Visual Abstract
Multiple myeloma (MM) is primarily a disease of older patients. Until recently, geriatric aspects in the context of MM have been poorly investigated. Treatment outcomes for geriatric patients with MM are often compromised by comorbidities and an enhanced susceptibility to adverse events from therapy. Assessment of patient frailty has become more frequent and will be useful in the context of significant and continuous advances in therapy. The recent emergence of immunotherapy with CD38 monoclonal antibodies and upcoming immunooncology drugs, such as bispecific antibodies, will lead to additional therapeutic progress. The applicability of these new molecules to older and frail patients is a key clinical question. Here, we present 2 patient cases derived from clinical practice. We review current frailty scores and standards of care for older, newly diagnosed patients with MM, including frail subgroups, and discuss ways to tailor treatment, as well as treatment perspectives in this population.
Introduction
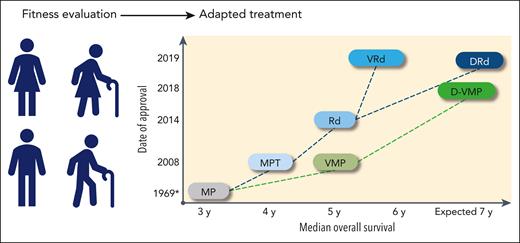
Multiple myeloma (MM), an incurable malignancy of plasma cells, is predominantly a disease of older adults; the median age at diagnosis is 69 years. Approximately one-third of patients are aged >75 years at diagnosis, and ∼10% are aged >85 years.1 The increased incidence of MM with age, combined with the aging population, is anticipated to generate nearly an 80% increase in the number of patients with MM aged >65 years diagnosed each year by 2030.2 Older patients are a heterogeneous population with an increased incidence of frailty; the term frailty refers to a subset of patients who are weaker and more vulnerable than their age-matched counterparts.3,4 The value of frailty assessment has only recently been recognized.5 Alkylating agents have been used for decades to treat MM, and for many years, dose reductions and delays have been the limited ways in which frailty has been accounted for. Furthermore, some geriatric patients have likely been treated in the past with palliation only, because of frailty.6 MM therapy has greatly improved since the late 1990s, and significant survival gains have been achieved, even in older patients (Figure 1). The MM treatment landscape is now rapidly evolving, which will reinforce the need for an operational frailty assessment to avoid suboptimal treatment and the potential for overtreatment in this population.
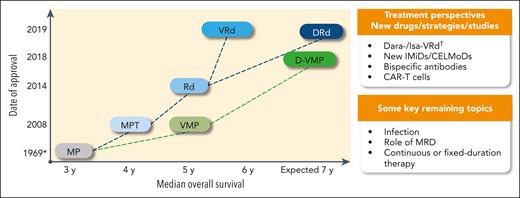
Treatment landscape and perspective in newly diagnosed transplant-ineligible patients: regimens, date of approval (European Medicines Agency), and OS. ∗ indicates the publication date, not an approval date; †, #NCT03319667 and #NCT03652064. CAR-T, chimeric antigen receptor T cell; CELMoDs, cereblon E3 ligase modulation drugs; Dara, daratumumab; IMiD, immunomodulatory drug; Isa, isatuximab; MRD, minimal residual disease.
Treatment landscape and perspective in newly diagnosed transplant-ineligible patients: regimens, date of approval (European Medicines Agency), and OS. ∗ indicates the publication date, not an approval date; †, #NCT03319667 and #NCT03652064. CAR-T, chimeric antigen receptor T cell; CELMoDs, cereblon E3 ligase modulation drugs; Dara, daratumumab; IMiD, immunomodulatory drug; Isa, isatuximab; MRD, minimal residual disease.
Patient case 1
Patient 1 is a 76-year-old man who has developed fatigue and rib pain over the past year, and these symptoms have limited his activities. He has an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 1, a history of thrombophlebitis in the right leg (6 months previously), mild obesity (body mass index = 31 kg/m2), and hypothyroidism that is being treated with levothyroxine. An initial evaluation revealed anemia, moderate renal impairment (creatinine clearance 60 mL/min), and a serum immunoglobulin A kappa M-protein concentration of 4.5 g/dL. A bone marrow biopsy showed kappa-restricted plasma cells comprising 80% of the bone marrow core, with no high-risk cytogenetic features. His hematologist performed a frailty assessment; the results indicated an activities of daily living score of 6, independent activities of daily living score of 7, and a Charlson Comorbidity Index (CCI) level of 1. A positron emission tomography scan showed extended and active lytic lesions in the ribs, bilaterally. The patient does not want to be treated in a clinical trial but can easily travel to a university cancer center for treatment.
Patient case 2
Patient 2 is an 86-year-old woman who has developed increasing fatigue and leg and dorsal back pain over the past 4 months. She has an ECOG PS of 2. She lives alone with support from her 2 daughters and was active until recently, but symptoms have now limited her activities. She lives in the countryside, 50 miles away from a cancer center. She has a history of chronic hypertension treated with diuretics, adult-onset diabetes mellitus treated with metformin, and mild hypercholesterolemia treated with a statin. Her cardiologist put her on low-dose aspirin a few years ago, and she takes acetaminophen for back pain (total of 5 medications). No specific frailty assessment was performed. An initial evaluation revealed anemia, normal renal function, and a serum immunoglobulin G kappa M-protein concentration of 2.5 g/dL. A bone marrow biopsy showed kappa-restricted plasma cells comprising 50% of the bone marrow core; these plasma cells harbor a translocation (4;14). A positron emission tomography scan showed active lytic lesions in the femurs as well as compression fractures at T5 and T7. The patient does not want to be treated in a clinical trial, would like to be treated as much as possible at home or near her home, and does not want to travel too frequently to a hospital.
General considerations on age and frailty assessment
Age negatively affects survival; progression-free survival (PFS) and overall survival (OS) are affected by age with a stepwise reduction in both with each added decade.1,7 In a recent study (not incorporating a CD38 antibody), the median PFS and OS were as low as 14 and 29 months, respectively, in patients aged >80 years.7 Interestingly, this study also noted that the relative contribution of molecular abnormalities, International Staging Score, and PS varied by age group, with molecular events being less and PS more contributive in older patients.7 However, treatment outcomes have slowly improved in recent years. In the EMMY real-life cohort, 561 patients aged ≥80 years were enrolled between 2017 and 2019 with potential access to CD38 antibodies; their median PFS was 19.3 months, and their median OS was 39.2 months.8 In a recent analysis from the International Myeloma Working Group (IMWG) Frailty Score cohort, 70 patients who were frail only by age (>80 years without comorbidities) had a median OS of 42.9 months, similar to the 190 patients who were frail for other conditions (OS, 41.6 months).9 Older and frail patients are still underrepresented in clinical studies, which makes treatment recommendations more difficult in this population.10 Reasons for exclusion have included predefined upper age limits or frailty, comorbidities, renal impairment, and concomitant use of multiple medications.2 Furthermore, even when considering the frail subpopulation of clinical studies, the real-life population is usually older and more vulnerable than the frail population in a clinical study.
Cure should remain the ultimate goal of therapy for every patient, including older and frail patients; however, it is generally accepted that treatment goals differ to a certain extent between younger and older patients. Some older patients may not prioritize prolongation of life at the expense of significant adverse events, leading to poor functional status and health-related quality of life (HRQOL).11 Fortunately, HRQOL is now examined in all registered MM studies, and some others.12-14 HRQOL results are a subtle balance between toxicity of therapy and symptoms of disease experienced by patients. Quality-of-life questionnaires do not capture everything that matters to patients, and older patients may also be more concerned about some non-HRQOL aspects such as transport and support from family and friends.15
Over the past 20 years, several tools have been developed to assess frailty in older adults in general,16,17 in patients with cancer,18 and more recently, specifically in patients with myeloma.19,20 The work in MM was pioneered <10 years ago by Palumbo et al, who published the first so-called IMWG Frailty Score.5 This work was seminal and drew the attention of the community to the frailty concept. Since then, several other scores from different countries and myeloma cooperative groups have been published, highlighting the value of frailty assessment (Table 1).5,14,19,21-26 An electronic frailty index based on data from administrative claims and electronic health records was also recently published (US Veterans Affairs Frailty Index).27 These scores, which have classified between 20% and 50% of patients as frail, have prognostic value and have consistently demonstrated increased rates of adverse events, treatment discontinuation, and worse PFS and OS in frail vs fit (or less frail) patients. Apart from scores, different approaches exist to measure muscle function and physical PS, and experts have recently recommended the use of a grip strength, 4-meter gait speed, or the Short Physical Performance Battery test to measure physical performance in daily practice.28 Gait speed is an easily obtained “vital sign” that predicts outcomes in patients aged ≥75 years with hematologic malignancies, including some patients with MM. Every 0.1 m/s decrease in gait speed was associated with higher mortality and unplanned hospitalizations.29 Frailty scores are useful but have several limitations. They are frequently considered to be time consuming, which is a significant barrier to their use in routine clinical practice. Age remains central in all scores, which is likely a weakness in contradiction with the idea that age, by itself, does not capture frailty. Among other limitations cited are the frequent use of the CCI, which does not appear optimal for hematologic malignancies; the lack of impact of polypharmacy and the variability in assessment of PS between clinicians. Sequential assessments of frailty should also be investigated. The diagnosis of frailty is usually clinical; however, the aforementioned limitations of scores have triggered attempts to identify and validate biomarkers.30-33 Studies on immunosenescence may also become important in the new era of immunotherapy.34 However, these parameters are not strictly associated with the biological changes of frailty and may fail to distinguish between frail and “healthy” older patients. Robust biomarkers of frailty are still lacking.
Key frailty scores
| . | IMWG5 . | Revised Myeloma Comorbidity Index22 . | UK Myeloma Research Alliance Myeloma Risk Profile31 . | Mayo risk score24 . | IFM simplified frailty25 . |
|---|---|---|---|---|---|
| No. of patients | 869 | NR | 2372 | 351 | 1618 |
| Frail patients, % | 30 | NR | 35 | 21 | 49 |
| OS in frail patients | 57% at 3 y | NR | mOS 20-25 mo | mOS 28 mo | mOS 42 mo |
| No. of groups | 3 | 3 | 3 | 4 | 2 |
| Parameters | Age, CCI, activities of daily living, independent activities of daily living | Estimated glomerular filtration rate, pulmonary function tests, frailty, age, cytogenetics, PS | Age, Revised International Staging System, C-reactive protein, PS | Age, N-terminal probrain natriuretic peptide, ECOG | Age, CCI, ECOG |
| Population | CT | CT, RW | CT | RW | CT |
| . | IMWG5 . | Revised Myeloma Comorbidity Index22 . | UK Myeloma Research Alliance Myeloma Risk Profile31 . | Mayo risk score24 . | IFM simplified frailty25 . |
|---|---|---|---|---|---|
| No. of patients | 869 | NR | 2372 | 351 | 1618 |
| Frail patients, % | 30 | NR | 35 | 21 | 49 |
| OS in frail patients | 57% at 3 y | NR | mOS 20-25 mo | mOS 28 mo | mOS 42 mo |
| No. of groups | 3 | 3 | 3 | 4 | 2 |
| Parameters | Age, CCI, activities of daily living, independent activities of daily living | Estimated glomerular filtration rate, pulmonary function tests, frailty, age, cytogenetics, PS | Age, Revised International Staging System, C-reactive protein, PS | Age, N-terminal probrain natriuretic peptide, ECOG | Age, CCI, ECOG |
| Population | CT | CT, RW | CT | RW | CT |
CT, clinical trial; mOS, median overall survival; NR, not reported; RW, real world.
Overall, there is now consensus that older patients with MM need geriatric assessment to identify frailty and vulnerabilities.35 Given the many parameters discussed earlier, and others yet to come, the development of new and better frailty scores in the future is probable.
Treatment strategy in older patients ineligible to receive transplantation
Newly diagnosed patients
The rapidly evolving landscape of MM therapy in the newly diagnosed MM population is described (for registered regimens, except MP [melphalan and prednisone]) in Figure 1.36-48 Additional regimens have been investigated but not approved.39,49,50 Before the recognition of frailty as an important issue, the Intergroupe Francophone Myélome (IFM) 01-01 with MP or MPT (melphalan, prednisone, and thalidomide) was specifically designed for patients aged ≥75 years,51 and the Rd (lenalidomide and dexamethasone) registration study also had an age subanalysis (≤75 vs >75 years).52 Treatment options are strongly correlated with drug access, and therefore, vary greatly across countries. Because autologous stem cell transplant has traditionally been considered as a potential option for patients aged <70 years without comorbidities,53 it is not reviewed here, and only approved regimens are discussed. Current standards of care are DRd (daratumumab, lenalidomide, and dexamethasone), D-VMP (daratumumab plus bortezomib, melphalan, and prednisone), and VRd (bortezomib, lenalidomide, and dexamethasone).53,54 If these options are not available, VMP or Rd could be recommended. Study populations and key efficacy results are shown in Table 2.
Phase 3 trials with older patients ineligible to receive transplantation
| . | MAIA DRd∗ . | ALCYONE D-VMP† . | SWOG VRd‡,§,‖ . | ENDURANCE VRd‡ . | TOURMALINE IRd¶ . | ENDURANCE KRd‡,¶ . | First Rd# . | MAIA Rd . | SWOG Rd‡,§ . | TOURMALINE Rd . | ALCYONE VMP . | CLARION VMP . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up (median), mo | 64.5 | 40.1 | 84 | NR | 53.3 | NR | NR | 64.5 | 84 | 55.8 | 40.1 | 22 |
| Number of patients | 368 | 350 | 235 | 542 | 351 | 545 | 535 | 369 | 225 | 354 | 356 | 477 |
| Age (median), y | 73 | 71 | 63 | 64 | 73 | 65 | 73 | 74 | 63 | 74 | 71 | 72 |
| <65 y, % | 1 | 10.3 | 61 | 50 | 3.1 | 50 | 6 | 1 | 53 | 2.3 | 6.7 | 7.3 |
| ≥70 y, % | 78 | NR | NR | 31 | NR | 32 | NR | 80 | NR | NR | NR | NR |
| ≥75 y, % | 43 | 29.7 | NR | NR | 43 | NR | 35 | 44 | NR | 44 | 30.1 | 30.4 |
| ORR, % | 93 | 91 | 90 | 84 | 82 | 87 | 81 | 81 | 79 | 80 | 74 | 79 |
| ≥Complete response, % | 51 | NR | 24 | 15 | 26 | 18 | 22 | 30 | 12 | 14 | NR | 23 |
| Minimal residual disease (10−5) | 32 | 28 | NR | NR | NR | NR | NR | 11 | NR | NR | 7 | 15.5 |
| PFS (median), mo | 61.9 | 36.4 | 34 (>65 y) | 34.4 | 35.3 | 34.6 | 26 | 34.4 | 24 (>65 y) | 21.8 | 19.3 | 22.1 |
| OS (median), mo | NR | NR | 65 (>65 y) | NR | NR | NR | 59 | 65.5 | 56 (>65 y) | NR | NR | NR |
| . | MAIA DRd∗ . | ALCYONE D-VMP† . | SWOG VRd‡,§,‖ . | ENDURANCE VRd‡ . | TOURMALINE IRd¶ . | ENDURANCE KRd‡,¶ . | First Rd# . | MAIA Rd . | SWOG Rd‡,§ . | TOURMALINE Rd . | ALCYONE VMP . | CLARION VMP . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up (median), mo | 64.5 | 40.1 | 84 | NR | 53.3 | NR | NR | 64.5 | 84 | 55.8 | 40.1 | 22 |
| Number of patients | 368 | 350 | 235 | 542 | 351 | 545 | 535 | 369 | 225 | 354 | 356 | 477 |
| Age (median), y | 73 | 71 | 63 | 64 | 73 | 65 | 73 | 74 | 63 | 74 | 71 | 72 |
| <65 y, % | 1 | 10.3 | 61 | 50 | 3.1 | 50 | 6 | 1 | 53 | 2.3 | 6.7 | 7.3 |
| ≥70 y, % | 78 | NR | NR | 31 | NR | 32 | NR | 80 | NR | NR | NR | NR |
| ≥75 y, % | 43 | 29.7 | NR | NR | 43 | NR | 35 | 44 | NR | 44 | 30.1 | 30.4 |
| ORR, % | 93 | 91 | 90 | 84 | 82 | 87 | 81 | 81 | 79 | 80 | 74 | 79 |
| ≥Complete response, % | 51 | NR | 24 | 15 | 26 | 18 | 22 | 30 | 12 | 14 | NR | 23 |
| Minimal residual disease (10−5) | 32 | 28 | NR | NR | NR | NR | NR | 11 | NR | NR | 7 | 15.5 |
| PFS (median), mo | 61.9 | 36.4 | 34 (>65 y) | 34.4 | 35.3 | 34.6 | 26 | 34.4 | 24 (>65 y) | 21.8 | 19.3 | 22.1 |
| OS (median), mo | NR | NR | 65 (>65 y) | NR | NR | NR | 59 | 65.5 | 56 (>65 y) | NR | NR | NR |
IRd, ixazomib-lenalidomide-dexamethasone; KRd, carfilzomib-lenalidomide-dexamethasone.
DRd, 28-day cycles of IV daratumumab (16 mg/kg once per week during cycles 1-2, once every 2 weeks in cycles 3-6, and once every 4 weeks thereafter) plus lenalidomide (25 mg on days 1-21 of each cycle) and dexamethasone (40 mg on days 1, 8, 15, and 22 of each cycle, with dose reduction to 20 mg if age is >75 years).46,47
D-VMP, 6-week cycles of subcutaneous bortezomib (1.3 mg/m2 of body surface area on days 1, 4, 8, 11, 22, 25, 29, and 32 of cycle 1 and on days 1, 8, 22, and 29 of cycles 2-9), melphalan (9 mg/m2 once daily on days 1-4 of each cycle), and prednisone (60 mg/m2 once daily on days 1-4 of each cycle), IV daratumumab (16 mg/kg once weekly during cycle 1, once every 3 weeks in cycles 2-9, and once every 4 weeks thereafter as maintenance therapy until disease progression).45,57
No intent for immediate transplant.
PFS and OS data from American Society of Hematology 2018 abstract.59
VRd, eight 21-day cycles of IV bortezomib (1.3 mg/m2 of body surface area on days 1, 4, 8, and 11) combined with lenalidomide (25 mg on days 1-14 of each cycle) and dexamethasone (20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12; VRd arm) or Rd alone (six 28-day cycles of lenalidomide 25 mg once a day on days 1-21 and dexamethasone 40 mg on days 1, 8, 15, and 22). On completion of induction, all patients received lenalidomide 25 mg once a day for 21 days plus dexamethasone 40 mg on days 1, 8, 15, and 22 of a 28-day cycle until disease progression.42,43,59
Not approved in first line.
Rd until disease progression.
DRd has emerged as a dominant standard of care.46-48 The registration study (MAIA) compared DRd with Rd alone, and PFS results with DRd were unprecedented in this population and associated with a high response rate (overall response rate [ORR], 93%; minimal residual disease negativity rate, 32%), meaning that virtually all patients achieved at least a partial response with this regimen.55,56 Treatment with DRd was manageable. The main differences in adverse events (grade ≥3) between DRd and Rd were for neutropenia (54% vs 37%) and infection (41% vs 29%; 19% vs 11% for pneumonia). D-VMP is the other main daratumumab-based standard of care. The registration study (ALCYONE) compared D-VMP with VMP alone and also showed high response rates (ORR, 91%; minimal residual disease negativity rate, 28%).45,55-57 The most common grade ≥3 adverse events with D-VMP and VMP alone were neutropenia (40% vs 39%), thrombocytopenia (34% vs 38%), and infections (23% vs 15%; 11% vs 4% for pneumonia). Importantly, both the MAIA and the ALCYONE studies demonstrated an OS benefit for DRd and D-VMP vs Rd and VMP (hazard ratio [HR], 0.68 and 0.60; respectively). The daratumumab arms of these studies were designed with IV daratumumab, but the drug is now prescribed subcutaneously based on the results of a randomized phase 3 noninferiority study in relapsed or refractory MM (COLUMBA).58 VRd is another recommended regimen, even though its development in older patients has not followed a straightforward registration pathway. The pivotal study was SWOG777 (VRd vs Rd alone), which was not specifically designed for older patients (only 91 patients in the VRd group were aged ≥65 years) (Table 2).44 In patients aged ≥65 years, median PFS was longer with VRd than with Rd (34 vs 24 months),59 and median OS was longer with VRd than with Rd (65 vs 56 months; HR, 0.77; not statistically significant).44 Adverse events were not reported specifically in older patients, but grade ≥3 gastrointestinal, neurological, and infectious adverse events were reported in 23%, 35%, and 18% of patients, respectively, in the intent-to-treat population.43 In the randomized phase 3 ENDURANCE study, which was conducted in patients with no intent for immediate transplant and no high-risk cytogenetic features, VRd and KRd achieved a similar median PFS of 34 months.50 Interestingly, a modified VRd regimen (VRd lite), more appropriate for older patients, was also investigated in a recent phase 2 study.44 The study enrolled 50 patients with a median age of 73 years (all patients were aged ≥65 years). Patients were treated with 9 5-week cycles of subcutaneous bortezomib once weekly on days 1, 8, 15, and 22; lenalidomide 15 mg on days 1 to 21, and low-dose dexamethasone followed by 6 cycles of consolidation and lenalidomide maintenance until disease progression. The median PFS was 41.9 months, and the safety profile was better than that with the original VRd regimen (eg, 2% of patients had grade ≥3 neurological toxicity). However, dose reductions still occurred in almost 80% of patients.60
Besides clinical studies, few real-life data have been published for VRd and Rd (no data yet for D-VMP or DRd). The IFM group presented data for 773 patients initiating a nontransplant first line of therapy between 2017 and 2019 (EMMY cohort), of whom 162 received Rd (21%; median age, 79 years) and 158 received VRd (20%; median age, 69 years).61 For patients who received Rd, the median time to next treatment by age (<75 or ≥75 years) was identical at ∼29 months. In contrast, for patients who received VRd, the median time to next treatment was almost 35 months in patients aged <75 years but only 17 months in patients aged ≥75 years. In the PEGASUS study,62 leveraging data from individual patients, an anchored indirect treatment comparison was made between patients given DRd in the MAIA study and patients treated with VRd from the Flatiron Health electronic health record–derived database. DRd was associated with a significantly lower risk of progression or death compared with VRd (HR, 0.68).
Patients who have relapsed
Treatment options at relapse were also recently reviewed.53,63 Of note, in a study investigating real-world outcomes in almost 5000 European patients, it was shown that only 61% and 38% of patients reached second- and third-line treatments, respectively.64 These results are clearly not optimal, and they are likely even worse in older and frail patients. It is important to keep in mind that most older patients will receive 1 to 3 lines of therapy, making the discussion on advanced MM in frail patients somewhat artificial. This also supports the consensus that the best treatment options, whatever they may be, should be used in front line. New immunotherapies have not yet been investigated in frail patients. To date, reports on the use of chimeric antigen receptor (CAR) T-cell therapy for older patients have been limited. In a recent older-patient subgroup analysis of the KarMMa study (#NCT03361748), treatment outcomes for idecabtagene vicleucel (bb2121) were similar in patients aged ≥70 years (n = 20, 4 patients aged >75 years) and those who were younger (aged ≥65 years, n = 45). However, none of the patients in the older age group were frail or >80 years of age, and all had an ECOG PS of 0 or 1.65 Few data in frail patients exist with carfilzomib.66
Frailty subanalyses and studies
The phase 2 HOVON 143 study was an important milestone in the field because it was the first study dedicated to frail patients (defined by the IMWG Frailty Index) and considered IDd (ixazomib, daratumumab, and low-dose dexamethasone).67 Sixty-five patients with a median age of 81 years (70-92 years) were treated with 9 induction cycles of IDd followed by maintenance with ID for a maximum of 2 years. The ORR was 78%, the median PFS was short at 13.8 months, and the 12-month OS was 78%. Patients who were frail based on age of >80 years alone had a better clinical outcome, with a median PFS of 21.6 months and a 12-month OS of 92%. Five patients died within 2 months of treatment initiation (8%), most from toxicity. Overall, the study highlights that even in the era of novel MM agents and with a presumably nontoxic regimen, treatment of frail patients remain challenging. The study will hopefully serve as a prototype for new trials in frail patients. Two other studies will have results in the next few years: the IFM 2017_03 trial (#NCT03993912),68 a dexamethasone sparing study for frail patients, and the UK Myeloma Research Alliance FiTNEss (Myeloma XIV)69 trial. It remains that there is currently no proven frailty-adjusted treatment approach.
Both daratumumab studies have presented a frailty subanalysis (simplified frailty score with age, CCI, and ECOG) (Table 3).48,70 In the ALCYONE study, 44.6% of patients were frail. After a median follow-up of 40.1 months, the frail patients in the D-VMP group had a shorter median PFS than the nonfrail patients (32.9 and 45.7 months, respectively).70 Similar median PFS was observed for frail and nonfrail patients treated with VMP (19.5 and 19.1 months, respectively). In the MAIA study, 46% of patients were frail.48 After a median follow-up of 36.4 months, the frail patients had a shorter median PFS than the nonfrail patients (not reached for DRd in both groups; 30.4 and 41.7 months for Rd in the frail and nonfrail subgroups, respectively); however, the PFS benefit of DRd over Rd was maintained across subgroups. In both studies, grade 3 or 4 adverse events, treatment-emergent adverse events with an outcome of death, and treatment discontinuation owing to treatment-emergent adverse events were slightly more frequent in the frail subgroups.
Frailty analysis in MAIA, ALCYONE, and HOVON143 study results
| . | MAIA48 . | ALCYONE70 . | HOVON14367 . | ||
|---|---|---|---|---|---|
| Follow-up (median), mo | 36.4∗ | 40.1∗ | 22.9 | ||
| Frail patients, % | 46.7 | 44.6 | 100 | ||
| . | MAIA48 . | ALCYONE70 . | HOVON14367 . | ||
|---|---|---|---|---|---|
| Follow-up (median), mo | 36.4∗ | 40.1∗ | 22.9 | ||
| Frail patients, % | 46.7 | 44.6 | 100 | ||
| . | DRd . | Rd . | D-VMP . | VMP . | IDd . |
|---|---|---|---|---|---|
| PFS in frail patients, (median), mo | NR | 30.4 | 32.9 | 19.5 | 13.8 |
| ORR, % | 87.2 | 78.1 | 88.3 | 72.4 | 78 |
| Death within 60 d, % | 6 | 3.6 | 4.4† | 5.3† | 8 |
| Grade ≥3 treatment-emergent adverse events, % | 94.6 | 89.2 | 79.4 | 81.5 | 30 (hematologic) 74 (nonhematologic) |
| Grade ≥3 pneumonia, % | 19.6 | 10.2 | 14.4 | 5.3 | NR |
| . | DRd . | Rd . | D-VMP . | VMP . | IDd . |
|---|---|---|---|---|---|
| PFS in frail patients, (median), mo | NR | 30.4 | 32.9 | 19.5 | 13.8 |
| ORR, % | 87.2 | 78.1 | 88.3 | 72.4 | 78 |
| Death within 60 d, % | 6 | 3.6 | 4.4† | 5.3† | 8 |
| Grade ≥3 treatment-emergent adverse events, % | 94.6 | 89.2 | 79.4 | 81.5 | 30 (hematologic) 74 (nonhematologic) |
| Grade ≥3 pneumonia, % | 19.6 | 10.2 | 14.4 | 5.3 | NR |
OS results not presented (not mature).
Unpublished, from clinical study report.
Specific recommendations for frail patients
Use of dexamethasone
The use of dexamethasone deserves special mention because it has been part of MM therapeutic regimens for almost 40 years.71,72 Although effective, regimens containing high-dose dexamethasone have been recognized for years as having significant toxicity, including neurological disorders, psychiatric complications, secondary diabetes mellitus, gastrointestinal adverse events, and susceptibility to infections.73,74 This led to the development (>10 years ago) of a low-dose dexamethasone schedule consisting of dexamethasone at 40 mg on days 1, 8, 15, and 22 of a 28-day cycle.75 This dose was then reduced to 20 mg on the same days for patients aged ≥75 years40 and even further to 10 mg every 2 to 4 weeks in a recent study dedicated to frail patients.67 Attempts have also been made to shorten treatment exposure, as in the recent study by the GIMEMA group in older, intermediate-fit patients. Patients were randomly allocated either to Rd given continuously or to Rd for 9 cycles followed by lenalidomide alone. Both arms were equally effective, and dexamethasone discontinuation was associated with a better safety profile.76 Earlier discontinuation after only 2 cycles is currently under investigation in the context of a lenalidomide and subcutaneous daratumumab regimen the in the IFM 2017_03 trial.68 Overall, it is anticipated that the use of dexamethasone will be considerably reduced or even abrogated in the future for older patients.
Suggested dose modifications of drugs and supportive care
Suggested dose modifications of key drugs in older patients have been published, and supportive measures have also been reported elsewhere.2,77,78 Briefly, patients may receive analgesics, bisphosphonates, and antithrombotic and antibiotic prophylaxis. Supplementation with calcium and vitamin D is recommended with bisphosphonates. Infection remains a concern, especially in frail patients. A predictive model for severe and early infection in older patients has been developed and may be applied to guide strategies for infection prevention.79 Addition of prophylactic levofloxacin to active myeloma treatment can be used in newly diagnosed MM.80 The use of prophylaxis against varicella-zoster virus is also frequent. An appropriate vaccination strategy is needed, including one against COVID-19.81,82 Patients must be instructed to avoid nonsteroidal anti-inflammatory drugs as well as other potentially nephrotoxic drugs. In patients failing to develop an immune response after COVID-19 vaccination, prophylactic or prompt initiation of COVID-19 treatment must be discussed.
Comments about cases 1 and 2
The patient in case 1 had a frailty assessment. He was considered to be intermediate frail by age only, according to the IMWG score, and frail according to the IFM score (with a score of 2, which is the minimum score indicating frailty). Of the 3 standards of care (DRd, D-VMP, and VRd), D-VMP is considered the most attractive because of the patient’s recent thromboembolic event. However, DRd and VRd (as VRd lite) would be more difficult to manage but not impossible. If there is no access to daratumumab, VMP is a reasonable option. In the future, bispecific antibodies may play a role in patient populations such as this.
The patient in case 2 had no frailty assessment but was considered frail at least by age per the IMWG Frailty Score and by age and ECOG PS per the IFM score. She was already taking several medications before the start of MM therapy, not unlike many older patients who take multiple medications to treat other medical conditions before being diagnosed with MM. In a recent study conducted in Belgium, the mean number of medications used per person aged ≥65 years was 3.5 (0-19); almost 50% of subjects took 1 to 4 medications, 25% took 5 to 8 (polypharmacy), and 8% took ≥9 (excessive polypharmacy).83 Considering a 2- or 3-drug MM regimen, and even the minimum of supportive medications, this would put a patient in an “excessive polypharmacy” situation. A clear understanding of, and compliance with, such complex combinations of drugs is a challenge and requires consistency of care, medical education, frequent visits, and possibly, help from relatives. We recommend either DRd or Rd as a first-line therapy. It is frequently recommended that frail patients receive a 2-drug rather than a 3-drug regimen, in this case, Rd instead of DRd. This recommendation could apply to patient 2, who is of advanced age, has comorbidities, and does not want to travel frequently to the hospital; however, the therapy of choice should be cautiously evaluated and discussed with patients and sometimes with their families. Excluding patients from receiving CD38 antibodies based on age or frailty is questionable, because these drugs are efficacious and can improve quality of life. For example, these antibodies are associated with a greater and faster reduction in bone pain and have an acceptable tolerability profile.13 Our preference would be to propose a “gentle” DRd regimen, such as dexamethasone limited to the initial cycles (eg, first 2 cycles) and a reduced lenalidomide dose (10 mg per day). An alternative schedule of daratumumab and fixed-duration (instead of continuous) therapy, although not yet investigated, might be considered.
Finally, the age at which MM is diagnosed correlates with the potential number of years of life lost, which is applicable to both the patients in cases 1 and 2. In a 2010 publication (patients enrolled between 1982-2002), an average of 8.1 years of life lost was reported in patients aged 70 to 79 years and 4.6 years in patients aged ≥80 years.84 This loss in years of life is now likely limited, provided that patients have access to new drugs and receive diligent care. Thus, in the optimal scenario, the years of life lost for the patients in cases 1 and 2 may be minimal or mitigated altogether.
Conclusion
Treatment perspectives include the use of new immunomodulatory drugs such as iberdomide, bispecific antibodies, or even CAR T-cell strategies (Figure 1). The degree of frailty that could lead to a contraindication to CAR T-cell therapy (concept of CAR T-cell therapy eligibility) or other new immunotherapies is undefined and needs to be investigated. Trying to define immunotherapeutic regimens with an appropriate risk-benefit balance for older patients is also needed. Furthermore, the continuous progress of MM therapy has possibly made us less focused on more basic aspects of patient care. Prevention and treatment of bone disease and infections, and supportive care, remain key issues. Early death is still observed in between 5% and 10% of frail patients, often because of infection, and efforts must be made to reduce this incidence. As discussed, frailty assessment has value and will hopefully lead to specific studies in frail patients. It would also be prudent to revise the way in which tolerability and quality of life are reported. Tolerability analyses should be harmonized across studies and not be limited to the most severe events; low-grade events should be reported, some of which impact quality of life and clinical outcome. Moreover, implementation of more diligent quality-of-life analyses would be desirable. Finally, most of the current regimens have been registered as continuous therapy; however, efforts should be made to assess the value of fixed-duration or response-driven therapy.
Significant progress has been made in the treatment of geriatric patients with MM, and an “operational cure” in a proportion of older patients with access to new drugs and regimens seems achievable in the future. Additional progress is yet to come, which is great news for patients and families. Some of these patients will benefit from long-term disease control and ultimately, will not directly die from MM.
Authorship
Contribution: All authors performed the literature review and wrote the manuscript.
Conflict-of-interest disclosure: All authors are consultants for Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, Regeneron, Roche, Sanofi, and Takeda.
Correspondence: Thierry Facon, Department of Hematology, University of Lille, Centre Hospitalier Universitaire Lille, Rue Michel Polonovski, 59000 Lille, France; email: thierry.facon@chu-lille.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal