In this issue of Blood, Krakow et al have explored the use of T-cell receptor (TCR) transduced T cells targeting the hematopoiesis-specific minor histocompatibility antigen HA-1 to control leukemia following allogeneic stem cell transplantation (allo-SCT).1 The notion that the therapeutic potential of allo-SCT is mainly dictated by alloreactive donor T cells recognizing polymorphic antigens differentially expressed on patient cells, including the malignant cells, and not on donor cells, while the detrimental graft-versus-host disease (GVHD) is also mediated by this immunologic phenomenon, has led to attempts to separate GVHD from the antitumor effect.
In the context of treating hematological malignancies with allo-SCT, the beneficial alloreactive antitumor effect does not necessarily need to be tumor specific. Posttransplantation, the chimeric status of the patient with hematopoiesis being primarily of donor origin and the malignancy being part of the hematopoietic system of the patient, allows targeting of hematopoietic tissue as tumor-specific immune therapy without harming nonhematopoietic tissue and normal donor hematopoiesis.2
Polymorphic proteins derived from genes containing single-nucleotide polymorphisms can cause polymorphic peptides presented in human leukocyte antigen (HLA) molecules on the cell membrane. In HLA-matched transplantation, these polymorphic peptides, if differentially expressed on patients and donor cells and recognized by T cells, are termed minor histocompatibility antigens (MiHAs).3 Donor T-cell responses against MiHAs derived from proteins that are broadly expressed in multiple recipient tissues can lead to GVHD. However, recognition of MiHAs derived from proteins with hematopoietic lineage restricted expression can result in an antipatient hematopoiesis and, thus, a tumor-specific immune response after transplantation. HA-1, an MiHA presented and recognized in the context of HLA-A∗02:01, is such an MiHA expressed on most hematopoietic cells.
Monitoring HA-1–specific T-cell responses in HA-1–mismatched patients after transplantation treated with donor lymphocyte infusions has indicated that donor immune responses against this MiHA may cause an antitumor effect without clinically significant GVHD.4 Despite successful generation of HA-1–specific donor-derived T-cell lines in vitro, their administration to patients with refractory leukemia after transplantation did not result in a beneficial clinical effect, possibly because of their inability to proliferate and expand in vivo.5
To address this issue, TCR gene therapeutic strategies were developed using high-affinity HA-1–specific TCRs. To prevent coinfusion of donor T cells capable of inducing GVHD, the initial study was performed using donor-derived virus-specific T cells specific for cytomegalovirus or Epstein-Barr virus that were equipped with an HA-1 TCR.6 Despite fulfilling the criteria of recognizing both the viral antigen and HA-1, administration of these TCR T-cell products in relatively small numbers to patients without prior lymphodepletion was safe, but insufficient in vivo expansion prevented the researchers from achieving a significant clinical effect.
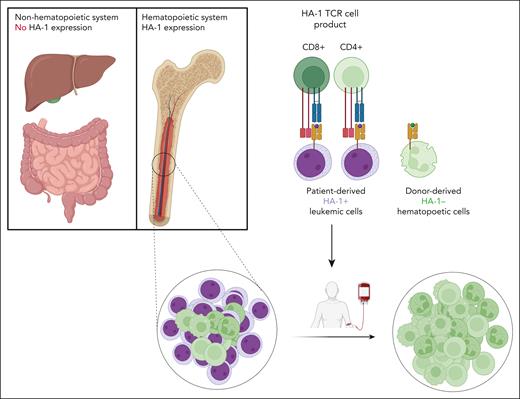
On the basis of enhanced understanding of the prerequisites for improved cellular immunotherapy using gene modified T cells, Krakow et al equipped the T cells not only with a high-affinity HA-1 TCR but also with the CD8 coreceptor essential for optimal binding of T cells to HA-1–positive target cells. This modification enabled both CD8 T cells and CD4 T cells to interact with HA-1–positive leukemic cells (see figure). To mitigate the potential risk of donor-derived T cells to cause GVHD after transplantation, naive T cells were depleted at the beginning of the manufacturing process, and HA-1 TCR-engineered CD4 and CD8 T cells were equipped with additional CD8, a CD34 tag, and a suicide gene. Subsequently, the cells were expanded, and at the end of the culture at day 20 the engineered T cells were enriched. Despite the relatively lengthy production process, highly enriched HA-1 TCR CD8 engineered T-cell products (HA-1 TCR T) were successfully generated. These products were then used to treat 9 patients with residual leukemia after HA-1–mismatched transplantation following lymphodepleting conditioning chemotherapy.
The HA-1 TCR cell product, composed of CD8 and CD4 T cells engineered with an HA-1 TCR and CD8 coreceptor, is infused into patients after allogeneic stem cell transplantation. The HA-1 TCR T-cell product only attacks the hematopoietic cells of the patient, including the malignant cells, whereas the nonhematopoietic tissue as well as donor-derived hematopoiesis are not attacked.
The HA-1 TCR cell product, composed of CD8 and CD4 T cells engineered with an HA-1 TCR and CD8 coreceptor, is infused into patients after allogeneic stem cell transplantation. The HA-1 TCR T-cell product only attacks the hematopoietic cells of the patient, including the malignant cells, whereas the nonhematopoietic tissue as well as donor-derived hematopoiesis are not attacked.
Following treatment with HA-1 TCR T, no dose-limiting toxicities were observed even at the highest dose of 30 × 106 TCR T/kg. Both CD4 and CD8 HA-1 TCR T cells appeared to be capable of interacting with the target cells, and in vivo expansion was observed in most cases. Long-term persistence of HA-1 TCR T could be documented in several cases. Although signs of efficacy were observed, all but 1 patient experienced relapse. The authors illustrated that this was not due to the inability of the HA-1 TCR T to recognize their target, and not due to downregulation of the target HA-1/HLA-A∗02:01 complex on the leukemic cells. Improved potency, higher or more frequent dosing of the product, and ability to proliferate more vigorously are probably required. As discussed by the authors, modifications are necessary for more impactful treatment of patients with gene-modified T cells specific for MiHAs. One potential strategy is to use CRISPR-Cas technology to replace the endogenous TCR by the introduced TCR, thus reducing competition between the introduced and endogenous TCR.7 Sustaining the immune response by vaccination strategies may also be a viable option.8 Alternatively, shortening the production process could enhance the potency and persistence of the engineered T cells. Given the need for caution in preventing GVHD of nonengineered T cells, it may be advantageous to equip donor T cells isolated from the patient after transplantation with the HA-1 TCR. These cells have a lower likelihood of inducing GVHD, eliminating the need for enrichment and subsequent expansion.
Successful control of hematological malignancies by MiHA-specific donor T cells has been associated with targeting of multiple antigens restricted to various HLA molecules, even in the absence of GVHD.9 Multiple targeting is likely to improve potency and efficacy, and will limit the risk of antigen loss and may, therefore, be necessary for elimination of the residual recipient malignant hematopoietic cells. Further identification of hematopoiesis-associated MiHAs and high-affinity TCRs recognizing these antigens will be helpful to compose future products.10 New transplantation strategies have decreased the toxicity of allo-SCT, but relapse after transplantation remains a major issue. The study of Krakow et al warrants further exploration of the use of TCR T products specific for MiHAs to control hematological malignancies after allo-SCT without causing GVHD.
Conflict-of-interest disclosure: J.H.F.F. and M.H.M.H. are coinventors on a patent describing HA-1 T-cell receptor therapy that is licensed to Miltenyi Biotec BV (Binding Proteins Specific for HA-1H and Uses Thereof), number NL2021789A, filed 10 October 2018.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal