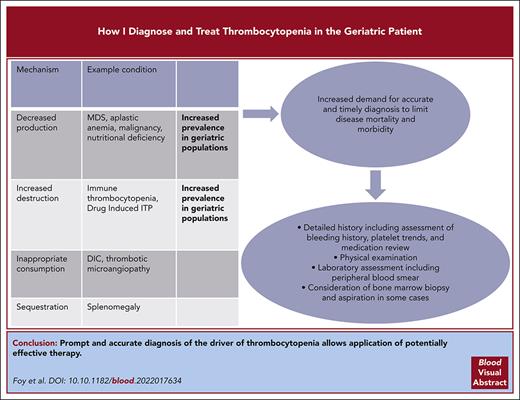
Visual Abstract
Thrombocytopenia in older individuals is a common but diagnostically challenging condition that has variable clinical impact to those who are affected. Diagnostic approach requires evaluation of the preexisting clinical conditions, detailed review of medications, and assessment for disorders that warrant urgent treatment. In this article, we describe a systematic approach to diagnosis of thrombocytopenia and present a schematic review for management strategies. Three clinical scenarios are presented that are relevant for their prevalence and management challenges in an older adult population. The first scenario addresses primary immune thrombocytopenia (ITP) and reviews different treatment options. The second one addresses complications of thrombocytopenia in management of the myelodysplastic syndrome. The last one reviews diagnostic challenges of drug-induced ITP.
Introduction
Thrombocytopenia, generally defined as a platelet count <150 000/μL, is more commonly identified in adults aged >60 years; mean platelet counts decline as populations age across decades.1 Multiple comorbidities and polypharmacy in older populations can complicate timely and accurate diagnosis of conditions causing thrombocytopenia. Bleeding associated with thrombocytopenia correlates poorly with platelet count, except when platelet counts decrease to <20 000/μL.2 Bleeding risk in older populations also depends upon platelet function, vascular integrity, antithrombotic therapy, coagulation defects, and regulation of fibrinolysis. Paradoxically, some patients with thrombocytopenia are at increased risk of thrombosis and warrant urgent treatment to prevent potentially life-threatening clotting complications.
The definition of “geriatric” is less well-established than the definition of thrombocytopenia. Studies of hematologic conditions in older patients primarily use chronologic age as an eligibility criterion. The process of aging is heterogeneous owing to differences in individual susceptibility and exposure to determinants of chronic disease.3 The concept of fragility was introduced as a way of identifying individuals who, at a given age, have a particularly labile health balance. Accurate selection of patients able to tolerate a specific therapy is crucial, and comprehensive geriatric assessment based upon age, comorbidities, and functional ability may help determine if a patient is fit (or not) for a given intervention. However, a precise definition of fragility is lacking. Although multiple tools have been developed for use in clinical and research settings, there are few studies comparing the predictive value of these instruments.4 Acknowledging the variability in definition, most of the studies cited here refer to patients aged at least >60 years and, in many cases, aged >75 years. In this submission, we use the terms “older” and “geriatric” interchangeably.
Although most thrombocytopenia may be clinically innocuous, more severe conditions have an outsized impact on morbidity and mortality in older patient populations. Up to 25% of patients who are critically ill develop drug-induced thrombocytopenia, and the overall incidence may be at least 10 cases per million population per year.5 For hospitalized populations, it has been estimated that ∼7% of tested blood specimens have platelet counts of <100 000/μL.6 Sequelae of thrombocytopenia include increased mortality, increased cost of care, and longer hospitalizations.7-9
Diagnostic approach to thrombocytopenia for older adults
General hematologists are well versed in the physiologic categorization of thrombocytopenia as arising from (1) decreased platelet production, (2) increased platelet destruction, (3) platelet consumption, or (4) platelet sequestration. The distribution of underlying causes is different in an older cohort than in a younger population. Some etiologies of thrombocytopenia may have multiple mechanistic effects. For example, medications may lower platelet counts through the development of drug-dependent platelet antibodies,12 suppression of megakaryocytopoiesis, or induction of thrombotic microangiopathy (TMA).13
Advanced age presents diagnostic challenges after thrombocytopenia has been established by complete blood count (CBC) and confirmed with peripheral smears. Because older populations have increased risk of malignancy, rheumatologic disease, liver disease, infection, bleeding disorders, thrombosis, and polypharmacy, detailed history-taking and medication review are required to accurately establish diagnosis. Initial questioning aims to establish the duration and progression of thrombocytopenia by reviewing prior medical records and laboratory measurements. The patient should be questioned on bleeding and thrombotic patterns, with special attention to the skin, gums, gastrointestinal tract, and central nervous system. Medical interviews might include an informed caregiver who can provide additional insights on medical history and medication adherence. This is particularly important for individuals with any level of dementia or delirium. A full medication history, including vaccinations and use of over-the-counter medications, dietary supplements, marijuana derivatives, or illicit drugs, is essential. This includes review of how often medications are taken according to recommended schedule and dosage.
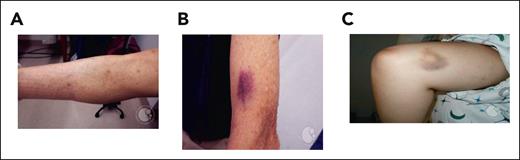
As with younger individuals, physical examination for older patients should focus on signs and symptoms of bleeding and thrombosis. The location of ecchymosis may delineate whether there is a traumatic component or a spontaneous nature to the contusions. The oral pharynx and hard palate may sometimes demonstrate petechiae. The patient’s neurologic examination including assessment of gait function is important to understand the risk associated with falls. Finally, physical examination may help to identify disease states that contribute to the patient’s thrombocytopenia, such as stigmata of liver disease, splenomegaly, and lymphadenopathy (Figure 1 shows physical findings of thrombocytopenia).
Cutaneous manifestations of thrombocytopenia including petechiae, purpura, and ecchymoses. (A) Petechiae: flat, red, pinpoint lesions often found in groups in dependent areas. (B) Purpura: purple discoloration caused by confluent petechiae in either the skin (dry) or mucosa (wet). (C) Ecchymoses: nontender areas of bleeding into the skin with extravasated blood and breakdown of heme pigments causing red, purple, green, and yellow coloration. These images were originally published in the American Society of Hematology Image Bank. Panel A was adapted from Peter Maslak, Petechiae-1, 2008; image 00003689. Panel B was adapted from John Lazarchick, Idiopathic Thrombocytopenic Purpura-1, 2011; image 00001367. Panel C was adapted from Peter Maslak, Ecchymoses-2, 2011; image 00003688.
Cutaneous manifestations of thrombocytopenia including petechiae, purpura, and ecchymoses. (A) Petechiae: flat, red, pinpoint lesions often found in groups in dependent areas. (B) Purpura: purple discoloration caused by confluent petechiae in either the skin (dry) or mucosa (wet). (C) Ecchymoses: nontender areas of bleeding into the skin with extravasated blood and breakdown of heme pigments causing red, purple, green, and yellow coloration. These images were originally published in the American Society of Hematology Image Bank. Panel A was adapted from Peter Maslak, Petechiae-1, 2008; image 00003689. Panel B was adapted from John Lazarchick, Idiopathic Thrombocytopenic Purpura-1, 2011; image 00001367. Panel C was adapted from Peter Maslak, Ecchymoses-2, 2011; image 00003688.
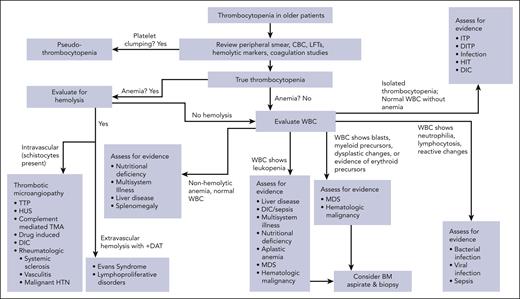
After the history and physical examination are complete, we recommend using a diagnostic algorithm to identify the differential information by the patient presentation and risk factors. This allows for choice of sequential testing (Figure 2 shows the diagnostic algorithm for thrombocytopenia in older adults). This approach requires initial review of CBC and peripheral smear to diagnose thrombocytopenia by excluding platelet clumping (so-called pseudothrombocytopenia) that occurs in vitro from exposure of sampled blood to EDTA in collection tubes. If suspicion for platelet clumping is raised, a repeat platelet count should be obtained in heparin or citrate rather than EDTA as a test tube anticoagulant.14 Review of CBC and peripheral smear also allows for identification of abnormal white and red blood cell changes, which may indicate alternative disease states. Abnormalities in the leukocyte lineage may include neutrophilia or lymphocytosis, circulating immature cells including blasts, or dysplastic changes.
Diagnostic strategy for geriatric patients with thrombocytopenia. Initial evaluation requires laboratory studies, including review of peripheral blood smear to exclude platelet clumping. After true thrombocytopenia is established, further assess for concurrent hemolytic anemia and white blood cell disorders to narrow differential diagnosis and alert clinicians to diagnoses that may warrant urgent action, such as TMA or suspicion for infection. When diagnosis is suspicious for hematologic malignancy or aplastic anemia, bone marrow aspiration and biopsy should be considered to establish diagnosis. BM, bone marrow; DIC, disseminated intravascular coagulation; LFT, liver function tests; HTN, hypertension; HUS, hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
Diagnostic strategy for geriatric patients with thrombocytopenia. Initial evaluation requires laboratory studies, including review of peripheral blood smear to exclude platelet clumping. After true thrombocytopenia is established, further assess for concurrent hemolytic anemia and white blood cell disorders to narrow differential diagnosis and alert clinicians to diagnoses that may warrant urgent action, such as TMA or suspicion for infection. When diagnosis is suspicious for hematologic malignancy or aplastic anemia, bone marrow aspiration and biopsy should be considered to establish diagnosis. BM, bone marrow; DIC, disseminated intravascular coagulation; LFT, liver function tests; HTN, hypertension; HUS, hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
For older patients, our approach to laboratory evaluation of thrombocytopenia initially evaluates for evidence of hemolysis (lactate dehydrogenase, haptoglobin, reticulocyte count, and direct antiglobulin test), hepatic function tests, nutritional markers (folate and vitamin B12), hepatitis C, and HIV testing, if these studies have not been performed in the preceding 6 to 12 months. Further evaluation includes screening for systemic autoimmune disease (antinuclear antibody and antiphospholipid antibodies), thyroid disease, and coagulation studies (fibrinogen level, prothrombin time, partial thromboplastin time, and D-dimer expression7) when initial evaluation raises concern. Use of reticulated platelets15 or immature platelet fraction16 can help to determine whether thrombocytopenia relates to bone marrow failure or increased destruction, although access to this testing is not available at all hematology laboratories.
Urgency of evaluation is amplified if initial assessment raises concern for a condition that warrants rapid administration of medical therapy. These conditions include concern for acute leukemia, severe aplastic anemia, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, disseminated intravascular coagulation, antibody-mediated platelet activation syndromes such as heparin-induced thrombocytopenia (HIT) or vaccine-induced immune thrombotic thrombocytopenia,17 and thrombocytopenia associated with bleeding.
Special consideration of thrombocytopenia for patients who are severely ill is warranted. Persons admitted to intensive care units (ICUs) are frequently thrombocytopenic at the time of admission, and many others develop thrombocytopenia during ICU stays.18 Lower platelet counts are associated with worsened mortality at ICUs19 and have been associated with renal impairment, continuous renal replacement therapy, acute lung injury, sepsis, extracorporeal membrane-oxygenator, and cardiac assist devices.20
Bone marrow aspiration and biopsy are not required for all older patients with thrombocytopenia because many times a clear culprit may be identified without examination of the marrow. However, the procedure is a generally safe one and should be performed when assessment of the peripheral blood points to alterations in other cell lineages or whenever there is suspicion of bone marrow failure syndrome. Bone marrow biopsy should also be considered if diagnosis of thrombocytopenia remains uncertain after initial assessment or if the platelet count fails to respond as anticipated to therapy. We do not consider any age to be a contraindication to bone marrow biopsy. The procedure can be more challenging for individuals with profound osteoporosis or significant orthopedic impairment.
Mechanisms of thrombocytopenia
Common causes of thrombocytopenia in older adults are listed in Table 1. Physiologically, these disorders result in thrombocytopenia due to decreased platelet production, increased platelet destruction, platelet consumption, and/or platelet sequestration. Medications and nonprescribed supplements may lower platelet counts through more than 1 mechanism: for example, gemcitabine regularly causes thrombocytopenia by decreasing platelet production21 but is also a recognized cause of TMA.
Causes of thrombocytopenia in older adults
| Decreased platelet production . | Increased platelet destruction . | Platelet consumption . | Splenic sequestration . |
|---|---|---|---|
| MDS | Primary ITP | Disseminated intravascular coagulation | Splenomegaly |
| Hematologic malignancy | DITP | TMA including TTP, HUS, atypical HUS, systemic sclerosis, and malignant hypertension | |
| Bone marrow metastasis | Secondary ITP associated with rheumatologic disease and infections | Sepsis/infection | |
| Aplastic anemia | Antiphospholipid antibody syndrome | ||
| Drug effect including alcohol | |||
| Nutritional deficiencies (vitamin B12, folate, and copper) | |||
| Liver disease | |||
| Sepsis/infection |
| Decreased platelet production . | Increased platelet destruction . | Platelet consumption . | Splenic sequestration . |
|---|---|---|---|
| MDS | Primary ITP | Disseminated intravascular coagulation | Splenomegaly |
| Hematologic malignancy | DITP | TMA including TTP, HUS, atypical HUS, systemic sclerosis, and malignant hypertension | |
| Bone marrow metastasis | Secondary ITP associated with rheumatologic disease and infections | Sepsis/infection | |
| Aplastic anemia | Antiphospholipid antibody syndrome | ||
| Drug effect including alcohol | |||
| Nutritional deficiencies (vitamin B12, folate, and copper) | |||
| Liver disease | |||
| Sepsis/infection |
HUS, hemolytic uremic syndrome; TTP, thrombocytopenic purpura.
Decreased production
Megakaryocyte dysfunction leading to decreased platelet production may be encountered in myelodysplastic syndrome (MDS) or acute leukemia, aplastic anemia, hematologic, or metastatic solid tumor malignancies. Bacterial and viral infections, sepsis, nutritional deficiencies, alcohol, and some classes of drugs can decrease platelet production. Thrombopoietin (TPO) acts as the hematologic growth factor preventing megakaryocyte apoptosis.22 Hepatic TPO production is typically constant, allowing circulating TPO levels to be regulated by the platelet mass.23 One of the causes of thrombocytopenia in liver disease is impaired platelet production by reduction of TPO.24
Increased destruction
Under normal physiologic conditions, platelets circulate in the peripheral blood for ∼10 days before they are removed from the circulation through apoptosis induced by senescence.25 Platelet destruction within the reticuloendothelium of the liver and spleen may be accelerated in immune thrombocytopenia (ITP) by interactions between platelet autoantibodies typically targeting platelet membrane glycoproteins and Fcγ receptors on phagocytic cells. Some drugs and vaccines can induce antiplatelet antibodies to bind specific platelet antigens. Both viral and bacterial infections can induce antibodies that cross-react with platelet antigens via molecular mimicry.26 Helper T cells reactive with platelet glycoproteins are activated in ITP and are associated with increased platelet autoantibody production.27 Disorders of immune regulation including systemic lupus erythematosus, antiphospholipid syndrome, rheumatoid arthritis, common variable immunodeficiency, chronic lymphocytic leukemia, and other lymphoproliferative disorders can induce platelet autoantibody formation. Although platelet production is often increased in ITP, production is insufficient to correct for loss in platelet mass due to antibody-mediated and T-cell–dependent apoptosis in megakaryocytes and decreased platelet shedding.28,29
Inappropriate consumption
Thrombocytopenia via platelet consumption is regularly seen in disorders that result in thrombus formation. Platelet counts typically decline within the first 4 days after surgery due to consumption.30 Dysregulated thrombus formation in both disseminated intravascular coagulation and TMA can result in thrombocytopenia. Although thrombocytopenia in these conditions may be mild compared with disorders of accelerated platelet destruction, these conditions are associated with impaired microvascular circulation, organ injury, and morbidity, particularly in older populations.31
Platelet sequestration
Approximately one-third of platelet mass is found in the spleen, and disorders that increase splenic volume result in reduction in the circulating number of platelets.32 Because sequestered platelets within the spleen remain functional, they act to decrease circulating TPO. This further contributes to thrombocytopenia by decreasing platelet production.33
Case 1
Patient 1 is a 74-year-old woman with osteoporosis and type 2 diabetes mellitus on alendronate and metformin who is found to have a platelet count of 19 000/μL after developing a petechial rash on her legs and epistaxis. Previous laboratory evaluations over the past decade had included regular observation of a normal CBC. Review of peripheral smear does not demonstrate abnormalities in leukocyte or erythrocyte morphology or quantity. She had a normal white blood cell (WBC) count and differential, hemoglobin, hematocrit, mean corpuscular volume (MCV), prothrombin time, partial thromboplastin time, fibrinogen, liver function tests, and creatinine. HIV and hepatitis virus serologic results were negative. The patient was presumptively diagnosed with ITP and started on dexamethasone for 4 days. After 5 days, the patient’s bleeding symptoms resolved, and her platelet count increased to 112 000/μL. After an additional 3 weeks, the patient’s platelet count decreased to 28 000/μL, and a repeat dexamethasone dose was administered. Therapy was complicated because of hyperglycemia, and she returned to her hematologist to discuss steroid-sparing therapies.
Diagnosis of ITP in older populations remains largely a diagnosis of exclusion. In the absence of other cytopenias, bone marrow biopsy may be deferred. Routine use of bone marrow biopsy for older populations when ITP is suspected is no longer suggested by either 2019 American Society of Hematology guidelines34 or the 2019 International Consensus Report on primary ITP.35 When other hematologic abnormalities are identified, bone marrow biopsy should be considered because the prevalence of MDS is increased among older adults.36 Evaluation for Helicobacter pylori with stool antigen or C-urea breath test should be considered if the patient resides within an area where it is endemic. The incidence of H pylori increases with age, and eradication is associated with improved platelet counts.37 Routine use of antiplatelet autoantibody testing is not recommended, given the test’s low sensitivity.38 Review of all prescribed and over-the-counter medications is essential to exclude drug-induced thrombocytopenia.
The decision to use medication to increase platelet counts in ITP aims to reduce the risk of severe bleeding complications while minimizing potential adverse reaction to therapy. Platelet count is an imperfect surrogate for bleeding risk, particularly when the platelet count is above 10 000/μL.39 In the absence of bleeding, some experts recommend avoiding treatment of ITP unless the platelet count decreases below 20 000/μL.40 It should be noted that the risk of bleeding with ITP increases 1.46 times for every 10 years of age,41 and risk of fatal hemorrhage is elevated for those aged >60 years.42 Guidelines on treatment of ITP for adults do not specifically make recommendations for older patients. Our practice involves treating ITP for anyone with bleeding and older patients with platelet counts of <30 000/μL and considering treatment for older patients with platelet counts between 30 000 and 50 000/μL if the patient requires surgery or antithrombotic therapy.
Most recent ITP treatment guidelines have recommended corticosteroids dose be limited to less than 6 weeks for initial treatment.34 Adverse effects of corticosteroids, including hyperglycemia, elevated blood pressure, fluid retention and osteoporosis, are often underestimated for older populations.43 Pulse dexamethasone (40 mg daily for 4 days) has been shown to be effective for persons aged >70 years44 and is associated with a higher platelet count at 14 days after administration compared with prednisone.45 High-dose IV immunoglobulin (IVIG) is effective as a first-line therapy for ITP in patients intolerant to corticosteroids. Combination corticosteroids with IVIG should be considered when there is severe bleeding, evidence of wet purpura, or a platelet count of <20 000/μL, because combination therapy more rapidly increases platelet count than corticosteroids alone.46 IVIG is well tolerated, but serious adverse events including arterial and venous thrombosis, acute kidney injury and fluid overload are more common among older adult populations.47
Although most older patients with ITP respond initially to corticosteroids and/or IVIG, the majority of patients have their disease relapse or progress to persistent or chronic ITP. Persistent ITP is defined as disease duration of 3 to 12 months, whereas chronic ITP requires >12 months' duration.48 There are few comparative trials of second-line agents for ITP management, and selection of therapy relies upon shared decision-making with patients balancing their preferences and potential for therapy-related complications.
TPO-receptor agonists (TPO-RAs) approved in the United States for treatment of ITP include the weekly subcutaneous injection romiplostim and 2 oral agents, eltrombopag and avatrombopag. TPO-RAs have high overall response rates in ITP therapy (70%-80%) but are associated with recurrent thrombocytopenia when treatment is discontinued.49,50 Selection between TPO-RAs for older populations depends upon different routes of administration (subcutaneous vs oral), potential food interactions, and need for daily vs weekly administration. Response rates with both romiplostim51 and eltrombopag52 were slightly higher in older populations than in younger adults. Potential adverse effects with TPO-RAs include headache, liver injury, thrombosis, and myelofibrosis. Thrombotic complications are more common in those aged >60 years on TPO-RAs.53 Bone marrow dysfunction and reticulin fibrosis appear to be reversible after TPO-RAs are discontinued.54
Rituximab, a chimeric anti-CD20 monoclonal antibody that decreases autoantibody production by depletion of blood cells, has been used in management of ITP for >20 years. Initial response rate in ITP is ∼60%, but decreases to ∼20% after 5 years.55 Risk of infection with rituximab for ITP therapy, including risk of severe infection and death is increased in populations aged >70 years.56 Given its effect on B cells, rituximab can be associated with impaired response to vaccination, hepatitis B reactivation, and progressive multifocal encephalopathy.
Fostamatinib is an oral Syk inhibitor that has been approved by the Food and Drug Administration for the treatment of chronic ITP on the basis of 2 clinical trials demonstrating a response rate of >40% in previously heavily treated patients with ITP.57 Approximately one-quarter of the 102 patients in the registration study were aged >64 years, and it appears that hypertension was a more common side effect in this older cohort than in younger cohorts. Likewise, older immunomodulatory agents, including dapsone, azathioprine, cyclosporin, hydroxychloroquine, mycophenolate mofetil, cyclophosphamide, and vinca alkaloids have all been used in secondary treatment of ITP without comparative data to guide decision making.
Splenectomy is an effective nonmedical therapy in ITP, with an initial response rate of >80% and long-term remissions of >60%.58 However, there have been concerns that splenectomy is less effective in adults aged >65 years and that advanced age is associated with increased risk of ITP relapse after splenectomy.59 Moreover, patients >60 years of age have increased risk of perioperative bleeding60 and increased risk of sepsis61 after splenectomy. Current guidelines recommend splenectomy be deferred at least 12 to 24 months following diagnosis of ITP. If splenectomy is considered or older patients, a laparoscopic technique is associated with fewer complications. Vaccination against encapsulated bacterium (streptococcus pneumoniae, haemophilus influenzae, and meningococcus) should be administered at least 4 weeks before splenectomy.
Case 2
Patient 2 is an 81-year-old man with a history of coronary artery disease and hypertension who is found to have a platelet count of 48 000/μL on routine CBC evaluation. Review of his medical history shows that his platelet count had slowly decreased from normal levels 7 years ago to its current nadir count. WBC was slightly reduced at 3.8/μL, although the absolute neutrophil count was 1.5. His hemoglobin level was 11.1 g/dL with an MCV of 108 fL. Folate and vitamin B12 levels were normal. The patient denied any changes to his activity level: he lives independently and performs all activities of daily living. He reported no bleeding or infection. He does not use alcohol. Bone marrow biopsy was performed and demonstrated a relative hypercellular marrow (40% cellular), multilineage dysplasia, ∼3% blasts on a 500-cell count differential, and an aberrant immunophenotype on flow cytometry. Cytogenetics showed deletion of the Y chromosome in all cells sampled, and a mutation in TET2 (variant allele frequency, 34%) was the only finding reported on a next-generation sequencing panel. He wanted to discuss the prognosis of his disease and potential therapeutic options.
MDSs are heterogeneous clonal disorders characterized by ineffective hematopoiesis, low circulating blood counts, and increased risk of progression to acute myelogenous leukemia (AML). Because clinical outcomes are varied in MDS, prognostication of the disease and individualized management plans are essential to optimize long-term outcomes. Thrombocytopenia in MDS is common, occurring in 40% to 60% of patients with the diagnosis.62 Thrombocytopenia is an independent risk factor for poor outcomes in MDS and is an essential part of prognostic calculation using the revised International Prognostic Scoring System criterion.63 Bone marrow biopsy is essential for the diagnosis of MDS and improves prognostication and identifies potential therapeutic targets.64 In MDS, severity of thrombocytopenia correlates with disease outcomes: patients with platelet counts >200 000/μL have survival advantage over those with platelet counts <30 000/μL.65 Platelet counts <50 000/μL are associated with increased risk of development of AML.66
Thrombocytopenia in MDS involves multiple mechanisms that decrease megakaryocytopoiesis and platelet shedding. Megakaryocytes in MDS are often dysplastic with a histologic appearance that includes large single nuclei and formation of so-called micromegakaryocytes.67 Bone marrow biopsy often shows intramedullary apoptosis of megakaryocytes.68 Further, megakaryocyte progenitor growth is often unresponsive to TPO signaling leading to expanded serum TPO levels.69 Platelets in MDS have a decreased lifespan that has been attributed to increased peripheral platelet destruction.
Bleeding in MDS relates to both the degree of thrombocytopenia and abnormalities within platelet function. Platelet aggregation is regularly impaired or absent when platelets are exposed to epinephrine, adenosine diphosphate, collagen, and/or ristocetin.70 Because of platelet dysfunction, bleeding in MDS can occur at levels greater than would be anticipated for a certain platelet count alone. Hemorrhage is not an uncommon cause of death in persons with MDS, accounting for 10% to 25% of deaths not related to AML.71
Management of thrombocytopenia and platelet dysfunction remains an essential component of MDS therapy, most commonly using prophylactic platelet transfusions. Although platelet transfusions are associated with improved platelet counts and may reduce bleeding complications, transfused platelets rarely circulate for more than 3 days.72
There is, indeed, debate on whether prophylactic platelet transfusions are required for patients with MDS. The current National Comprehensive Cancer Network guidance states that “platelet transfusions are generally not used routinely in patients with thrombocytopenia in the absence of bleeding unless the platelet count is <10 000/μL.”73 Other groups, including the American Society of Clinical Oncology has published that prophylactic transfusions are not mandatory even at low counts in clinically stable, nonbleeding patients with MDS who are not undergoing active therapy.74 For patients with a variety of myeloid malignancies, 2 large randomized studies have examined whether there is evidence to definitively support prophylactic platelet transfusions.75,76 Both studies were conducted in Europe. The authors of both articles have leaned against recommending prophylactic transfusions, except for patients with acute leukemia. In our practice, we often recommend transfusions for patients with MDS having counts even higher than 10 × 103/μL when there is evidence of sepsis, hyperleukocytosis, or coagulation abnormalities. We discuss with older patients the need to treat the underlying MDS when platelet counts decrease to severely low levels, that is, less than 20 × 103 to 30 × 103/μL, and the risk/benefit of prophylactic platelet transfusion for patients who opt against therapy and whose counts are regularly ≤10 × 103/μL.
Frequent platelet transfusion can lead to alloimmunization, which can limit access to future functional transfusions. Additional risks of platelet transfusion include allergic reaction, fever, infection, transfusion-associated lung injury, and post-transfusion purpura.
Treatment of MDS for all age groups, especially in individuals for whom stem cell transplantation is not feasible or safe, remains a significant unmet need. Although improvements in MDS related therapies have all demonstrated improved platelet counts, none directly targeting mechanisms of thrombocytopenia. Treatment of MDS with hypomethylating agents (decitabine and azacytidine) showed complete hematologic response, including platelet recovery, in only about 7% to 17% of patients.77,78 Both agents were associated with grade 3 to 4 thrombocytopenia in >50% of the cases. We advocate for enrolling patients in clinical trials of novel therapies for MDS whenever possible.
TPO-RAs have been studied in several small clinical trials with International Prognostic Scoring System low and intermediate risk disease. Meta-analysis of 4 trials comparing romiplostim with placebo showed that romiplostim decreased the bleeding rate and number of platelet transfusions without increased risk of AML progression.79 However, the largest clinical trial investigating use of romiplostim in thrombocytopenia with MDS was prematurely stopped, given the concern of increased blasts in the romiplostim arm.80 Some clinical trials combining TPO-RAs with hypomethylating agents or lenalidomide were initially promising. The combination of romiplostim with azacitidine showed a decreased number of bleeding incidents and a reduction in platelet transfusion compared to azacitidine and placebo.81 Unfortunately a randomized, placebo-controlled trial with azacitidine with and without eltrombopag failed to demonstrate platelet recovery and trended toward progression to acute leukemia.82 None of these trials specifically targeted the older population.
After review of the treatment options, this patient chose observation alone. His platelet count and neutrophils continued to decline over the subsequent year. After additional discussion, the patient was treated with an oral hypomethylating agent. Treatment was complicated because of 2 episodes of febrile neutropenia requiring hospitalization. Partial response, including improvement in platelet count, was noted after his fourth month of therapy.
Case 3
Patient 3 is an 82-year-old female resident of a skilled nursing facility, with a history of seizures and ischemic cardiomyopathy, who is found have an acute decrease in the platelet count from 168 000 to 8000/μL while hospitalized for heart failure exacerbation and lower extremity cellulitis. Her platelet count began to decrease on hospital day 5 and reached a nadir on hospital day 8. The patient’s hemoglobin, WBC count, and MCV remain at baseline levels. She was started on furosemide, vancomycin, and meropenem, with improvement in heart failure and infection. She has been on venous thromboembolism prophylaxis with unfractionated heparin. Outpatient medications, including lisinopril, metoprolol, aspirin, digoxin, and valproic acid, were continued throughout her hospitalization. Drug-induced immune thrombocytopenia (DITP) was suspected. Digoxin was discontinued, and vancomycin was replaced by amoxicillin plus doxycycline. HIT was considered unlikely, given a low pretest probability assessment. Her home medications were continued. The patient was transfused with 1 unit of single donor platelets, and over the next 5 days, her platelet count returned to normal. Vancomycin-dependent platelet-reactive antibodies were identified and confirmed a diagnosis of DITP.
Thrombocytopenia caused by drugs is a common yet clinically challenging diagnosis that is particularly prevalent in older populations. The condition is likely underreported, although it is notable that nearly 1 quarter of persons who are critically ill are at risk for DITP.5 Although the incidence of DITP in older populations is unknown, geriatric patients more commonly use medications commonly associated with DITP.83 Drugs can induce thrombocytopenia through different mechanisms, including direct toxicity to hematopoietic stem cells (such as chemotherapeutics and valproic acid), and specifically to megakaryocytes (linezolid,84 thiazide diuretics, and antiviral agents85). However, DITP has a unique mechanism of thrombocytopenia caused by drug-dependent platelet antibodies resulting in increased platelet destruction. In some cases (such as with eptifibatide), drug-dependent antibodies have been shown to cause megakaryocyte destruction.86 DITP typically occurs within 5 to 10 days after initial drug exposure or within 24 hours of reexposure to a previously used medication. The platelet count typically decreases to <20 000/μL, and bleeding symptoms are common.87 The mechanism of HIT and related disorders (4719 spontaneous HIT and adenovirus-associated vaccine–induced thrombotic thrombocytopenia) is related to the formation of platelet-activating autoantibodies, requiring clinical suspicion on the part of the physician, specific diagnostic assays, and initiation of antithrombotic therapy.88
Diagnosis of DITP requires investigative history-taking that is often more complicated in patients with polypharmacy. Hundreds of drugs and vaccinations (including COVID-19 vaccines) have been demonstrated to induce drug-dependent antibodies.89 Drugs and vaccinations most commonly implicated in DITP are listed in Table 2. Five clinical criteria can retrospectively help establish a diagnosis of DITP: (1) exposure to the drug prior to thrombocytopenia, (2) recovery of platelet count after drug cessation, (3) other potential causative drugs were reintroduced after platelet recovery, (4) other causes of thrombocytopenia were excluded, and (5) reexposure to the candidate drug caused recurrent thrombocytopenia.91 Laboratory detection of drug-dependent platelet antibodies can confirm DITP, although testing may be limited by accessibility. Flow cytometry shows drug dependence, immunoglobulin binding, and platelet specificity according to standardization guidelines.92
Drugs and vaccines commonly associated with drug-dependent antiplatelet antibodies
| Antimicrobials |
| β-Lactams, including penicillin and cephalosporins |
| Piperacillin |
| Rifampin |
| Quinine |
| Trimethoprim-sulfamethoxazole |
| Vancomycin |
| Cardiovascular medications and anticoagulants |
| Abciximab∗ |
| Eptifibatide |
| Heparin and low-molecular-weight heparins |
| Quinidine |
| Tirofiban |
| Antiseizure medications |
| Carbamazepine |
| Phenytoin |
| Vaccines |
| COVID-19 vaccine |
| MMR vaccine |
| Antimicrobials |
| β-Lactams, including penicillin and cephalosporins |
| Piperacillin |
| Rifampin |
| Quinine |
| Trimethoprim-sulfamethoxazole |
| Vancomycin |
| Cardiovascular medications and anticoagulants |
| Abciximab∗ |
| Eptifibatide |
| Heparin and low-molecular-weight heparins |
| Quinidine |
| Tirofiban |
| Antiseizure medications |
| Carbamazepine |
| Phenytoin |
| Vaccines |
| COVID-19 vaccine |
| MMR vaccine |
Adapted from Curtis et al with permission.90
MMR, measles, mumps, and rubella.
No longer available in the United States.
As a matter of clinical practice, we use drug-dependent antibody testing for cases in which the cause of thrombocytopenia is uncertain. The clinical sensitivity and specificity of assays for DITP are largely unknown because it is difficult to obtain the large number of samples required to validate such data, but very high laboratory sensitivity and specificity have been documented for vancomycin.93 Treatment of DITP involves discontinuation of offending agents with anticipated platelet recovery within 4 to 5 half-lives of the responsible drug or metabolite. For patients with a platelet count of <10 000 μL or acute bleeding, response to IVIG, prednisone, and platelet transfusions has been reported.17
Conclusions
Thrombocytopenia in older adults remains both a diagnostic and therapeutic challenge. Medical comorbidities and polypharmacy increase the risk of both bleeding and thrombotic complications in patients with thrombocytopenia. Accurate history, physical examination, and laboratory analysis, including peripheral smear, are essential for prompt diagnosis. Treatment of thrombocytopenia depends largely on correct diagnosis. Treatment of ITP in older adults is challenging, with goals to urgently increase the platelet count to a level high enough to achieve adequate control and prevention of bleeding and then use subsequent therapy to limit adverse effects. Initial attempts at therapy designed to improve platelet counts in MDS were promising, but the use of TPO-RAs has stalled, given the concern of both efficacy and safety. Therapies to manage thrombocytopenia and bleeding disfunction for patients with MDS are clearly needed. DITP remains a diagnostic challenge, although laboratory assays may become a relevant tool for identification of culprit drugs associated thrombocytopenia in the older population. High suspicion, early recognition, and discontinuation of offending therapy remain essential to improvement.
Authorship
Contribution: P.F. wrote the manuscript; and P.F., K.D.F., and L.C.M. edited the manuscript.
Conflict-of-interest disclosure: P.F. has performed consultancy for Rigel and AstraZeneca. K.D.F. has performed consultancy for Bayer, Genentech, Siemens, Takeda, and Werfen. L.C.M. has clinical trial funding from Jazz Pharmaceuticals; has performed consulting for AbbVie, Curio Science, Celgene, Sierra Oncology, and Incyte; and has received honoraria from the Society of Hematologic Oncology, the American Society of Hematology, and the American Board of Internal Medicine.
Correspondence: Patrick Foy, Department of Medicine, Division of Hematology Oncology, Medical College of Wisconsin, 9200 W Wisconsin Ave, Milwaukee, WI 53226; email: pfoy@mcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal