In this issue of Blood, Guerrero et al1 provide new insights into the molecular mechanisms underlying the establishment of immune memory within the hematopoietic hierarchy and highlight the role of granulocyte-macrophage colony-stimulating factor (GM-CSF) in orchestrating divergent immune responses at different hematopoietic stages (see figure). The authors propose that the existence and the coordination of these opposing immune memory programs, driven by GM-CSF, may be essential to ensure an efficient, yet controlled, innate immune response to secondary challenges.
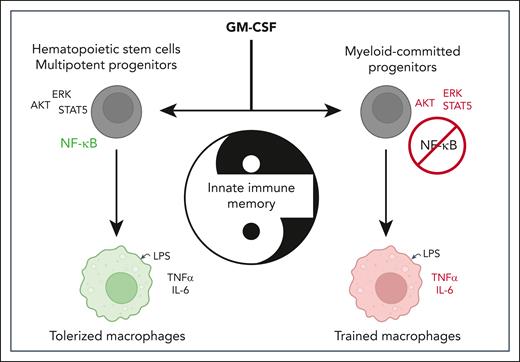
GM-CSF signaling strength drives distinct NF-κB responses in the hematopoietic hierarchy, thereby leading to the establishment of divergent innate immune memory programs. IL6, interleukin 6; TNFα, tumor necrosis factor α.
GM-CSF signaling strength drives distinct NF-κB responses in the hematopoietic hierarchy, thereby leading to the establishment of divergent innate immune memory programs. IL6, interleukin 6; TNFα, tumor necrosis factor α.
The concept of innate immune memory refers to the ability of the innate immune cells to adapt their functional characteristics following an initial challenge, thereby altering their response to secondary homo- or heterologous challenges.2 This adaptation can lead to an enhanced secondary immune response, known as “trained immunity,” or a repressed response, termed “immune tolerance.” Although short-lived immune cells such as monocytes, macrophages, or neutrophils are its ultimate effectors, the innate memory is sustained over time by alterations occurring in the hematopoietic stem and progenitor cell (HSPC) compartments present in the bone marrow.3,4 Recent studies have identified a variety of inducers of immune memory in HSPCs, including microbial agents/compounds (eg, bacillus Calmette-Guerin vaccine, β-glucans, lipopolysaccharide [LPS]), endogenous sterile factors (eg, mevalonate, oxidized low-density lipoprotein), or immune mediators such as inflammatory cytokines and growth factors. However, our understanding of the conditions under which this type of memory is acquired remains limited. Key questions revolve around how the nature, the intensity, and the duration of the initial challenge direct the immune memory toward a phenotype of immune training or immune tolerance. Similarly, how the immune memory becomes established and harmonized within the complex HSPC hierarchy present in the bone marrow has not been established.
In this context, Guerrero et al revisit the role of the growth factor GM-CSF in the establishment of immune memory across the hematopoietic hierarchy by comparing its impact on myeloid-committed progenitors (ie, common granulocyte-macrophage progenitors [GMPs]) and on the more immature multipotent compartments (phenotypically defined as Lin–ckit+Sca1+ [LKS+] cells). The authors show that GM-CSF treatment induces 2 opposite memory programs at these different hematopoietic stages, as macrophages derived from GMP display a trained proinflammatory cytokine production and macrophages derived from LKS+ progenitors show a tolerized phenotype. Through meticulous mechanistic analyses, they identify NF-κB as the key determinant of these divergent programs. They show that NF-κB nuclear translocation is required for the induction of the tolerization program in early LKS+ progenitors. Conversely, they demonstrate that NF-κB is inactive in myeloid-committed progenitors undergoing immune training and that its activation is sufficient to reorient these progenitors toward a tolerized phenotype. As such, the study establishes that NF-κB under GM-CSF control acts as an essential switch regulating the tolerization/training programs in HSPCs.
The study demonstrates that competing memory programs can develop across the hematopoietic hierarchy in response to a single inducer. From a biological perspective, this balance between trained mature progenitors and tolerized early progenitors may provide a temporal harmonization of the immune memory. This balance may be beneficial in enhancing the immediate immune response to protect against infections while limiting the risks associated with a prolonged inflammatory response. Further investigation is warranted to evaluate the extent of this multilayered memory within the early HSPC compartments, as the study suggests the existence of additional levels of heterogeneity. Indeed, the study reveals that the tolerization program is attenuated in hematopoietic stem cells (HSCs) compared with downstream multipotent progenitors. It also shows that the memory programs can be sustained through primary but not secondary transplantation. These results can be interpreted in the context of the well-established heterogeneity of the HSC compartment5 and suggest the existence of HSC subsets susceptible or refractory to memory induction.6 The study also demonstrates that the memory programs established in myeloid-committed progenitors and in immature multipotent compartments translate into functionally distinct monocytes and macrophages. These findings underscore the existence and the significance of the diverse paths of myeloid differentiation that connect HSPCs to specific myeloid subsets. In doing so, it extends the scope of recent studies deciphering the developmental origin of monocyte diversity,7,8 but it also raises new questions about the mechanisms sustaining these memory programs during the hematopoietic differentiation process.
Collectively, the study shows that innate immune memory should be viewed as collection of distinct memory programs that develop in the hematopoietic hierarchy. It highlights the importance of considering the dynamic interplay between trained and tolerized immune programs in HSPCs as it may contribute to shape the immune response over time. It also suggests the potential benefits in targeting these competing memory programs through specific inducers such as GM-CSF for enhancing immune function or treating immune-related disorders.
Conflict-of-interest disclosure: D.R. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal