may not accompany CRS Symptoms: delirium, dysgraphia, tremor, lethargy, difficulty concentrating, agitation, confusion, expressive aphasia, apraxia, depressed level of consciousness, encephalopathy, and seizures Recommendations: patients and caregivers need to be educated on symptoms and patients cannot drive or operate heavy machinery if symptomatic Workup and evaluation:
| |
| ICE scoring system | |
| Orientation to year, month, city, hospital | 4 points |
| Naming 3 objects | 3 points |
| Following simple commands | 1 point |
| Writing standard sentence | 1 point |
| Attention to count backward from 100 by 10 | 1 point |
| ICANS grading | Management |
| Grade 1: ICE 7-9 or depressed level of consciousness but awakens spontaneously |
|
| Grade 2: ICE 3-6 or depressed level of consciousness but awakens to voice |
|
| Grade 3: ICE 0-2 or depressed level of consciousness but awakens to tactile stimulus or any clinical seizure that resolves rapidly or focal/local edema on neuroimaging |
|
| Grade 4: ICE is 0 or patient is unarousable or requires vigorous or repetitive tactile stimuli, or life-threatening prolonged seizure (>5 min) or repetitive seizures without return to baseline or deep focal motor weakness or diffuse cerebral edema on neuroimaging |
|
CT, computed tomography; EEG, electroencephalogram; ICE, immune effector cell encephalopathy; ICU, intensive care unit; LP, lumbar puncture; MRI, magnetic resonance imaging; PE, physical examination.
Management of BsAb-related neurotoxicity should be multidisciplinary with consideration for neurology involvement and, depending on the severity of the clinical picture, an intensivist and/or neurointensivist with expertise in the treatment of ICANS. Additionally, should BsAb neurotoxicity occur, clinicians should consider additional corticosteroid prophylaxis, hospitalization when appropriate, and closer monitoring after a subsequent dose. Finally, given their rarity, alternate etiologies for the neurologic findings should always be considered with appropriate evaluation and treatment as needed.
Evaluation and management of other toxicities
Tumor flare reaction
Tumor flare reaction occurs rarely in the setting of BsAb treatment. It is characterized by short-term volumetric increase in lymphoma lesions accompanied by erythema, pain, and fever, and can result in local compression or organ dysfunction.14 Like CRS, it occurs most frequently after the first dose, or first target dose of a BsAb, and may occur together with other symptoms of CRS. For patients with large tumor lesions near vital structures, such as the airway or the mediastinum, it is critical that providers have mitigation steps in place. Irradiation of a high-risk site may be considered in certain cases if there is significant concern for vital organ compromise at baseline. These patients will require close monitoring, consideration of inpatient drug administration, and consideration of consultation with appropriate teams (eg, otolaryngology) at experienced centers. Tumor flare reaction typically responds rapidly to corticosteroid therapy.
Cytopenias
Growth factor support can be considered for patients who develop neutropenia while on treatment. Consideration should be given to withholding BsAb therapy as per the prescribing label, balancing the patient’s disease control with risks associated with neutropenia. BsAb therapy may be continued in patients with concern for underlying lymphoma causing cytopenias due to either marrow infiltration or organomegaly with sequestration. Clinicians should be mindful of the potential increased bleeding risk when thrombocytopenia and fever co-occur, and follow institutional transfusion practices for the treatment of anemia or thrombocytopenia, and consider withholding drug as per the prescribing information.
Infections
Treatment should be withheld for patients with active infection, particularly because concurrent active infection and immune cell stimulation may heighten the risk and severity of immune toxicity. Patients affected by COVID-19, should be managed as per local institutional guidelines. Because of profound B-cell depletion, prolonged COVID-19 positivity can be observed in many patients. The panel recommends individualized consultation with infectious disease experts to determine additional therapies that may be warranted and appropriate timing for resumption of therapy. Finally, as with other B-cell depleting therapies, hypogammaglobulinemia can be seen in patients treated with BsAbs. Immunoglobulin levels should be monitored regularly and IV immunoglobulin replacement should be considered for individuals with recurrent infections, as per institutional standards.
Prophylaxis against Pneumocystis jirovecii pneumonia and varicella-zoster virus is universally recommended by the group, especially because patients have received prior lymphoma-directed therapies and receive steroid prophylaxis with step-up dosing. Although the duration of prophylaxis is not established, some members of the panel recommended continuing prophylaxis for up to 6 months after treatment discontinuation. In patients with a history of latent hepatitis B, specific antiviral therapy is recommended as it is with other CD20-directed therapies.15,16 Although vaccine response may be impaired in patients receiving BsAbs, the panel also recommends that patients receive standard vaccinations, including those against influenza and COVID-19, as indicated.
Tumor lysis syndrome
There is, at present, no indication that the risk of tumor lysis syndrome after BsAbs is significantly different from that of other therapies. Therefore, the panel recommends that tumor lysis syndrome risk assessment follow the standard of care for patients with lymphoma.17,18 Tumor lysis syndrome monitoring and prophylaxis should be performed in patients at increased risk based on factors such as disease histology, tumor burden, and baseline renal function.
Discussion
Several CD3×CD20 BsAbs have been approved by regulatory agencies for the treatment of patients with indolent or aggressive B-NHL and will be increasingly adopted by both academic and community practice physicians. Although the arrival of these agents for routine clinical use has been eagerly anticipated, their distinct toxicity profiles should be recognized by health care providers and their teams at treating facilities. Specifically, although often mild, BsAb-associated CRS can lead to potentially life-threatening complications and, as such, requires vigilant and proactive monitoring and management.
One important task for the panel was to highlight key differences between neurologic toxicities seen with CAR T-cell and BsAb therapy with practical management implications. Specifically, given low rates of ICANS-like toxicity seen with CD3×CD20 BsAbs, regular neurologic assessments, testing for encephalopathy, and driving restrictions were not universally recommended for patients who are asymptomatic.
Several additional considerations deserve emphasis. First, it is paramount to note that the recommendations may not be applicable in all cases and should never substitute for individual clinical judgment, especially in patients with comorbidities that would make high-grade CRS harder to withstand or manage. Such patients should have more aggressive monitoring and management of early CRS and, in extreme circumstances, consideration of alternative therapies. Second, we envision these recommendations to be part of an ongoing effort to provide up-to-date guidance for clinicians. The panel and the Lymphoma Research Foundation are committed to updating this document as additional information or agents become available. Third, much work remains to be done to better understand patient- and disease-related risk factors for severe toxicity after the administration of CD3×CD20 BsAbs. Development and refinement of risk stratification tools may allow for more specific monitoring and management recommendations in the near future.19 Furthermore, a deeper understanding of the mechanism of CRS and neurologic toxicity with these agents may improve treatment algorithms.
Ultimately, BsAbs are likely to significantly alter treatment paradigms across a broad range of lymphoma subtypes and clinical settings. Continued investigation of their toxicity and efficacy and efforts to collect additional information that can be used to generate broadly applicable guidance for clinicians and researchers, will be required for optimal deployment of these potentially practice-changing treatments. We are hopeful that the present guidelines may serve to facilitate BsAb adoption in the hematology/oncology community.
Acknowledgment
The authors thank Maddie Koppin, Mission Cancer and Blood, Des Moines, IA, for help with writing the manuscript.
Authorship
Contribution: J.L.C. and L.F. designed the research, created surveys, led discussions, provided recommendations, and wrote the manuscript; Y.H.K. and T.G. designed the research, provided recommendations, and wrote the manuscript; and R.B., L.N., C.T., R.U., N.B., V.N., J.W., J.O., K.P., J.B., J.S.A., M.L., N.N.S., A.A., M.K., B.P., P.C., I.F., A.H., J.S., M.M., P.A., B.K., S.S., A.Z., L.E.B., M.H., T.P., and M.D. provided recommendations, participated in discussions, and provided feedback on the manuscript.
Conflict-of-interest disclosure: J.L.C. reports consulting for ADC Therapeutics, Seagen, Kite, Incyte/MorphoSys, and Regeneron; and received research funding from Genentech/Roche, Merck, AbbVie, and Bayer. T.G. reports consulting for and serving as advisory board member/speaker for AbbVie, Adaptive Biotechnologies, ADC Therapeutics, AstraZeneca, Bristol Myers Squibb, BeiGene, Genentech, Genmab-National Lymphoma Steering Committee, Gilead, Kite, Lilly, and TG Therapeutics. L.F. received consulting fees from, and served on advisory boards for Roche/Genentech, Genmab, AbbVie, Seagen, ADC Therapeutics, AstraZeneca, Evolveimmune, and Ipsen; and reports research funding from Roche, Genentech, Genmab, AbbVie, and Innate Pharma. R.B. is currently employed by Ipsen. L.N. reports honoraria from AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Genentech, Genmab, Gilead/Kite, Janssen, Incyte, Merck, Novartis, Regeneron, and Takeda; and reports research support from Bristol Myers Squibb, Daiichi Sankyo, Genentech, Genmab, Gilead/Kite, Janssen, Merck, Novartis, and Takeda. K.P. reports consultancy with AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Bristol Myers Squibb, Caribou, Epizyme, Genentech/Roche, Kite, Loxo, MEI Pharma, Merck, Morphosys, Nurix, Pharmacyclics/Janssen, Sana Biotechnology, TG Therapeutics, Trillium Therapeutics/Pfizer, and Xencor; and reports research funding from Adaptive Biotechnologies, AstraZeneca, CRISPR Therapeutics, Curis Inc, Epizyme, Fate Therapeutics, Genentech/Roche, Kite, MEI Pharma, Merck, Nurix, Pharmacyclics/Janssen, Sunesis Pharmaceuticals, Trillium Therapeutics/Pfizer, and Xencor. V.N. reports advisory board membership/consulting for Genmab, AbbVie, and ADC Therapeutics. A.A. reports consulting for Genentech, Ipsen, and Janssen Biotech. J.S.A. reports consulting for AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Caribou Biosciences, Cellectar, Century Therapeutics, Epizyme, Genentech, Genmab, Incyte, Interius, Janssen, Kite Pharma, Lilly, and Takeda; and reports research support (to the institution) from Bristol Myers Squibb, Cellectis, Merck, Mustang Bio, and Seagen. P.A. reports consultancy for Merck, Bristol Myers Squibb, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech/Roche, Xencor, Foresight, and ATB Therapeutics; received research funding from Kite; received research funding (institutional) from Merck, Bristol Myers Squibb, Affimed, Adaptive, Tensha, Otsuka, Sigma Tau, Genentech/Roche, IGM Biosciences, AstraZeneca; and reports honoraria from Merck and Bristol Myers Squibb. B.K. reports consulting for Genentech/Roche, AbbVie, Genmab PA, Merck, Bristol Myers Squibb, ADC Therapeutics, GenMab, Enterome, Genentech/Roche, ATB Therapeutics, and Foresight; and reports research funding from Kite and research funding (to the institution) from Merck, Bristol Myers Squibb, Adaptive, Genentech, IGM Biosciences, AstraZeneca. A.Z. reports consulting for Genentech, Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnology, MorphoSys, AbbVie, AstraZeneca, and MEI Pharma; received research finding from MEI Pharmaceuticals, Genentech, Roche, and BeiGene; serves as data monitoring committee chair for BeiGene; and serves as data monitoring committee member for Bristol Myers Squibb, Celgene, and Juno. M.H. reports consulting for AbbVie, AstraZeneca, Genmab, Janssen, Merck, Roche, and Takeda; reports honoraria from AbbVie, AstraZeneca, Genmab, Janssen, Merck, Roche, and Takeda; and received research support from AbbVie, Celgene, AstraZeneca, Bristol Myers Squibb, Genentech, Genmab, Incyte, Janssen, Roche, Novartis, Merck, and Takeda. T.P. is a consultant for AbbVie, AstraZeneca, ADC Therapeutics, Bayer, BeiGene, Bristol Myers Squibb, Eli Lilly, Genmab, Genentech, Ipsen, Incyte, Morphosys, Pharmacyclics, Xencor; and reports research funding from Genentech, Sobi, and AbbVie. M.D. reports research funding from, and advisory board membership with Roche, Novartis, AbbVie, Kite/Gilead, Eli Lilly/Loxo, Merck Sharp & Dohme, and Takeda; received speaker fees from Roche, Novartis, AbbVie, Kite/Gilead Genmab, and Janssen; reports advisory board membership with Roche, Novartis, AbbVie, and Kite/Gilead; and serves on the advisory boards of Nkarta, Interius, Janssen, Adicet Bio, Genmab, Bristol Myers Squibb, AstraZeneca, and Merck Sharp & Dohme. The remaining authors declare no competing financial interests.
Correspondence: Lorenzo Falchi, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; email: falchil@mskcc.org.
References
Author notes
J.L.C., T.G., L.F., and Y.H.K. contributed equally to this study.
L.E.B., M.H., T.P., and M.D. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal