Visual Abstract
von Willebrand disease (VWD) is the most common bleeding disorder and especially milder type 1 VWD might not be cared for in specialty clinics. VW factor levels rise with age, but the rise of these levels does not necessarily correlate with bleeding risk. A recent bleeding history combined with recent labs are important for hemostatic management decision during surgical interventions. Antifibrinolytics appear safe in the population of older adults, whereas desmopressin (DDAVP) should be used cautiously. Where needed, factor concentrates present a great treatment option. Acquired von Willebrand syndrome is vastly underrecognized, but likely to surface in the aging, especially in the setting of comorbidities, such as plasma-cell dyscrasias. Intravenous immunoglobulin can be an effective treatment in this scenario, but potentially increases thrombotic risk.
Introduction
Managing inherited and acquired bleeding disorders in older patients (age ≥65 years) requires a holistic approach that incorporates each patient’s underlying comorbidities and risk factors for treatment related complications. von Willebrand disease (VWD) is the most prevalent inherited bleeding disorder (0.8%-1%),1,2 and most patients with milder disease are likely not receiving care in a center for specialty bleeding disorders.3 Acquired VW syndrome (AVWS) is associated with lympho- or myeloproliferative disorders, solid tumors, autoimmune disorders, and conditions altering cardiac circulation. It is likely underreported and recognized increasingly with advancing age of the patient.4 In this article, we highlight things to consider when taking care of an older patient with a congenital or AVW disorder.
Case-based discussion
Case 1: managing type 1 VWD in an older patient
A 77-year-old woman with type 1 VWD, hypertension, and osteoarthritis presents for preoperative recommendations for a hip replacement. She was diagnosed with type 1 VWD in her early 20s when her VW factor antigen (VWF:Ag) was 27 IU/dL, and her VWF ristocetin cofactor activity (VWF:RCo) was 29 IU/dL. She has a remote history of bleeding consistent with type 1 VWD including epistaxis in childhood that was successfully treated with cauterization and desmopressin (DDAVP), as well as heavy menstrual bleeding that improved after starting combined oral contraception. In the last several years, she has not had atypical bleeding, such as prolonged epistaxis or easy bruising. She underwent laparoscopic cholecystectomy 5 years ago without hemostatic prophylaxis and had no abnormal bleeding. Her last documented VWF levels were when she was aged 42 years with a VWF:Ag of 42 IU/dL and VWF:RCo of 44 IU/dL.
Impact of aging on clotting factors
Coagulation factor activities, including VWF, are anticipated to change with age (Table 1). Both VWF:Ag and VWF:RCo increase significantly with age in people without VWD,9 especially in those who are not blood group O.9,10 In cohorts of healthy individuals, rising VWF levels and activities have been associated with increased thrombogenicity. A longitudinal follow-up of patients with type 1 VWD observed a similar increase and suggests that 20% to 50% of patients develop normalized VWF:RCo (>50 IU/dL) with age.19 A study of 31 patients with type 1 VWD showed an increase in VWF:Ag and VWF:RCo over time with 58% of patients having normalization of both VWF:Ag and VWF:RCo to ≥50 IU/dL but also noted that not everyone experienced this increase.19 A larger study of 195 patients with type 1 VWD showed that this increase was mainly in those with mild type 1 VWD (baseline VWF:Ag and VWF:RCo from >30 to <50 IU/dL).20 The rate of change in the VWF:Ag and VWF:RCo does not appear to be affected by sex. Although VWF:Ag and VWF:RCo increase with age in type 1 VWD, the bleeding phenotype does not necessarily improve.21 It remains essential to quantify each patient’s bleeding score because symptoms are the strongest predictor of outcomes and inversely correlate with VWF:Ag and VWF:RCo, irrespective of age, sex, and blood group.22
Changes in coagulation factors with age
| Clotting factor . | Changes with aging . |
|---|---|
| Fibrinogen | Increases5,6 |
| Thrombin | Unaffected |
| FV | Increases7 |
| FX | Unaffected |
| FVII | Increases5,7 |
| FVIII | Increases5 |
| FIX | Increases7 |
| FXI | Increases7 |
| FXII | Depends on the reference (unaffected vs increases) |
| FXIII | Increases8 |
| VWF | VWF Ag increases with age, especially in those who have non-O type blood9-11 |
| Protein C | Protein C Ag increases with aging (∼4% by decade); in women this is primarily seen after menopause12 |
| Protein S | Increases with age; lower and more variable increase in women owing to increased total protein S but no change in free protein S (increase in C4b-BP levels with normal aging); may be affected by serum lipids (higher protein S a/w higher cholesterol and triglyceride levels)13,14 |
| PAI-1 | Increases (especially in association with myocardial infarction, obesity, insulin resistance, atherosclerosis, malignancy, inflammation, and psychological stress)15 |
| D-dimer | Increases (especially in association with comorbidities, such as infection, heart disease, and malignancy)16 |
| Platelet count | Decreased (more pronounced in males)17 |
| Platelet activation | Increases18 |
| Bleeding time | Shortens18 |
| Clotting factor . | Changes with aging . |
|---|---|
| Fibrinogen | Increases5,6 |
| Thrombin | Unaffected |
| FV | Increases7 |
| FX | Unaffected |
| FVII | Increases5,7 |
| FVIII | Increases5 |
| FIX | Increases7 |
| FXI | Increases7 |
| FXII | Depends on the reference (unaffected vs increases) |
| FXIII | Increases8 |
| VWF | VWF Ag increases with age, especially in those who have non-O type blood9-11 |
| Protein C | Protein C Ag increases with aging (∼4% by decade); in women this is primarily seen after menopause12 |
| Protein S | Increases with age; lower and more variable increase in women owing to increased total protein S but no change in free protein S (increase in C4b-BP levels with normal aging); may be affected by serum lipids (higher protein S a/w higher cholesterol and triglyceride levels)13,14 |
| PAI-1 | Increases (especially in association with myocardial infarction, obesity, insulin resistance, atherosclerosis, malignancy, inflammation, and psychological stress)15 |
| D-dimer | Increases (especially in association with comorbidities, such as infection, heart disease, and malignancy)16 |
| Platelet count | Decreased (more pronounced in males)17 |
| Platelet activation | Increases18 |
| Bleeding time | Shortens18 |
a/w, associated with; BP, binding protein; PAI-1, plasminogen activator inhibitor-1.
The rise in VWF levels with aging may be related to an increase in inflammation and underlying comorbidities because VWF is an acute phase reactant; in the WiN study of 333 patients with type 1 VWD, the association between aging and rising VWF was not present after adjusting for comorbidities.23 It is also possible that the underlying mutation driving VWD affects whether VWF levels are responsive to age and inflammation because VWF levels appear to be minimally affected by aging in forms of type 2 VWD.24
Case 1 continued
For surgical planning it is important to consider current rather than historical clotting factor activities and to assess a recent bleeding history. Before formulating a treatment plan for her hip replacement, this patient’s repeat VWF:Ag, VWF:RCo, and factor VIII (FVIII) were 65, 64, and 90 IU/dL, respectively. She asks whether she should receive DDAVP before surgery given her positive experience with it in the remote past.
Does age affect treatment decisions for VWD?
DDAVP
DDAVP increases endogenous VWF levels transiently and can result in hyponatremia and tachyphylaxis with ongoing use regardless of the age of the patient. Because of these limitations, alternative hemostatic agents, such as VWF products are favored following procedures that require higher VWF levels for hemostatic control beyond the initial perioperative period (>24-48 hours). This is particularly important in patients who require longer healing times owing to advanced age and/or underlying comorbidities.
In general, the use of DDAVP is not favored in older patients with VWD or mild hemophilia A because of a theoretical increased risk for adverse events. Careful consideration must be given to the patient’s comorbidities and sensitivity to fluid shifts and risk for seizure. Theoretically, DDAVP may increase the risk of thrombosis in older patients especially in the setting of a rising FVIII levels, however there is minimal data available to support this theoretical concern. Case reports have documented arterial thrombotic events after DDAVP infusion, but these events occurred in patients with strong underlying risk factors.25-27 DDAVP has not been shown to increase the risk of venous or arterial thrombosis in populations without bleeding disorders at high risks for thrombotic events, such as in the perioperative setting, as well as after intracranial hemorrhage while on antiplatelet therapy28 or with concurrent alcohol abuse.29 Until more data are available in older patients with bleeding disorders, DDAVP should be used cautiously particularly in the setting of elevated baseline FVIII levels, underlying cardiac risk factors, or sensitive to fluid shifts.
Antifibrinolytics
Although there are limited data on the use of antifibrinolytics in older patients with bleeding disorders, there are significant safety data showing no increased risk of thrombosis in older patients without bleeding disorders including in the setting of traumatic injuries,30 orthopedic,31-33 and cardiac surgeries.34 A meta-analysis of over 40 000 nonsurgical patients showed no increased risk of thrombosis, including stroke, myocardial infarction, pulmonary embolism, and deep vein thrombosis with tranexamic acid.35 Notably, patients without bleeding disorders typically receive antifibrinolytics for shorter durations, but a recent trial of patients with hematologic malignancies did not show an increased risk of thrombosis after an average of 2 weeks of tranexamic acid administration.36 Given the available extensive and reassuring safety data that included populations of older individuals, antifibrinolytics are an excellent option for older patients with bleeding disorders.
VWF clotting factor concentrates
The indications for VWF replacement concentrates and target VWF:RCo activity for major and minor surgeries have been previously described.37 Several plasma-derived (also contain FVIII) and 1 recombinant (does not contain FVIII) VWF concentrate are available in the United States (Table 2). In theory, the recombinant product may be a safer hemostatic agent in an older patient with type 1 VWD, if baseline FVIII levels are normal or increased, because the product does not provide external FVIII. However, the exogenous VWF from recombinant VWF will stabilize and potentially further increase endogenous FVIII. Regardless of the product selected, VWF and FVIII monitoring is necessary to gauge response. There are no studies comparing VWF products in older patients with VWD, and limited data on the risk of thrombosis with plasma-derived VWF products are available.
VWF products licensed to treat VWD in the United States
| Product . | Indication . | Dosage (adults)∗ . | Approximate VWF:RCo-to-FVIII ratio in vial . | Reference . |
|---|---|---|---|---|
| Plasma derived | ||||
| Alphanate | Surgical and/or invasive procedures in adult and pediatric patients with VWD in whom DDAVP is either ineffective or contraindicated. It is not indicated for patients with severe VWD (type 3) undergoing major surgery | Preoperative dose of 60 IU VWF:RCo/kg body weight; subsequent doses of 40-60 IU VWF:RCo/kg body weight. | 1.2:1 | 38,39 |
| Humate P | (1) Treatment of spontaneous and trauma-induced bleeding episodes, and (2) prevention of excessive bleeding during and after surgery. This applies to patients with severe VWD as well as patients with mild to moderate VWD in which the use of DDAVP is known or suspected to be inadequate | (Target peak plasma VWF:RCo level – baseline plasma VWF:RCo level)/in vivo recovery in patient × body weight (BW) in kg | 2.4:1 | 40,41 |
| Wilate | (1) On-demand treatment and control of bleeding episodes, (2) perioperative management of bleeding | Required IU = BW in kg × desired VWF:RCo rise (%) (IU/dL) × 0.5 (IU/kg per IU/dL) | 1:1 | 42 |
| Recombinant | ||||
| Vonvendi | (1) On-demand treatment and control of bleeding episodes1 (2) Perioperative management of bleeding1 (3) Routine prophylaxis to reduce the frequency of bleeding episodes in patients with severe type 3 VWD receiving on-demand therapy | Give 12-24 h before surgery to allow the endogenous FVIII levels to increase to at least 30 IU/dL (minor surgery) or 60 IU/dL (major surgery) | n/a | 43 |
| Product . | Indication . | Dosage (adults)∗ . | Approximate VWF:RCo-to-FVIII ratio in vial . | Reference . |
|---|---|---|---|---|
| Plasma derived | ||||
| Alphanate | Surgical and/or invasive procedures in adult and pediatric patients with VWD in whom DDAVP is either ineffective or contraindicated. It is not indicated for patients with severe VWD (type 3) undergoing major surgery | Preoperative dose of 60 IU VWF:RCo/kg body weight; subsequent doses of 40-60 IU VWF:RCo/kg body weight. | 1.2:1 | 38,39 |
| Humate P | (1) Treatment of spontaneous and trauma-induced bleeding episodes, and (2) prevention of excessive bleeding during and after surgery. This applies to patients with severe VWD as well as patients with mild to moderate VWD in which the use of DDAVP is known or suspected to be inadequate | (Target peak plasma VWF:RCo level – baseline plasma VWF:RCo level)/in vivo recovery in patient × body weight (BW) in kg | 2.4:1 | 40,41 |
| Wilate | (1) On-demand treatment and control of bleeding episodes, (2) perioperative management of bleeding | Required IU = BW in kg × desired VWF:RCo rise (%) (IU/dL) × 0.5 (IU/kg per IU/dL) | 1:1 | 42 |
| Recombinant | ||||
| Vonvendi | (1) On-demand treatment and control of bleeding episodes1 (2) Perioperative management of bleeding1 (3) Routine prophylaxis to reduce the frequency of bleeding episodes in patients with severe type 3 VWD receiving on-demand therapy | Give 12-24 h before surgery to allow the endogenous FVIII levels to increase to at least 30 IU/dL (minor surgery) or 60 IU/dL (major surgery) | n/a | 43 |
n/a, not applicable.
Plasma-derived VWF/FVIII concentrates can be dosed based on the FVIII or VWF in the vial, and it is important to specify that the dosing is given based on the VWF:RCo units in the vial.
Case 1 continued
Given the patient’s lack of recent bleeding symptoms and normalized levels of VWF:Ag, VWF:RCo, and FVIII with aging, the patient was prescribed no additional hemostatic agents besides tranexamic acid after her hip replacement. She was also advised to discontinue any drugs or supplements that could affect platelet function in the 2 weeks preceding surgery and to limit the use of nonsteroidal anti-inflammatory drugs post operatively. She underwent hip replacement without any atypical bleeding or adverse event.
The 2021 combined guidelines on VWD from the International Society on Thrombosis and Haemostasis (ISTH), American Society of Hematology (ASH), National Hemophilia Foundation (NHF), and World Federation of Hemophilia (WFH) suggest “reconsidering” rather than removing a diagnosis of type 1 VWD when VWF levels rise with age, especially because the correlation between rising VWF levels and risk of bleeding has not been established.44 In the absence of bleeding symptoms the decision to reconsider or remove the diagnosis of VWD for this patient should be considered and should be one of shared decision-making as per the 2021 ISTH, ASH, NHF, and WFH combined guidelines.
Role of postoperative thromboprophylaxis
The decision to use medical thromboprophylaxis depends on the patient’s bleeding and clotting history, VWF levels and risk for thrombosis associated with the procedure. For many patients with VWD, the bleeding risk from medical thromboprophylaxis outweighs any benefit. Prophylactic anticoagulation may be useful after higher risk surgeries for patients with low bleeding scores whose VWF levels have normalized with aging. In our practice, we do not use postoperative prophylactic anticoagulation for patients with bleeding disorders unless they have negligible bleeding scores and normalized VWF levels with aging (similar to the patient in case 1). If prophylactic anticoagulation is used, the patient should be closely monitored for bleeding symptoms with a low threshold to discontinue anticoagulation.
Case 2: thrombotic event after IV immunoglobulin (IVIG) for AVWS
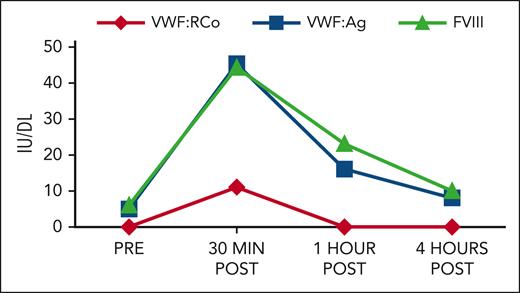
A 72-year-old man with obesity and amyloid light chain (AL) amyloidosis, which is complicated by cardiomyopathy, and chronic renal insufficiency on maintenance daratumumab presented with hematochezia after undergoing biopsies during a colonoscopy. He denied any abnormal bleeding earlier in life but had noticed increased bruising over the last year. Coagulation testing revealed a prolonged activated partial thromboplastin time (aPTT) of 50 seconds (reference range 26-37 seconds) with a normal prothrombin time and fibrinogen. Subsequent testing showed normal coagulation factors except for a FVIII of 13 IU/dL, VWF:Ag of 10 IU/dL, and a VWF:RCo below the limit of detection. A pharmacokinetic study receiving a 100% corrective dose of a plasma-derived VWF:FVIII concentrate showed initial increase of VWF:Ag and RCo, but a significantly decreased half-life (Figure 1), and he was diagnosed with AVWS.
Response to PDVWF/FVIII concentrate (50 U/kg dose). PDVWF, plasma derived von Willebrand factor.
Response to PDVWF/FVIII concentrate (50 U/kg dose). PDVWF, plasma derived von Willebrand factor.
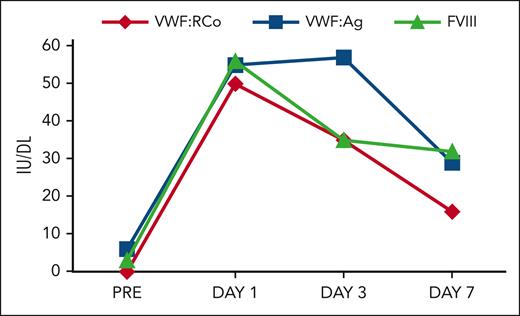
The patient received 500 mg/kg of IVIG with subsequent normalization of his VWF:Ag, VWF:RCo, and FVIII (Figure 2). Repeat colonoscopy showed bleeding at the site of prior biopsies, which resolved after receiving IVIG. The patient was discharged and continued to require IVIG once per month. In retrospect, the patient had increased bruising and an elevated aPTT for at least 2 years before diagnosis of AVWS.
AVWS
Acquired bleeding disorders are more common in older patients and should be considered when patients present with new atypical bleeding and/or abnormal coagulation testing (Table 3). Most patients with amyloidosis who develop an acquired bleeding disorder have factor X deficiency,52 but AVWS and acquired hemophilia A can occur and should be considered especially in the setting of an isolated prolongation in the aPTT.
Acquired factor deficiencies associated with bleeding
| Deficiency . | Prolonged aPTT and PT . | Prolonged aPTT, nl PT . | Prolonged PT, nl aPTT . | Normal aPTT and PT∗ . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fibrinogen . | FII . | FV45 . | FX46 . | FVIII . | FIX . | FXI . | FVII . | FXIII47-49 . | |
| Predisposing factors | DIC Liver disease Trauma MGUS, MM50 Asparaginase51 | Liver disease Sepsis Vitamin K deficiency | Inhibitor Topical bovine thrombin Antibiotics | Amyloidosis Liver disease Vitamin K deficiency | Inhibitor DIC AVWS with reduced FVIII | Inhibitor DIC Liver disease Vitamin K deficiency | Inhibitor DIC Liver disease | Liver disease Sepsis Vitamin K deficiency Vitamin K antagonist | Inhibitor Liver disease Post op Cardiovascular bypass Inflammatory bowel disease |
| Deficiency . | Prolonged aPTT and PT . | Prolonged aPTT, nl PT . | Prolonged PT, nl aPTT . | Normal aPTT and PT∗ . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fibrinogen . | FII . | FV45 . | FX46 . | FVIII . | FIX . | FXI . | FVII . | FXIII47-49 . | |
| Predisposing factors | DIC Liver disease Trauma MGUS, MM50 Asparaginase51 | Liver disease Sepsis Vitamin K deficiency | Inhibitor Topical bovine thrombin Antibiotics | Amyloidosis Liver disease Vitamin K deficiency | Inhibitor DIC AVWS with reduced FVIII | Inhibitor DIC Liver disease Vitamin K deficiency | Inhibitor DIC Liver disease | Liver disease Sepsis Vitamin K deficiency Vitamin K antagonist | Inhibitor Liver disease Post op Cardiovascular bypass Inflammatory bowel disease |
DIC, disseminated intravascular coagulation; MGUS, monoclonal gammopathy of unknown significance; nl, normal; PT, prothrombin time.
Acquired platelet dysfunction from medications/exposures should also be considered when a patient presents with new bleeding symptoms with a normal aPTT and PT.
Our patient presented with gastrointestinal bleeding, which may be more common in cases of AVWS secondary to plasma-cell dyscrasias based on an international registry53 of patients with AVWS from multiple causes including from mechanical destruction of VWF with loss of high molecular weight multimers and altered clearance in the setting of aortic valve stenosis, mitral valve regurgitation, and left ventricular assist devices.4 In some cases, AVWS will resolve with treatment of the underlying disease, such as after valve replacement.54,55 In patients with underlying plasma-cell dyscrasias, autoantibodies increase clearance of VWF and can persist despite adequate treatment of the plasma-cell dyscrasia. VWF containing concentrates and DDAVP are only transiently effective (return to baseline within 4 hours), but IVIG can lead to more sustained VWF levels (peak at 4 days and return to baseline in 21 days) in AVWS from plasma-cell dyscrasias.56 This seems to be more reliable in AVWS related to IgG monoclonal gammopathy of unknown significance over IgM monoclonal gammopathy of unknown significance, in which responses to IVIG have been varied.56,57 IVIG can be administered 3 to 4 days before surgery (suggested dose of 1 g/kg per day for 2 days) or can be administered every 3 weeks (suggested dose 1g/kg) to sustain chronic higher VWF levels to prevent bleeding. In our experience, doses and dose intervals can be titrated to response. Thalidomide58 and lenalidomide or dexamethasone59 have been used successfully to treat gastrointestinal bleeding in AVWS. Rituximab has been used with inconsistent response.60,61
Case 2 continued
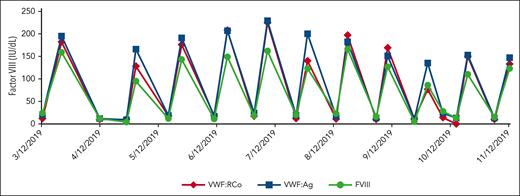
The patient received IVIG 1 g/kg every 3 weeks (Figure 3).
Receiving IVIG 1 g/kg. The graph shows the factor VIII response to receiving IVIG 1 g/kg every 3 weeks (prior to dose adjustment and thrombotic event).
Receiving IVIG 1 g/kg. The graph shows the factor VIII response to receiving IVIG 1 g/kg every 3 weeks (prior to dose adjustment and thrombotic event).
Approximately 1 year after diagnosis of AVWS, his IVIG dose was increased to 1.5 g/kg in an attempt to increase his nadir VWF:RCo. Shortly thereafter, he represented with a submassive pulmonary embolism (acute onset dyspnea and chest pain) 2 days after receiving IVIG. His FVIII was 314 IU/dL 4 days before the thrombotic event. The patient also reported decreased mobility over the prior year related to chronic knee and back osteoarthritis. He was started on a heparin drip and bridged to warfarin with a goal international normalized ratio (INR) of 2 to 3. Warfarin was selected, given the patient’s renal insufficiency and risk of acute bleeding if his VWF:RCo were to decline while being on anticoagulation. His IVIG dose was switched to twice per month instead of monthly and was reduced to 0.2 g/kg to maintain lowest (nadir) VWF:RCo ≥30 IU/dL while avoiding “overshooting” FVIII >200 IU/dL after redosing IVIG.
Risk of IVIG and thrombosis
The risk of thrombosis with IVIG is unknown. Literature to date has reported from 0.5% to 17% rate of arterial and venous thrombosis with IVIG.62 One of the largest studies found a thrombosis rate of 1% to 2% based on medical claims data for a retrospective cohort of 11 785 patients treated with IVIG.63 This study identified that thrombotic events were most likely to occur within 24 hours of IVIG use with the highest rates of thrombosis in patients aged >45 years and/or with preexisting hypercoagulable state. Subcutaneous immunoglobulin (vivaglobin) appeared to carry a higher risk for thrombosis than IVIG. In the experience of the UK Biobank of patients receiving IVIG, 14 794 of 502 492 (2.9%) had a thromboembolic event with the highest risk occurring in individuals with a prior history of thrombosis.64 A meta-analysis of 4129 patients in 31 randomized controlled trials of IVIG suggested that IVIG does not increase the risk of arterial and venous thrombosis, however the study population in this meta-analysis was younger (median age 47) and may have been at lower risk for thrombosis.62 Other risk factors for thrombosis with IVIG identified in the literature include faster infusion rates, higher doses of IVIG, atherosclerosis, IVIG use for autoimmune disorders (especially immune thrombocytopenia), and potentially higher levels of factor XI in the IVIG formulation.63-67 It is unclear if medical thromboprophylaxis reduces the risk of thrombosis from IVIG.
Other potential adverse events from IVIG include immediate infusion reaction (eg, flushing, fever, fatigue, chills, rash, hypotension, and arrhythmia) and delayed infusion reactions (eg, transfusion-related lung injury and hemolysis), rare neurological complications, such as posterior reversible encephalopathy syndrome and renal dysfunction.65 The risk of renal injury from IVIG is highest in older patients, as well as those with diabetes mellitus, preexisting renal dysfunction, or dehydration.65,68
Case 2 continued
After 3 months of therapeutic (INR, 1.9-3.0) anticoagulation with warfarin, the patient presented with a persistent bleed from a traumatic skin wound on his thigh, as well as 3 days of dark stools. His INR was 5.0, and he had a VWF:RCo of 45 IU/dL and FVIII activity of 86 IU/dL. Warfarin was reversed with oral vitamin K, and he received a low dose of IVIG (0.1 g/kg) with resolution of his bleeding. The patient reported that he recently started a new diet pill that contained multiple supplements with potential interactions with warfarin. After his INR decreased to <2, the patient was transitioned to apixaban 2.5 mg by mouth twice daily for secondary prevention of a venous thromboembolism. IVIG was continued at 0.2 g/kg every 2 weeks.
Case 3: managing antiplatelet therapy in a patient with type 1 VWD and coronary artery disease (CAD)
An 82-year-old man with type 1 VWD, hyperlipidemia, tobacco use, and insulin-dependent diabetes mellitus presented with acute onset chest pain with an elevated troponin and electrocardiogram changes consistent with an acute ST elevation myocardial infarction. At baseline, the patient experienced easy bruising, intermittent epistaxis, and bleeding after surgery and trauma. His most recent VWF:RCo was 32 IU/dL, VWF:Ag 30 IU/dL, and FVIII of 65 IU/dL. Before undergoing left heart catheterization, the patient received a VWF factor product to achieve VWF:RCo >50 IU/dL. Catheterization revealed a left anterior descending infarction requiring angioplasty and stent placement. The cardiologist asked whether the patient may receive dual antiplatelet therapy for 1 year.
Managing antiplatelet therapy
VWD may be protective against CAD.69 However, although rare, CAD requiring antiplatelet therapy can occur. In our practice, we aim to achieve a VWF:RCo >50 IU/dL before catheterization and in the subsequent 24 to 48 hours. Ideally, close communication between interventional cardiology and hematology occurs to discuss the choice of stent and duration of anticoagulation. There are no data to guide which stent to select in this patient population, and limited case reports70 suggest that patients with VWD can fare well with both bare metal and drug eluting stents. Although current practice patterns for patients without bleeding disorders favor dual antiplatelet therapy for at least 1 year after stent placement, we discuss switching to single agent antiplatelet therapy with our cardiology colleagues as soon as feasible in patients with bleeding disorders. There are no data on the goal VWF activity while on antiplatelet therapy. We closely monitor patients for bleeding symptoms and consider using concurrent VWF product prophylaxis, if concerning bleeding symptoms occur.
Conclusion
Treatment of older adult patients with acquired or congenital VW disorder should be tailored to each individual and incorporate the patient’s comorbidities and treatment preferences. Some risk factors for adverse events from hemostatic therapies increase with age, such as the risk of thrombosis owing to rising clotting factors. Other side effects may also be more common with advanced age, such as the risk of seizures and inability to tolerate fluid shifts with DDAVP for older patients with mild hemophilia A, VWD, or AVWS. Acquired bleeding disorders should also be considered for older patients presented with new bleeding and/or coagulation abnormalities.
Authorship
Contribution: J.P. and R.K.-J. conceptualized, wrote, and edited this manuscript.
Conflict-of-interest disclosure: J.N.P. is a paid consultant for TeraImmune. R.K.-J. has been a paid consultant for Biomarin, Pfizer, and Genentech/Roche, has been an educational speaker for Genentech/Roche and Takeda and is receiving research funding from Genentech.
Correspondence: Rebecca Kruse-Jarres, Washington Center for Bleeding Disorders, University of Washington, 701 Pike St, Ste 1900, Seattle, WA 98101; email: rkj@wacbd.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal