In this issue of Blood, Woyach et al present a beautiful study of mutational profiling for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) at the time of progression on treatment with a covalent Bruton tyrosine kinase inhibitor (BTKi) in ELEVATE-RR, a phase 3 study comparing ibrutinib and acalabrutinib.1
BTKis block B-cell receptor signaling to impair survival and growth of CLL cells, and development of these agents has transformed the therapeutic landscape for this chronic disease. Although effective, acquired resistance limits the use of both covalent (ibrutinib, acalabrutinib, zanubrutinib) and noncovalent (pirtobrutinib) BTKis. Until recently, much of our understanding of mechanisms of resistance to covalent BTKis arose from study of ibrutinib-treated patients. In these patients, resistance mechanisms included BTK C481 mutations and mutations downstream of BTK in PLCγ2.2-4
Covalent BTKis irreversibly bind to BTK at the 481 cysteine residue. The most common BTK C481 mutation is C481S, which alters the binding site confirmation for covalent BTKis, thus inhibiting effective drug binding and allowing for BTK signaling despite the presence of covalent BTKis. Noncovalent BTKis have been designed to maintain activity against CLL with both wild-type and C481-mutated BTK.5 With the development of noncovalent BTKis, analysis of patient samples at the time of progression on pirtobrutinib led to discovery of additional mechanisms of resistance including alternate site BTK mutations.6,7 These alternate BTK mutations impair drug binding and include gatekeeper mutations and “kinase-dead” mutations, which disable BTK kinase activity but maintain downstream B-cell receptor signaling through a scaffolding function, which promotes physical interactions and activity of kinases involved in B-cell receptor signaling.8
Though these additional resistance mechanisms were discovered in the setting of noncovalent BTKi resistance, understanding if these “non-C481” mutation mechanisms of resistance occur during treatment with covalent BTKis may have significant clinical implications, particularly for treatment sequencing. As CLL is a chronic disease requiring long-term control, we must consider not only the efficacy and toxicity of a selected line of therapy, but also the impact that this treatment might have on future lines of therapy. Understanding whether resistance mutations developing during treatment with covalent BTKis impact subsequent efficacy of noncovalent BTKis has implications when the goal is, in many cases, decades of disease control across multiple lines of therapy.
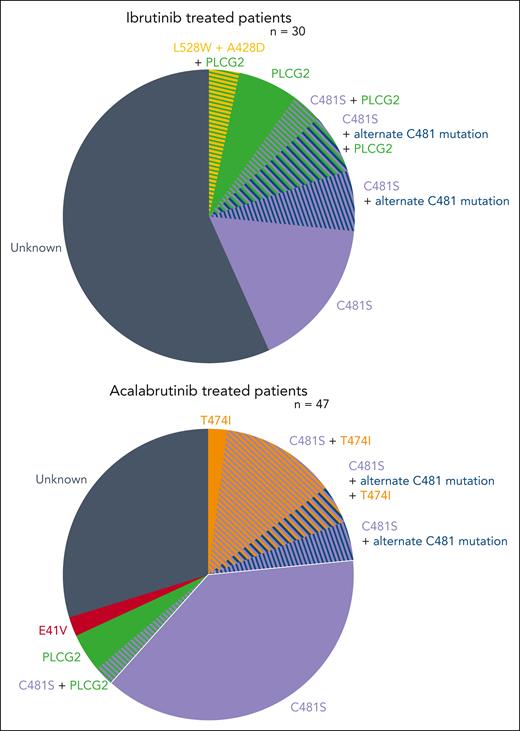
Among peripheral blood samples studied by Woyach et al from 47 acalabrutinib-treated and 30 ibrutinib-treated patients with paired baseline and CLL progression, BTK mutations were observed in 66% of those progressing on acalabrutinib and 37% progressing on ibrutinib. Among those who developed BTK mutations, C418S was the most common mutation (94% of BTK mutations in acalabrutinib-treated patients, 91% of BTK mutations in ibrutinib-treated patients). However, additional non-C481S mutations also arose in these patients. Specifically, gatekeeper mutations (BTK T474I) were observed in 9 patients treated with acalabrutinib, with 8 co-occurring with C481 mutations. One ibrutinib-treated patient developed concurrent kinase-dead L528W and A428D mutations. An additional novel BTK E41V mutation was observed in 1 patient treated with acalabrutinib; preclinical testing suggests that this mutation may not independently confer BTKi resistance. These samples were also examined for emergence of non-BTK mutations. New PLCγ2 mutations, which occur downstream of BTK in the B-cell receptor pathway, were observed in 6% of the acalabrutinib-treated patients and 20% of the ibrutinib-treated patients (see figure).
These data add significantly to our understanding of the occurrence of non-C481 mutations and variation in mutational patterns for those treated with covalent BTKis. As gatekeeper and kinase-dead mutations may impact efficacy of both covalent and noncovalent BTKis, emergence of these mutations during treatment with covalent inhibitors may confer cross-resistance to noncovalent BTKis in a future line of therapy. Thus, we will need to closely consider the optimal sequencing strategy for these patients because directly proceeding from covalent to noncovalent BTKis in sequential lines may not be effective. Whether an intervening line of therapy between covalent and noncovalent BTKis may exert selective pressure to eliminate these resistant clones, thus sensitizing to noncovalent BTKi in a future line, is a worthy topic for exploration.
Notably, this is a high-risk group of patients with relapsed or refractory disease and unfavorable cytogenetics including del17p and del11q. Given that many patients had aberrant TP53 at baseline, it is possible that this group is particularly susceptible to genetic instability and clonal evolution. Future studies examining clonal evolution and emergence of non-C481 BTK mutations in other populations will add to our understanding of emerging resistance to covalent BTKis in a broad population of patients with CLL. These studies will also allow us to confirm if the differences in mutational patterns observed in ibrutinib- and acalabrutinib-treated patients in this study are intrinsic to treatment with these agents or a consequence of the population in which they are studied.
Interestingly, when patients treated with ibrutinib and acalabrutinib were considered together, median progression-free survival was significantly longer for those who developed BTK resistance mutations than for those without BTK mutations. We are yet to understand mechanisms of resistance for these patients without identified mutations in BTK or downstream in the B-cell receptor pathway. Understanding drivers of resistance and potential for differential resistance mechanisms across compartments (peripheral blood, bone marrow, lymph nodes) for patients without identified resistance mutations is an area ripe for further exploration of underlying pathobiology.
These data documenting emergence of alternate mechanisms of resistance for those treated with covalent BTKis draw to mind topics of future exploration and strategies for mitigating resistance. Studies exploring these trends in other populations and with other BTKis will inform risk of cross-resistance across lines of therapy and risk groups. The effects of treatment with non-BTK pathway-directed inhibitors or alternate BTK-targeting agents on clonal evolution in patients with these resistance patterns, along with clinical response to these agents in these settings, will inform strategies for overcoming cross-resistance. Although treatments with covalent BTKis have used treat-to-progression strategies, time-limited BTKi-containing regimens are also being developed. Early data from these studies suggest that time-limited therapy may mitigate emergence of resistance with potential retreatment opportunity in subsequent lines.9 These data highlight that the future of CLL-directed sequencing strategy may not only consider prior lines of therapy and reasons for discontinuation, but also emergent mechanisms for resistance as we aim to deliver the optimal treatment strategy for each patient.
In this study, mutational profiling was performed on paired samples from patients with relapsed or refractory CLL who were treated with covalent BTKis (ibrutinib or acalabrutinib) in the ELEVATE-RR study. Here we can see the distribution of BTK and B-cell receptor pathway mutations that developed during treatment with ibrutinib or acalabrutinib.
In this study, mutational profiling was performed on paired samples from patients with relapsed or refractory CLL who were treated with covalent BTKis (ibrutinib or acalabrutinib) in the ELEVATE-RR study. Here we can see the distribution of BTK and B-cell receptor pathway mutations that developed during treatment with ibrutinib or acalabrutinib.
Conflict-of-interest disclosure: L.E.R. has served as a consultant for AbbVie, Ascentage, AstraZeneca, Beigene, Janssen, Loxo Oncology, Pharmacyclics, Pfizer, TG Therapeutics; is a member of a data safety monitoring committee for Ascentage; served as a Continuing Medical Education speaker for DAVA, Curio, Medscape, and PeerView; holds minority ownership interest in Abbott Laboratories; received travel support from Loxo Oncology; and has received research funding (paid to the institution) from Adaptive Biotechnologies, AstraZeneca, Genentech, AbbVie, Pfizer, Loxo Oncology, Aptose Biosciences, Dren Bio, and Qilu Puget Sound Biotherapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal