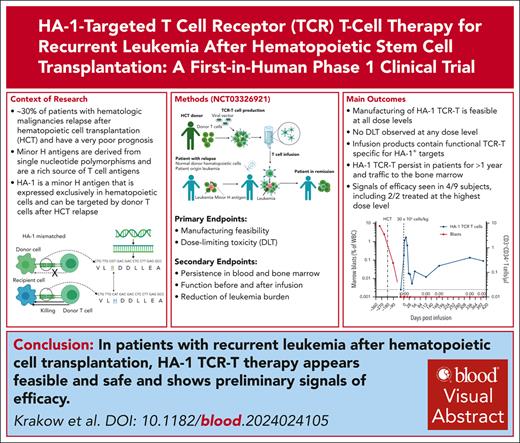
Key Points
This trial used a class I–restricted TCR and a CD8 coreceptor to enable antigen–specific CD8+ and CD4+ T-cell function.
Primary end points of feasibility and tolerability were met in a phase 1 clinical trial of HA-1 TCR-T for recurrent leukemia after HCT.
Visual Abstract
Relapse is the leading cause of death after allogeneic hematopoietic stem cell transplantation (HCT) for leukemia. T cells engineered by gene transfer to express T cell receptors (TCR; TCR-T) specific for hematopoietic-restricted minor histocompatibility (H) antigens may provide a potent selective antileukemic effect post-HCT. We conducted a phase 1 clinical trial using a novel TCR-T product targeting the minor H antigen, HA-1, to treat or consolidate treatment of persistent or recurrent leukemia and myeloid neoplasms. The primary objective was to evaluate the feasibility and safety of administration of HA-1 TCR-T after HCT. CD8+ and CD4+ T cells expressing the HA-1 TCR and a CD8 coreceptor were successfully manufactured from HA-1–disparate HCT donors. One or more infusions of HA-1 TCR-T following lymphodepleting chemotherapy were administered to 9 HCT recipients who had developed disease recurrence after HCT. TCR-T cells expanded and persisted in vivo after adoptive transfer. No dose-limiting toxicities occurred. Although the study was not designed to assess efficacy, 4 patients achieved or maintained complete remissions following lymphodepletion and HA-1 TCR-T, with 1 patient still in remission at >2 years. Single-cell RNA sequencing of relapsing/progressive leukemia after TCR-T therapy identified upregulated molecules associated with T-cell dysfunction or cancer cell survival. HA-1 TCR-T therapy appears feasible and safe and shows preliminary signals of efficacy. This clinical trial was registered at ClinicalTrials.gov as #NCT03326921.
Introduction
Allogeneic hematopoietic stem cell transplantation (HCT) is a standard, frequently curative consolidation therapy for most acute leukemia subtypes and myeloid neoplasms. However, disease relapse after HCT occurs in >30% of patients, and prognosis following relapse is poor, with 2-year overall survival rates of <20%.1,2 Patients who relapse within 6 months of HCT have a dismal prognosis, with 2-year survival rates of ∼4%.2 Promising results have been achieved using T cells genetically modified to express a Wilms Tumor 1 (WT1)–specific T-cell receptor (TCR) to prevent acute myeloid leukemia (AML) relapse after HCT. However, genetically modified T cells (TCR-T) and chimeric antigen receptor (CAR) T cells have not been effective for treating recurrent AML after HCT.3,4
Minor histocompatibility (H) antigens are peptides derived from normal polymorphic self-proteins that differ in amino acid sequence between HCT recipients and donors, and are presented by HLA molecules on recipient cells.5 In the post-HCT setting, minor H antigens that are only expressed by hematopoietic-lineage cells represent potential leukemia-specific targets for TCR-T. Specifically, following HCT in recipient–donor pairs that are suitably HLA-matched but mismatched for a minor H antigen, recurrent recipient-origin leukemia will present the minor H antigen to donor T cells. In contrast, donor–derived normal hematopoietic cells that engraft in the recipient lack the recipient minor H antigen genotype and cannot be recognized by donor T cells. Importantly, hematopoietic–restricted minor H antigens are not expressed on recipient nonhematopoietic tissues, minimizing the risk of graft-versus-host disease (GVHD) mediated by the donor-derived TCR-T.5
The gene HMHA-1 (ARHGAP45) encodes a hematopoietic–restricted minor H antigen, specifically a polymorphic peptide (VLHDDLLEA, “HA-1+”, genotype RS_1801284 A/G or A/A) presented by the common HLA-A∗02:01 molecule.6,7 In the post-HCT setting, HA-1–negative (VLRDDLLEA, “HA-1−”, genotype RS_1801284 G/G) donor T cells may be primed or genetically modified to express HA-1–specific TCR to deliver an antileukemic effect.5,7,8 We isolated a high-affinity HA-1–specific TCR. We then cloned the TCR alpha and beta chains into a lentiviral vector that was also engineered to encode CD8 coreceptor alpha and beta chains to promote function of the class I–restricted TCR in transduced CD4+ T cells, along with an inducible caspase-9 suicide switch that can be triggered by the dimerizer agent rimiducid, and a CD34–CD20 epitope for enrichment of HA-1 TCR-T during manufacturing and tracking after infusion.7,9-11
Herein, we present the results of a first-in-human phase 1 trial (NCT03326921) of HA-1 TCR-T therapy in 9 HLA-A∗02:01+ HA-1+ patients with recurrent leukemia after HCT from an HA-1– donor. To our knowledge, this is the first reported TCR-T trial to include CD4+ T cells transduced with a class I–restricted TCR and both chains of the CD8 coreceptor in the cell product. We demonstrate successful manufacture and administration of HA-1 TCR-T without significant treatment-related toxicities. CD4+ and CD8+ TCR-T expanded and persisted in blood and bone marrow (BM) after infusion. Although the study was not designed to assess efficacy, a therapeutic benefit, as defined by prespecified protocol criteria, was observed in 4 of 9 patients. This trial establishes the feasibility, safety, and antileukemic activity of HA-1 TCR-T in patients with recurrent leukemia after HCT.
Methods
HA-1 TCR-T manufacture
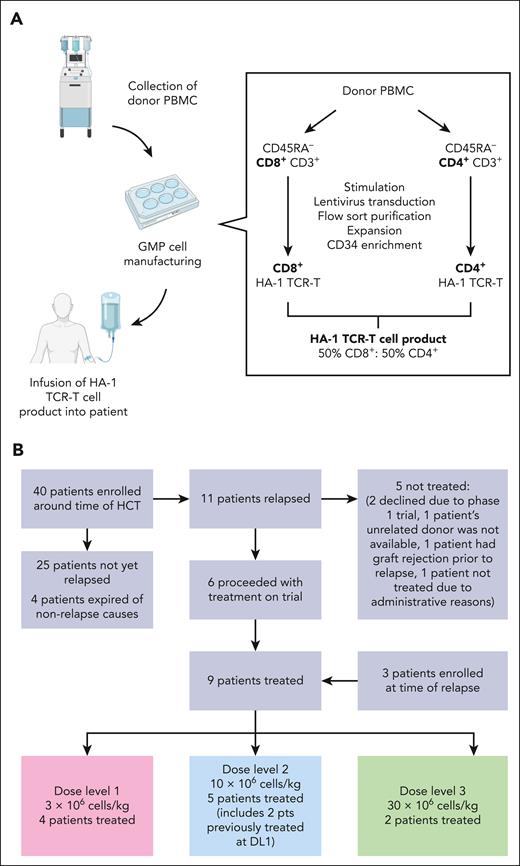
As described previously, HA-1 TCR-T is an HCT-donor–derived TCR-T product expressing the following elements: (1) an HA-1-specific TCR; (2) a CD8 coreceptor to promote the function of the class I–restricted TCR in CD4+ T cells; (3) an inducible caspase 9 safety switch to enable elimination of the HA-1 TCR-T in the event of toxicity; and (4) a CD34–CD20 epitope to facilitate selection of the engineered cell product and tracking of the transferred HA-1 TCR-T.7 HA-1 TCR-T products were generated by activation, lentiviral HA-1 TCR construct transduction, enrichment, and expansion of HCT-donor memory T cells (TM) (Figure 1A; supplemental Method, available on the Blood website).
Clinical trial and manufacturing outline. (A) Schematic of clinical trial donor collection, GMP manufacturing, and infusion process. The process entailed the following: (1) collecting HCT-donor peripheral blood mononuclear cells (PBMC) by apheresis; (2) removing CD45RA+ cells from PBMC to reduce the risk of GVHD associated with donor naïve T cells and to skew the product toward a central memory T-cell (TCM) phenotype to enhance in vivo persistence; (3) separating the T cells into predominantly CD4+ and CD8+ fractions to control the CD4:CD8 ratio in the cell product; (4) stimulating and transducing CD4+ and CD8+ T cells in parallel cultures; (5) sorting for T cells expressing the TCR and CD34 tag on a fluorescence-activated cell sorter; (6) expanding; (7) enriching transduced cells using CD34+ antibody–conjugated immunomagnetic beads and a magnetic column; and (8) evaluating the final product. Refer to supplemental Method online for additional details. (B) CONSORT diagram depicting patient enrollment and treatment. The reasons for not treating patients included the following: 2 patients declined treatment within a phase 1 trial, 1 patient’s unrelated donor was not available, 1 patient had graft rejection before relapse, and 1 patient was not treated because of administrative reasons.
Clinical trial and manufacturing outline. (A) Schematic of clinical trial donor collection, GMP manufacturing, and infusion process. The process entailed the following: (1) collecting HCT-donor peripheral blood mononuclear cells (PBMC) by apheresis; (2) removing CD45RA+ cells from PBMC to reduce the risk of GVHD associated with donor naïve T cells and to skew the product toward a central memory T-cell (TCM) phenotype to enhance in vivo persistence; (3) separating the T cells into predominantly CD4+ and CD8+ fractions to control the CD4:CD8 ratio in the cell product; (4) stimulating and transducing CD4+ and CD8+ T cells in parallel cultures; (5) sorting for T cells expressing the TCR and CD34 tag on a fluorescence-activated cell sorter; (6) expanding; (7) enriching transduced cells using CD34+ antibody–conjugated immunomagnetic beads and a magnetic column; and (8) evaluating the final product. Refer to supplemental Method online for additional details. (B) CONSORT diagram depicting patient enrollment and treatment. The reasons for not treating patients included the following: 2 patients declined treatment within a phase 1 trial, 1 patient’s unrelated donor was not available, 1 patient had graft rejection before relapse, and 1 patient was not treated because of administrative reasons.
Study design
Patients with leukemia undergoing HCT and their HCT donors were genotyped for the HA-1 polymorphism before or at the time of post-HCT relapse. Patients with a suitable mismatch (patient: HLA-A∗02:01+ RS_1801284 A/G or A/A; donor: HLA-A∗02:01⁻ or RS_1801284 G/G) were offered enrollment. HA-1 TCR-T were manufactured and infused if recurrent leukemia was identified in the BM using clinical testing after HCT and if other treatment eligibility criteria were met, including provision of written informed consent. The inclusion and exclusion criteria are outlined in supplemental Method. The trial used a 3-by-3 dose-escalation design, starting at 3 × 106 TCR-T/kg, escalating to 30 × 106 TCR-T/kg (Figure 1B). Patients received lymphodepleting chemotherapy followed by ≥1 HA-1 TCR-T infusions (Table 1; supplemental Table 1). Primary end points were the feasibility of manufacturing and administering HA-1 TCR CD8+ TM and CD4+ TM, and the dose-limiting toxicity of HA-1 TCR-T. Major secondary end points were duration of in vivo persistence of transferred CD8+ and CD4+ HA-1 TCR-T in peripheral blood, and the presence, proportion, and persistence of HA-1 TCR-T in the BM. The study (NCT03326921) was approved by the Fred Hutchinson Cancer Center Institutional Review Board (IRB) and conducted under US Food and Drug Administration Investigational New Drug Application 17678. Refer to supplemental Method for details.
Clinical features of patients treated with HA-1 TCR-T
| Participant . | Age, y . | Disease . | Cytogenetic/molecular . | HCT to first relapse, d . | HCT to TCR-T, d . | Disease stage . | Lymphodepletion (doses) . | TCR-T dose level . | CRS . | ICANS . | GVHD after TCR-T . | Other relevant AE (maximal grade) . | Disease outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | B-ALL | Ph-like RCSD1-ABL2 + complex | 987 | >1000 | 1. MRD– CR 2. MRD+ CR 3. MRD+ CR | 1. Fludarabine (3) 2. Fludarabine (3) 3. Fludarabine (3) | 1. DL1 2. DL2 3. DL2 | No | No | No | No | 1. Maintained CR 6 mo 2. Progressive disease 3. Progressive disease |
| 2 | 58 | AML | del 5, -7, other | 195 | 257 | MRD– CR | Fludarabine (3) | DL1 | No | No | No | Neutropenia (4)∗ | Progressive disease |
| 3 | 2 | T-ALL | t(8:14), biallelic loss of CDKN2A and CDKN2B | 71 | 1. 181 2. 236 3. 546 | 1. MRD+ CR 2. MRD+ CR 3. Relapse | 1. Fludarabine (2) 2. Nil 3. NECTAR | 1. DL1 2. DL2 3. DL2 | No | No | No | Neutropenia (4)†, fatigue (2)† | 1. Response with MRD 2. MRD– CR 3. Progressive disease |
| 4 | 64 | MDS-AML | -5, -7, complex | 134 | 177 | MRD+ CR | Fludarabine (2) | DL1 | No | No | Grade 2 stage 1 acute GI | Infection (3-5)∗,‡ | Progressive disease |
| 5 | 51 | AML | t(2:8) U2AF1, IDH1 | 381 | 1. 453 2. 557 | 1. MRD+ CR 2. MRD+ CR | 1. Fludarabine (3) 2. Nil | 1. DL2 2. DL2 | No | No | No | Fever (1)† d 0-1, self-resolving | Progressive disease |
| 6 | 62 | AML | Complex, TP53 | 83 | 161 | MRD+ CR | Fludarabine (1) | DL2 | No | No | No | Fever (1)† d 0-1, self-resolving, Neutropenia (3)∗ | Progressive disease |
| 7 | 23 | AUL | Complex | 184 after second HCT | 267 after second HCT | MRD− CRi | Fludarabine (3) | DL2 | No | No | No | Neutropenia∗ (4), infection-related fever (3) through day 3∗,§ | Progressive disease |
| 8 | 66 | MDS-EB1 | Complex, -7, TP53 | 84 | 1. 179 2. 589 | MRD− CRi | Fludarabine (2) | 1. DL3 2. DL2 | No | No | Grade 1 acute skin, subsequent mild chronic | Fever (2)† with rigors, transient nausea, d 0-1, self-resolving | Maintained CR >27 mo |
| 9 | 57 | AML | t(11:19), KMT2a | 30 | 204 | Refractory relapse (38% blasts) | Clofarabine and cytarabine | DL3 | No | No | No | Infusion reaction (4)† Neutropenia (4)∗ | CRi at 3 and 5 wk Relapse at 12 wk |
| Participant . | Age, y . | Disease . | Cytogenetic/molecular . | HCT to first relapse, d . | HCT to TCR-T, d . | Disease stage . | Lymphodepletion (doses) . | TCR-T dose level . | CRS . | ICANS . | GVHD after TCR-T . | Other relevant AE (maximal grade) . | Disease outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | B-ALL | Ph-like RCSD1-ABL2 + complex | 987 | >1000 | 1. MRD– CR 2. MRD+ CR 3. MRD+ CR | 1. Fludarabine (3) 2. Fludarabine (3) 3. Fludarabine (3) | 1. DL1 2. DL2 3. DL2 | No | No | No | No | 1. Maintained CR 6 mo 2. Progressive disease 3. Progressive disease |
| 2 | 58 | AML | del 5, -7, other | 195 | 257 | MRD– CR | Fludarabine (3) | DL1 | No | No | No | Neutropenia (4)∗ | Progressive disease |
| 3 | 2 | T-ALL | t(8:14), biallelic loss of CDKN2A and CDKN2B | 71 | 1. 181 2. 236 3. 546 | 1. MRD+ CR 2. MRD+ CR 3. Relapse | 1. Fludarabine (2) 2. Nil 3. NECTAR | 1. DL1 2. DL2 3. DL2 | No | No | No | Neutropenia (4)†, fatigue (2)† | 1. Response with MRD 2. MRD– CR 3. Progressive disease |
| 4 | 64 | MDS-AML | -5, -7, complex | 134 | 177 | MRD+ CR | Fludarabine (2) | DL1 | No | No | Grade 2 stage 1 acute GI | Infection (3-5)∗,‡ | Progressive disease |
| 5 | 51 | AML | t(2:8) U2AF1, IDH1 | 381 | 1. 453 2. 557 | 1. MRD+ CR 2. MRD+ CR | 1. Fludarabine (3) 2. Nil | 1. DL2 2. DL2 | No | No | No | Fever (1)† d 0-1, self-resolving | Progressive disease |
| 6 | 62 | AML | Complex, TP53 | 83 | 161 | MRD+ CR | Fludarabine (1) | DL2 | No | No | No | Fever (1)† d 0-1, self-resolving, Neutropenia (3)∗ | Progressive disease |
| 7 | 23 | AUL | Complex | 184 after second HCT | 267 after second HCT | MRD− CRi | Fludarabine (3) | DL2 | No | No | No | Neutropenia∗ (4), infection-related fever (3) through day 3∗,§ | Progressive disease |
| 8 | 66 | MDS-EB1 | Complex, -7, TP53 | 84 | 1. 179 2. 589 | MRD− CRi | Fludarabine (2) | 1. DL3 2. DL2 | No | No | Grade 1 acute skin, subsequent mild chronic | Fever (2)† with rigors, transient nausea, d 0-1, self-resolving | Maintained CR >27 mo |
| 9 | 57 | AML | t(11:19), KMT2a | 30 | 204 | Refractory relapse (38% blasts) | Clofarabine and cytarabine | DL3 | No | No | No | Infusion reaction (4)† Neutropenia (4)∗ | CRi at 3 and 5 wk Relapse at 12 wk |
AE, adverse events; AUL, acute undifferentiated leukemia; Cri, CR with incomplete hematologic recovery; EB, excess blasts; GI, gastrointestinal; NECTAR, nelarabine, etoposide, and cyclophosphamide; Ph, Philadelphia chromosome.
Unrelated to TCR T-cell infusion.
Possibly or probably related to TCR T-cell infusion. Note: Cytopenias were short-term and self-resolving unless they were caused by progressive leukemia.
Infection = environmentally-acquired, severe Legionella pneumonia ultimately led to death with multiorgan failure concurrent with progressive AML, not attributed to TCR T-cell infusion.
Infection = methicillin-susceptible Staphylococcus aureus pneumonia and septicemia.
Toxicity assessment
Adverse events for the first 28 days after TCR-T infusion were graded using the Common Terminology Criteria for Adverse Events, version 5. Cytokine release syndrome and neurotoxicity were graded using the American Society for Transplantation and Cellular Therapy criteria.12
Response assessment and translational analysis
Blood and BM samples were obtained before and after treatment to evaluate expansion, persistence, and function of TCR-T and the impact of treatment on leukemic burden. A potential therapeutic effect was defined in the trial protocol as (1) decreased blast percentage in the marrow over a period of 30 days, or (2) lack of progression over 3 months for patients who had attained remission without measurable residual disease (MRD) following bridging therapy before administration of TCR-T, as such patients commonly relapse rapidly.
We quantified TCR-T in recipient blood and marrow and evaluated the expression of cell-surface molecules and TCR-T function (target cell lysis and cytokine production) after infusion. Single-cell RNA sequencing was performed on residual leukemia to evaluate potential routes of escape from HA-1 TCR-T, specifically by examining the expression of genes known or implicated in immune escape and performing unbiased pathway analysis on genes with differential expression in neoplastic cells before and after HA-1 TCR-T infusion. Laboratory assay details are included in supplemental Method.
Patients (or guardians) and related donors provided informed written consent to participate in the IRB-approved protocol #9716 (NCT03326921) in accordance with the Declaration of Helsinki on consent forms approved by the Fred Hutchinson Cancer Center IRB and in accordance with an assurance filed with and approved by the US Department of Health and Human Services (Federalwide Assurance 00001920). Unrelated donors provided consent through processes facilitated by the National Marrow Donor Program and, where applicable, the Deutsche Knochenmarkspenderdatei and Anthony Nolan registries.
Results
Patients treated
Nine patients with recurrent myeloid malignancies (n = 7) or ALL (n = 2) after HCT were treated with HA-1 TCR-T following lymphodepletion with fludarabine monotherapy (n = 8) or clofarabine and cytarabine (n = 1) to facilitate TCR-T expansion (Table 1). Four patients received multiple infusions at the discretion of the principal investigator and treating physician, with approval by the US Food and Drug Administration. All patients and donors were HLA-A∗02:01–positive (supplemental Table 1). Most patients had developed recurrent leukemia within 6 months of HCT, and all had adverse-risk cytogenetic and/or molecular features. The extremely poor prognosis of this cohort is evident from diagnosis and previous treatment details (supplemental Table 1).
Manufacturing and infusion product evaluation
At the start of manufacturing, HCT-donor peripheral blood mononuclear cells were depleted of CD45RA+ cells to reduce the risk of GVHD associated with donor naïve T cells13,14 and to skew the product toward a central memory T cell (TCM) phenotype to enhance in vivo persistence.15 T cells were separated into predominantly CD4+ and CD8+ fractions to control the CD4:CD8 ratio in the cell product (target ratio 1:1) before transduction, enrichment, and expansion of HCT-donor TM (Figure 1A; “Methods”; supplemental Method). Manufacturing was successful for all 9 patients at the intended dose level.
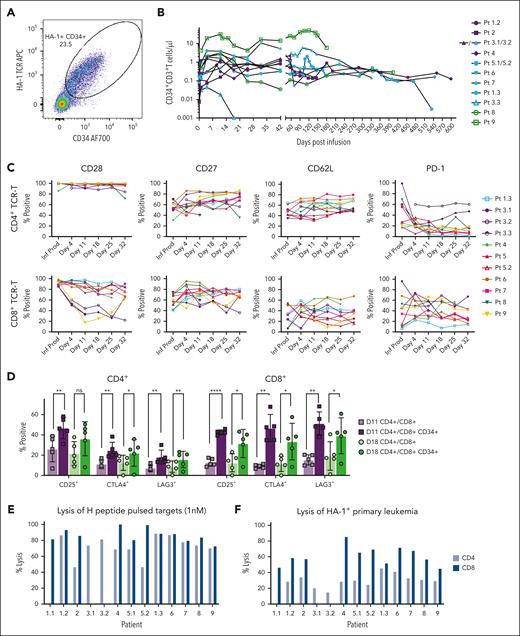
We assessed the functional and phenotypic characteristics of HA-1 TCR-T infusion products. Multiparameter flow cytometry (MFC) was used to assess expression of the CD34 tag encoded in the transgene. All final products were highly enriched for TCR-T, with most cells (60% to >90%) expressing the CD34 tag (Figure 2A; supplemental Figure 1A). The HA-1 TCR-T product was functional, as shown by degranulation in response to HA-1+ primary leukemia (Figure 2B) and lysis of HA-1+ peptide–pulsed T2 cells and HA-1+ primary leukemia in chromium release assays (Figure 2C-D). Negative control targets were not recognized (supplemental Figure 1B-D). Additionally, infusion products proliferated in response to coculture with HA-1+, but not HA-1–, peptide–pulsed T2 cells (supplemental Figure 1E-F). Partly because of the depletion of CD45RA+ cells in the first manufacturing step, HA-1 TCR-T had a predominantly TCM phenotype with expression of molecules associated with survival and self-renewal (CD28,16 CD27,17 and CD12718) and lymph node homing (CD62L and CCR719,20) (Figure 2E-F). After expansion in culture, a fraction of the TCR-T product also expressed cell-surface molecules associated with activation and/or inhibition (including CD38, CD69, TIM3, LAG3, and PD-1; Figure 2E-F). All products contained polyfunctional TCR-T that produced interleukin-2 (IL-2), interferon-γ, and/or tumor necrosis factor-α in response to HA-1 antigen stimulation (Figure 2G).
Functional and phenotypic characterization of HA-1 TCR-infusion products. (A) Percentage of cells expressing the CD34 tag within the CD4 (light blue bars) and CD8 (dark blue bars) infusion products. Cell products 1.1, 1.2, and 1.3 are 3 separate products made for patient 1. (B) Degranulation of CD4 (light blue bars) and CD8 (dark blue bars) infusion products when cocultured with primary HA-1+ AML (effector:stimulator = 1:1) measured by CD107a flow cytometric assay. Absence of bars indicates that data were not available (eg, product 3, 3.3, 5 CD4). (C-D) Lysis of HA-1 peptide–pulsed (1nM) T2 cells (C) or primary HA-1+ AML (D) in 4-hour chromium release assay by CD4 (light blue bars) and CD8 (dark blue bars) cell infusion products (effector:target = 20:1). (E-F) Expression of T-cell differentiation markers (left), activation markers (middle), and activation/inhibition markers (right) in CD4 (n = 11) (E) and CD8 (n = 12) (F) infusion products. Horizontal line represents the median. Each color represents an individual patient. (G) Percentage of CD4 HA-1 TCR-T (in CD4 infusion products, n = 8) or CD8 TCR-T (in CD8 [n = 12] or CD4 infusion products [n = 8]) secreting interferon (IFN)γ (red), IL-2 (blue), tumor necrosis factor (TNF)α (green), individually (unfilled bars) or combinations of cytokines (filled bars) in response to HA-1 peptide–pulsed (1nM) T2 cells by intracellular cytokine staining. Error bars indicate standard error of the mean. Statistics determined by an unpaired t test (2-sided P value: ∗P <. 05, ∗∗P < .01; ∗∗∗P < .001).
Functional and phenotypic characterization of HA-1 TCR-infusion products. (A) Percentage of cells expressing the CD34 tag within the CD4 (light blue bars) and CD8 (dark blue bars) infusion products. Cell products 1.1, 1.2, and 1.3 are 3 separate products made for patient 1. (B) Degranulation of CD4 (light blue bars) and CD8 (dark blue bars) infusion products when cocultured with primary HA-1+ AML (effector:stimulator = 1:1) measured by CD107a flow cytometric assay. Absence of bars indicates that data were not available (eg, product 3, 3.3, 5 CD4). (C-D) Lysis of HA-1 peptide–pulsed (1nM) T2 cells (C) or primary HA-1+ AML (D) in 4-hour chromium release assay by CD4 (light blue bars) and CD8 (dark blue bars) cell infusion products (effector:target = 20:1). (E-F) Expression of T-cell differentiation markers (left), activation markers (middle), and activation/inhibition markers (right) in CD4 (n = 11) (E) and CD8 (n = 12) (F) infusion products. Horizontal line represents the median. Each color represents an individual patient. (G) Percentage of CD4 HA-1 TCR-T (in CD4 infusion products, n = 8) or CD8 TCR-T (in CD8 [n = 12] or CD4 infusion products [n = 8]) secreting interferon (IFN)γ (red), IL-2 (blue), tumor necrosis factor (TNF)α (green), individually (unfilled bars) or combinations of cytokines (filled bars) in response to HA-1 peptide–pulsed (1nM) T2 cells by intracellular cytokine staining. Error bars indicate standard error of the mean. Statistics determined by an unpaired t test (2-sided P value: ∗P <. 05, ∗∗P < .01; ∗∗∗P < .001).
Because of the cell selection process (supplemental Method), there was a population of CD8+ CD4- TCR-T (2%-30%) in the predominantly CD4+ fraction of the cell product. These “contaminating” CD8+ TCR-T produced more cytokines than CD4+ TCR-T from the same culture or CD8+ TCR-T grown alone and exhibited phenotypic differences (Figure 2G; supplemental Figure 2A-B). We analyzed the frequency of CD8+ TCR clonotypes identified uniquely in the CD8+-infusion product and in the CD4+-infusion product and found no difference in relative clone sizes between CD8+ clonotypes unique to either product at baseline (supplemental Figure 2Ci). However, CD8+ T-cell clonotypes found only in the CD4+-infusion product comprised a higher proportion of all clonotypes and total TCR counts at each postinfusion time point compared with CD8+ T-cell clonotypes found only in the CD8+-infusion product (supplemental Figure 2Cii-Ciii), suggesting that CD8+ clonotypes originating from the CD4+ product survived and/or expanded to a greater extent after infusion.
Persistence and expansion of HA-1 TCR-T
HA-1 TCR-T were quantified in patient blood and marrow after infusion using MFC (Figure 3A). Postinfusion TCR-T were detectable in all patients’ blood and marrow samples (Figure 3B; supplemental Figure 3A). In 10 of 14 total infusions, peak TCR-T expansion in the blood occurred 4 to 11 days after infusion and ranged from 0.7 CD3+ TCR-T/μL (patient 4, dose level 1 [DL1]) to 31 CD3+ TCR-T/μL (patient 9, DL3). CD4+ TCR-T consistently expanded to greater numbers than CD8+ TCR-T (P = .0373) (supplemental Figure 3B). TCR-T were detectable at nearly all time points for 8 out of 9 patients and persisted beyond 12 months. The greatest expansion of TCR-T was in patient 9, who had a high burden of AML and received more intensive lymphodepleting chemotherapy than other participants.
Persistence, phenotype, and function of TCR-T after infusion. (A) HA-1 TCR-T by MFC in a representative patient 18 days after infusion indicating CD34 tag and HA-1 TCR dextramer staining. (B) Peripheral blood CD3+CD34+ HA-1 TCR-T (cells per μL) over time across all patients (n = 9) at dose level (DL) 1 (purple), DL2 (blue), and DL3 (green). Note: Infusion 3.3 was delivered at the time of ∼80% BM blasts, and the massive and rapidly increasing tumor burden likely induced T-cell death. (C) TCR-T phenotype by MFC on gated CD34+ cells for CD4 and CD8 infusion products and recipient blood. (D) Activation/inhibition markers on gated CD34+ cells and bulk CD4+ and CD8+ T cells on days 11 (purple) and 18 (green) after infusion. Error bars show mean and standard deviation. Statistics determined by paired t test (2-sided P value ∗P < .05, ∗∗P < .01; ∗∗∗∗P < .0001). (E-F) Lysis in a chromium release assay of HA-1 peptide–pulsed (1nM) T2 cells (E) or HA-1+ primary AML (F) at an effector:target ratio of 20:1 by HA-1 TCR-T recovered from recipient blood after infusion. CD4 (light blue bars) and CD8 (dark blue bars) TCR-T were sorted from patient blood at approximately day 32 after infusion and expanded with anti-CD3 monoclonal antibody and IL-2 and sorted by MFC before the functional assay. The patient and infusion numbers are indicated by 1.1, 1.2, etc. Absence of a bar indicates that data were not available (eg, no CD8+ HA-1 TCR-T were successfully isolated and expanded from patient 3).
Persistence, phenotype, and function of TCR-T after infusion. (A) HA-1 TCR-T by MFC in a representative patient 18 days after infusion indicating CD34 tag and HA-1 TCR dextramer staining. (B) Peripheral blood CD3+CD34+ HA-1 TCR-T (cells per μL) over time across all patients (n = 9) at dose level (DL) 1 (purple), DL2 (blue), and DL3 (green). Note: Infusion 3.3 was delivered at the time of ∼80% BM blasts, and the massive and rapidly increasing tumor burden likely induced T-cell death. (C) TCR-T phenotype by MFC on gated CD34+ cells for CD4 and CD8 infusion products and recipient blood. (D) Activation/inhibition markers on gated CD34+ cells and bulk CD4+ and CD8+ T cells on days 11 (purple) and 18 (green) after infusion. Error bars show mean and standard deviation. Statistics determined by paired t test (2-sided P value ∗P < .05, ∗∗P < .01; ∗∗∗∗P < .0001). (E-F) Lysis in a chromium release assay of HA-1 peptide–pulsed (1nM) T2 cells (E) or HA-1+ primary AML (F) at an effector:target ratio of 20:1 by HA-1 TCR-T recovered from recipient blood after infusion. CD4 (light blue bars) and CD8 (dark blue bars) TCR-T were sorted from patient blood at approximately day 32 after infusion and expanded with anti-CD3 monoclonal antibody and IL-2 and sorted by MFC before the functional assay. The patient and infusion numbers are indicated by 1.1, 1.2, etc. Absence of a bar indicates that data were not available (eg, no CD8+ HA-1 TCR-T were successfully isolated and expanded from patient 3).
Evaluating the postinfusion immunophenotype of TCR-T in patient blood and marrow longitudinally, we observed that TCR-T maintained a largely TCM phenotype after infusion, characterized by CD28, CD27, and CD62L expression (Figure 3C). Notably, CD28 expression decreased on CD8+ TCR-T over time in patients 3 and 9, who had the greatest leukemic burden at the time of TCR-T, likely reflecting activation and differentiation of the TCR-T upon recognition of leukemia. Expression of activation (CD25) and activation/inhibition molecules (CTLA4 and LAG3) was significantly increased on HA-1 TCR-T after infusion compared with endogenous, untransduced T cells, also consistent with in vivo activation of the TCR-T (Figure 3D). The phenotype of TCR-T in marrow samples was similar to TCR-T in peripheral blood (supplemental Figure 3C).
Functional activity of TCR-T after infusion was assessed using TCR-T isolated and expanded from recipient blood samples. Ex vivo isolated and expanded CD8+ and CD4+ TCR-T retained cytolytic capacity and cytokine production against HA-1+ peptide–pulsed T2 cells and a third-party HA-1+ HLA-A∗02:01+ primary AML, and not HA-1− counterparts (Figure 3E-F; supplemental Figure 4). Notably, we observed HA-1-specific killing of targets by TCR-T in chromium release assays in vitro where the ratio of TCR-T cells to targets was relatively high (effector to target ratio, 20:1), even with TCR-T retrieved from patients with progressive or relapsed disease, indicating their continued functionality and specificity and suggesting that TCR-T retain potential for delivering an antileukemic effect when adequate TCR-T to leukemic cell ratios can be achieved.
Treatment outcomes
Infusions were well tolerated by all patients with no cytokine release syndrome, immune effector cell–associated neurotoxicity syndrome, or dose-limiting toxicity. The most common adverse event following infusion was brief, self-resolving fever (Table 1; supplemental Table 2). Inflammatory serum proteins were not significantly elevated after infusion except in 2 cases: patient 4, who had a severe concurrent infection with preinfusion cytokine elevations; and patient 9, who had the greatest TCR-T expansion, which was associated with self-limited elevations of IL-2Rα, IL-6, tumor necrosis factor R1, IL-10, CRP, TIM3, and IL-15 (supplemental Figure 5). Mild acute GVHD was diagnosed clinically in 2 patients and was not attributed to TCR-T but rather to withdrawal of immunosuppression before TCR-T infusion (supplemental Table 3).
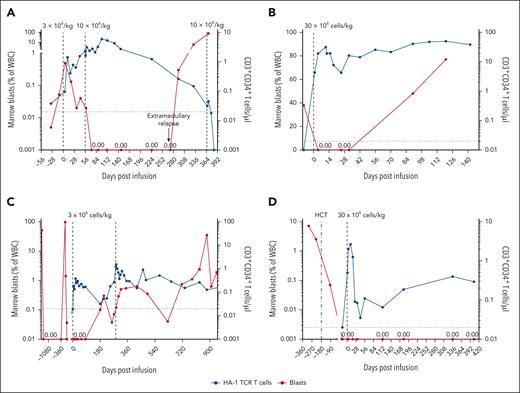
Although the trial was not designed to evaluate efficacy, disease status was assessed by BM aspiration before and after HA-1 TCR-T. Four of 9 patients met the protocol criteria for a potential therapeutic effect, including both patients treated at DL3 (Figure 4; Table 1). Two of these patients achieved a reduction of marrow blasts lasting >30 days (one with T-cell ALL [T-ALL] and the other with AML) (Figure 4A-B), whereas the other 2 had achieved remission with bridging chemotherapy before lymphodepletion and TCR-T, and sustained it following TCR-T (3-6 months and >27 months, B-cell acute lymphoblastic leukemia and myelodysplastic syndrome [MDS]) (Figure 4C-D).
Patient clinical course. (A-D) Marrow blasts as percentage of white blood cells (WBC) (red lines, left y-axis) and absolute count of HA-1 TCR-T (blue lines, right y-axis) are shown for the 4 participants with treatment response. Vertical dashed lines denote HCT or infusion time points. The horizontal dashed line indicates limit of detection for HA-1 TCR-T (0.02 cells per μl). (A) Patient 3 received 2 escalating dose infusions (DL1, DL2), the first at the time of rapidly proliferating T-ALL, resulting in a 6-month remission. Concurrent with falling blood concentrations of TCR-T, he experienced relapse. A third DL infusion given in the context of overwhelming, chemorefractory marrow and extramedullary T-ALL had no effect. (B) Patient 9 had 38% AML in the marrow before receiving a single DL3 infusion, resulting in a complete remission of up to 12 weeks (CRi documented at 3 and 5 weeks, relapse at 12 weeks). (C) Patient 1 entered the trial in an MRD-negative state after serial salvage therapies. He received 1 TCR-T infusion (DL1, CD8 only) with no subsequent pharmacologic maintenance, sustained MRD-negative status through 3 months after infusion, with eventual MRD detection at month 6. He received a second (DL1) and third (DL2, not shown) infusion in the context of MRD, but did not regain MRD-negative CR. (D) Patient 8 had high-risk, TP53+ MDS. She received 1 infusion (DL3) after salvage with hypomethylating therapy and venetoclax and sustained MRD-negative remission following HA-1 TCR-T.
Patient clinical course. (A-D) Marrow blasts as percentage of white blood cells (WBC) (red lines, left y-axis) and absolute count of HA-1 TCR-T (blue lines, right y-axis) are shown for the 4 participants with treatment response. Vertical dashed lines denote HCT or infusion time points. The horizontal dashed line indicates limit of detection for HA-1 TCR-T (0.02 cells per μl). (A) Patient 3 received 2 escalating dose infusions (DL1, DL2), the first at the time of rapidly proliferating T-ALL, resulting in a 6-month remission. Concurrent with falling blood concentrations of TCR-T, he experienced relapse. A third DL infusion given in the context of overwhelming, chemorefractory marrow and extramedullary T-ALL had no effect. (B) Patient 9 had 38% AML in the marrow before receiving a single DL3 infusion, resulting in a complete remission of up to 12 weeks (CRi documented at 3 and 5 weeks, relapse at 12 weeks). (C) Patient 1 entered the trial in an MRD-negative state after serial salvage therapies. He received 1 TCR-T infusion (DL1, CD8 only) with no subsequent pharmacologic maintenance, sustained MRD-negative status through 3 months after infusion, with eventual MRD detection at month 6. He received a second (DL1) and third (DL2, not shown) infusion in the context of MRD, but did not regain MRD-negative CR. (D) Patient 8 had high-risk, TP53+ MDS. She received 1 infusion (DL3) after salvage with hypomethylating therapy and venetoclax and sustained MRD-negative remission following HA-1 TCR-T.
Patient 3 achieved a reduction of marrow blasts with TCR-T. He presented with an aggressive, chemotherapy-resistant T-ALL that recurred 70 days after high-dose total body irradiation–based HCT. He was treated with HA-1 TCR-T at the time of rapidly rising leukemic burden despite bridging chemotherapy. Actively relapsing T-ALL after HCT is expected to relentlessly progress without intervention and often despite intervention. His first infusion (DL1) was followed by reduction but not complete elimination of marrow blasts, whereas the second (DL2), administered 56 days after the first and without lymphodepleting chemotherapy, induced a complete remission (CR) lasting 6 months (Figure 4A). Ultimately, concurrent with falling concentrations of TCR-T in the blood, disease recurred, initially at an extramedullary site.
Patient 9 had persistent complex karyotype, KMT2A-rearranged AML only 30 days after HCT conditioned with cladribine, cytarabine, mitoxantrone, and low-dose total body irradiation. AML progressed despite azacitidine and venetoclax, and she entered the trial with 38% marrow blasts. Following clofarabine and cytarabine for lymphodepletion and 1 HA-1 TCR-T infusion at DL3, she achieved and sustained CR lasting up to 12 weeks (CR with incomplete hematologic recovery documented at 3 and 5 weeks, relapse at 12 weeks) (Figure 4B), surpassing the remission duration provided by HCT. Whether the response was due primarily to clofarabine and cytarabine, HA-1 TCR-T, or both cannot be definitively ascertained. However, the in vivo expansion of CD4+ and CD8+ TCR-T was greatest in this patient. Thus, the intensive lymphodepletion may have enabled sufficient TCR-T expansion to deliver a therapeutic T-cell–mediated effect despite the heavy disease burden.
Patient 1 relapsed with Ph-like B-cell acute lymphoblastic leukemia after HCT, and again after CAR T-cell therapy with CD19-negative leukemia escape. Bridging treatment with 1 cycle of inotuzumab ozogamicin (InO) 3 months before TCR-T induced a CR deepened by the addition of dasatinib, and he entered the trial in an MRD-negative state. He received 1 TCR-T infusion (DL1) with no subsequent pharmacologic maintenance, sustained MRD-negativity through month 3 after infusion, with eventual MRD at month 6 (Figure 4C). Patients treated with InO who receive ≤3 treatment cycles almost always experience recurrence within 6 months (median 3 months) of InO treatment,21 and dasatinib effects are not expected to endure following drug discontinuation. Thus, bridging therapy is unlikely to account for the 3- to 6-month remission after TCR-T.
Patient 8 had MDS with MRD recurrence 84 days after HCT despite 2 cycles of prophylactic decitabine-cedazuridine after HCT. She regained MRD-negativity by adding venetoclax; however, given the high-risk features of her MDS (refractoriness to intensive pre-HCT chemotherapy, early post-HCT relapse, TP53 mutations, and complex karyotype), only brief remission was expected. Nevertheless, she sustained remission for >27 months after her first TCR-T infusion (DL3) (Figure 4D), having received no maintenance therapy except for a discretionary boost of TCR-T (without lymphodepletion) 410 days after her first infusion due to declining TCR-T concentrations.
Encouragingly, adoptive transfer of HA-1 TCR-T was well tolerated and showed promising antileukemic activity despite infusion of relatively low cell doses in most patients.
Leukemia escape evaluation
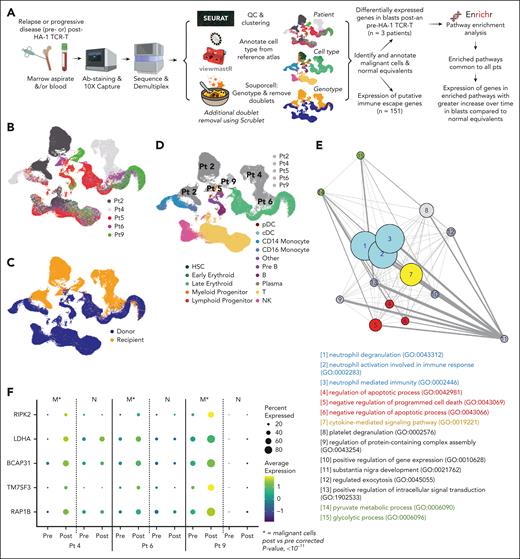
Although we observed probable clinical responses in 4 patients, all but 1 experienced subsequent disease progression or relapse. Quantitative insufficiency of TCR-T probably contributed to leukemia progression given the relatively low frequency achieved in vivo at the lower dose levels in this phase 1 dose-escalation trial. To explore additional contributors to resistance, we used single-cell RNA sequencing to study neoplastic cells that progressed after HA-1 TCR-T (workflow in Figure 5A). Adequate peripheral blood mononuclear cell and marrow samples were obtained from 5 patients with myeloid malignancies progressing 3 to 21 weeks after TCR-T infusion. Preinfusion disease samples were also procured from 3 patients. We captured 140 708 total cells, then removed low-quality cells and multiplets, yielding 84 355 high-quality single cells for analysis. We performed dimensionality reduction and clustering (Figure 5B), and classified cells as being of recipient or donor origin by using a genetic demultiplexing algorithm22 that identifies single-nucleotide variants in the raw sequence data (Figure 5C). To annotate cell type, we classified cell profiles on the basis of their similarity to a known reference atlas of healthy BM mononuclear cells23 (supplemental Figure 6A). Putatively malignant cells of recipient origin with disordered gene expression characteristic of neoplasia clearly clustered separately from donor cells (Figure 5D). Orthogonal validation of neoplastic cells in 4 patients and annotation of cells derived from paired preinfusion and postinfusion samples is presented in supplemental Figure 6B-D and Table 1.24,25
Single-cell RNA sequencing analysis of patient leukemia. (A) Schematic of single-cell RNA-sequencing experimental design and data processing pipeline. (B) Uniform manifold approximation and projection (UMAP) of combined patient data annotated by patient. (C) UMAP of combined patient data annotated by genotype, determined by the souporcell algorithm. (D) UMAP of combined patient data annotated by patient malignant cells and cell type, determined by (B-C) and viewmastR algorithm, respectively. (E) Network map of significantly enriched Gene Ontology (GO) Biological Processes 2021 pathways (P < .05) in genes upregulated (log2FC ≥ 0.25) in blasts after HA-1 TCR-T compared with before the treatment in all 3 patients with preinfusion and postinfusion samples. Bubble size corresponds to larger –log (average adjusted P value) for each pathway. Blue, neutrophil-related pathways; red, cell death/survival; yellow, cytokine signaling; green, metabolism; gray, other processes. (F) Bubble plots illustrate the expression patterns of 5 genes. These genes were identified from the significantly enriched pathways and had a greater log2 fold-change after the infusion in malignant cells compared with the change in nonmalignant cells (log2FC malignant ≥1.5× log2FC normal) in all 3 patients with pre- and post-HA-1 TCR-T samples. (E-F) Postinfusion sample time points: patient 4, day 29; patient 6, day 18; patient 9, day 90. viewmastR images in panel A are from https://github.com/furlan-lab/viewmastR/blob/main/man/figures/viewmaster.png.
Single-cell RNA sequencing analysis of patient leukemia. (A) Schematic of single-cell RNA-sequencing experimental design and data processing pipeline. (B) Uniform manifold approximation and projection (UMAP) of combined patient data annotated by patient. (C) UMAP of combined patient data annotated by genotype, determined by the souporcell algorithm. (D) UMAP of combined patient data annotated by patient malignant cells and cell type, determined by (B-C) and viewmastR algorithm, respectively. (E) Network map of significantly enriched Gene Ontology (GO) Biological Processes 2021 pathways (P < .05) in genes upregulated (log2FC ≥ 0.25) in blasts after HA-1 TCR-T compared with before the treatment in all 3 patients with preinfusion and postinfusion samples. Bubble size corresponds to larger –log (average adjusted P value) for each pathway. Blue, neutrophil-related pathways; red, cell death/survival; yellow, cytokine signaling; green, metabolism; gray, other processes. (F) Bubble plots illustrate the expression patterns of 5 genes. These genes were identified from the significantly enriched pathways and had a greater log2 fold-change after the infusion in malignant cells compared with the change in nonmalignant cells (log2FC malignant ≥1.5× log2FC normal) in all 3 patients with pre- and post-HA-1 TCR-T samples. (E-F) Postinfusion sample time points: patient 4, day 29; patient 6, day 18; patient 9, day 90. viewmastR images in panel A are from https://github.com/furlan-lab/viewmastR/blob/main/man/figures/viewmaster.png.
We did not observe downregulated expression of either HA-1 (HMHA1/ARHGAP45) or HLA-A genes in any patient within our single-cell data set, nor did we observe decreased HLA-A2 protein on the surface of patient leukemia cells by MFC (supplemental Figure 7A-C). We then examined the expression of 151 genes known or implicated in leukemic escape from immune surveillance (supplemental Figure 7D; supplemental Table 4). Nine genes, including TGFB1 and CD47, had increased expression in blasts after infusion that was both statistically significant and favored leukemia resistance to cell therapy (supplemental Figure 7E-F; supplemental Table 5A). No convincing consistent gene downregulation after infusion that favored immune escape, was observed.
We then applied an unbiased approach to explore immune escape by performing pathway enrichment analysis using the Enrichr database26,27 and Gene Ontology Biological Pathways 202128,29 on genes in neoplastic cells with differential expression after compared with before HA-1 TCR-T. Fifteen pathways were significantly enriched after treatment (ie, lymphodepletion and HA-1 TCR-T) in all 3 patients who had AML/MDS with paired pretreatment and posttreatment samples (Figure 5E). Analysis of genes within enriched pathways identified 5 genes that were significantly increased (Bonferroni-corrected P value <10-11) and increased to a greater degree in post- vs pre-infusion blasts compared to change in post- vs pre-infusion normal equivalent cells in all 3 paired samples, highlighting potential treatment-driven changes occurring specifically in malignant cells (Figure 5F; supplemental Tables 5B and 6). Two of the identified genes were previously associated with induction of T-cell-dysfunction (RIPK2, LDHA).30-33
Discussion
Post-HCT relapse of acute leukemia is typically rapidly fatal.1,2 In a phase 1 trial, we have shown that adoptively transferring donor T cells engineered with a TCR specific for HA-1 is safe up to the highest cell dose tested, 30 × 106/kg. TCR-T expanded and persisted in patient blood and marrow. Four of 9 heavily pretreated patients with myeloid neoplasms and ALL bearing very poor prognoses met the a priori criteria for a potential therapeutic effect, including the 2 patients treated at the highest dose level. These results lay the foundation for further dose-escalation and larger trials to assess efficacy.
HA-1–directed therapy is restricted to patients who express the common HLA allele A∗02:01+ and have the correct recipient/donor genotype pairing, meaning that HA-1 TCR-T can provide semipersonalized immunotherapy or immunoprophylaxis for 10% to 15% of the HCT population.6,34 Importantly, because HA-1 is expressed across hematopoietic cells, treatment could potentially be applied to any hematologic malignancy for which HCT is indicated, in contrast to current cellular therapies such as CAR T-cell therapy that target lineage-specific antigens. Moreover, additional minor H antigens are likely to be suitable targets for TCR-T in patients with other HLA types and minor H antigen genotypes.8
Although the current study used HA-1 TCR-T for treating recurrent disease after HCT, the excellent tolerability of the T-cell product in patients aged 2 to 66 suggests that HA-1 TCR-T should also be evaluated as prophylaxis against relapse. The demonstration of the safety of HA-1 as a target for immunotherapy in our treatment trial is more definitive than previous observations by other investigators in a small trial of HA-1 TCR-T as relapse prophylaxis. In that trial, manufacturing challenges were encountered, and TCR-T, which were infused into 5 of the 9 enrolled patients, mostly at very low doses, could be detected at minimal to low levels in only 3 patients, all of whom had limited follow-up (7-19 weeks) due to leukemia or HCT complications.35 In contrast, we infused 9 out of 9 patients, could detect the TCR-T in all, and followed them for a median of 31 weeks (range, 4-120).
CD4+ T-cell help has long been recognized as important in antitumor responses,36-41 providing prosurvival and proinflammatory cytokines and increasing cytotoxic CD8+ cell function, tumor-homing, and persistence. Antigen-specific CD4+ CAR T cell, CD4+ T cells expressing or transduced with native class II TCRs, and CD4+ T cells transduced with class I TCRs without CD8 coreceptors as part of a mixed CD8+ and CD4+ TCR-T product42-48 have been clinically evaluated. However, our study is, to our knowledge, the first reported trial to use a class I–restricted TCR with a CD8 coreceptor to enable antigen-specific CD4+ T-cell function. The expansion and persistence of CD4+ TCR-T exceeded that of CD8+ TCR-T in all patients. CD4+ and CD8+ HA-1 TCR-T both demonstrated leukemia-reactivity when recovered from patient blood following infusion.
Although we observed clinical improvements in leukemic burden and maintenance of remissions in some patients, in all but 1 patient the response was ultimately insufficient to prevent leukemia progression. An adequate number of gene-modified T cells is necessary for rapid and sustained elimination of malignancy.49-52 Although HA-1 TCR-T expanded and persisted in all patients, relapses occurred in 2 patients (patients 3 and 9) after HA-1 TCR-T numbers declined. Therefore, the following modifications should be considered to augment peak TCR-T levels and prolong their high-level persistence in future trials: (1) treating patients at dose level 3 or higher; (2) administering more intense lymphodepleting chemotherapy to augment the initial expansion of TCR-T; (3) culturing CD4+ and CD8+ TCR-T together, as coculture was associated with improved CD8+ HA-1 TCR-T cytokine production and expansion and persistence in vivo (Figure 2G; supplemental Figure 2); (4) administering repeated doses; and (5) considering vaccine boosts to expose TCR-T to antigen intermittently and maintain TCR-T numbers.53-57
To generate hypotheses about how leukemia might evade TCR-T, we examined single-cell gene expression in recurrent disease after HA-1 TCR-T. We identified 3 genes that have previously been reported to be overexpressed in cancer cells and for which an association of cancer cell gene expression and/or protein expression in the microenvironment with T-cell dysfunction has been observed. Namely, LDHA32,33 and RIPK230,31 had increased expression following TCR-T in the unbiased analysis, and TGFB158-61 was significantly increased following treatment in the analysis of our predefined list of 151 putative immune escape genes and also apparent in the unbiased analysis (supplemental Tables 5 and 6), although it did not meet the stringent criteria for inclusion in Figure 5F. Both the predefined gene and unbiased analyses also identified increased expression following treatment of multiple genes associated with antiapoptotic activity and cell survival (supplemental Table 5). Taken together, these exploratory hypothesis-generating data suggest that TGFβ and lactic acid generation by leukemia cells increase after TCR-T therapy, which could lead to T-cell dysfunction, whereas leukemia cells themselves may become more resistant to cell death. Several upregulated genes have inhibitors that could be used in conjunction with T-cell immunotherapy (supplemental Table 5).
In summary, this trial represents progress toward a greatly needed new therapy for patients with post-HCT relapse. The study met the primary objectives of demonstrating manufacturing feasibility and patient safety. We used both CD8+ and CD4+ TCR-T, and to our knowledge, demonstrated for the first time clinically that CD4+ T cells can be successfully redirected to target an HLA class I–presented antigen by incorporating a CD8 coreceptor in the transgene construct. We showed that HA-1 can be targeted to treat post-HCT leukemia relapse; despite most patients receiving relatively low doses of TCR-T, we observed signals of efficacy in 4 out of 9 patients, including 1 who remains in remission >27 months after treatment. TCR-T therapy targeting HA-1, and possibly other minor H antigens, represents a promising option warranting further clinical development.
Acknowledgments
The authors thank the following individuals and groups: Zita Mears and the Fred Hutchinson Cancer Center (FHCC) Therapeutic Products Program; Kari Stricker, Bethany Murphy, Carrie Angeles and FHCC Clinical Research Support; Andrew Berger and the FHCC Flow Cytometry Core Facility; Megan Hickner, Jeff Huguenor and the FHCC Immunotherapy Integrated Research Center; Mirjam Heemskerk, Shruti Bhise, M. Nicole Nazzaro, Vipul Sheth, Matthew Ulrickson, Erin Guest-Hall, Basir Haque, National Marrow Donor Program, Deutsche Knochenmarkspenderdatei; and Anthony Nolan. Bellicum Pharmaceuticals contributed rimiducid.
This study was sponsored primarily by ElevateBio with research funding to E.F.K. (clinical) and M. Bleakley (laboratory). Initial and additional funding sources also included the National Institutes of Health, National Cancer Institute (NCI K23CA154532 and P30 CA015704-46), Damon Runyon Cancer Research Foundation, Alex’s Lemonade Stand Foundation, Hyundai Hope On Wheels, Leukemia & Lymphoma Society Translational Research Program, the Gerdin Family Foundation, and the Bezos Family. M. Bleakley is the Gerdin Family Endowed Chair for Leukemia Research. She was a Damon Runyon-Richard Lumsden Foundation Clinical Investigator, supported in part by the Damon Runyon Cancer Research Foundation and the Richard Lumsden Foundation (CI-57-11), and received support from an Alex’s Lemonade Stand Foundation Bio-Therapeutics Impact Grant. M.A.B. is an Amy Strelzer Manasevit Research Program Scholar.
Authorship
Contribution: M. Bleakley was responsible for the conceptualization of the trial and for funding acquisition. E.F.K., T.A.G., and M. Bleakley developed the methodology; E.F.K., C.S., M.A.B., M. Bar, R.D.C., A.D., B.G.T., D.G.M., and A.G.C. participated in the investigation, specifically the conduct of the trial; E.F.K., C.S., and N.V. had project administration responsibility for the trial; E.F.K., C.S., A.E.D., and M. Bleakley supervised the trial; T.M.C., R.G.D., and M. Bleakley contributed to resource development, specifically the development of TCR-T; E.F.K., T.A.G., M. Brault, C.S., T.M.C., M.A.B, R.G.B., K.B.W., S.B.K., A.C.Y., C.C.S.Y., S.N.F., and M. Bleakley participated in the investigation, specifically the acquisition or analysis of data. No author was precluded from access to the primary clinical trial data. E.F.K., T.A.G., M. Brault, C.S., T.M.C., M.A.B., R.G.B., K.B.W., S.B.K., A.C.Y., A.E.D., C.C.S.Y., S.R.R., P.D.G., A.G.C., E.W.N., S.N.F., and M. Bleakley participated in the investigation, specifically the design, interpretation, and/or validation of the reported experiments or results. S.N.F. provided computing resources; E.F.K., M. Brault, M.A.B., and M. Bleakley wrote the original manuscript; E.F.K., M. Brault, M.A.B., S.N.F., T.M.C., R.G.B., A.C.Y., and M. Bleakley contributed to data visualization; M. Bleakley supervised all aspects of the research; and all authors were responsible for reviewing and editing the manuscript.
Conflict-of-interest disclosure: M. Bleakley and R.G.D. are coinventors on a patent describing HA-1 T-cell receptor therapy that was previously licensed to ElevateBio and has recently been licensed to PromiCell Therapeutics (TCRS SPECIFIC FOR MINOR H ANTIGEN HA-1 AND USES THEREOF; US 10,538,577 B2 Jan 21 2020; Inventors M. Bleakley, Robson Dossa, Daniel Sommermeyer). M. Bleakley received funding from HighPassBio, an ElevateBio portfolio company, and was a scientific advisory board member and had equity in HighPassBio. HighPassBio provided funding for the clinical trial on which E.F.K. was the principal investigator. At the time of the research, A.E.D. was an employee of ElevateBio Technologies and is currently an owner of stock in ElevateBio LLC. E.F.K. served on an advisory board for TScan Therapeutics. R.D.C. has received research funding from Amgen, Kite/Gilead, Incyte, Merck, Pfizer, Servier, and Vanda Pharmaceuticals; consultancy/honoraria from Amgen, Jazz, Kite/Gilead, and Pfizer; and membership on a board or advisory committee for Autolus and PeproMene Bio. The remaining authors declare no competing financial interests.
The current affiliation of R.G.D. is Arovella Therapeutics, Melbourne, VIC, Australia.
The current affiliation of M. Bar is Bristol-Myers Squibb, Summit, NJ.
Correspondence: Marie Bleakley, Translational Science and Therapeutics, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109; email: mbleakle@fredhutch.org.
References
Author notes
E.F.K. and M. Brault contributed equally to this study.
The code used to reproduce all single-cell genomic analysis are available at https://github.com/furlan-lab/HA1_paper. Single-cell gene expression data have been uploaded to the Gene Expression Omnibus database (RRID: SCR_005012, accession code: GSE 244663). Data are also available in the supplemental Methods.
Original data are available from the corresponding author, Marie Bleakley (mbleakle@fredhutch.org), on request.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Functional and phenotypic characterization of HA-1 TCR-infusion products. (A) Percentage of cells expressing the CD34 tag within the CD4 (light blue bars) and CD8 (dark blue bars) infusion products. Cell products 1.1, 1.2, and 1.3 are 3 separate products made for patient 1. (B) Degranulation of CD4 (light blue bars) and CD8 (dark blue bars) infusion products when cocultured with primary HA-1+ AML (effector:stimulator = 1:1) measured by CD107a flow cytometric assay. Absence of bars indicates that data were not available (eg, product 3, 3.3, 5 CD4). (C-D) Lysis of HA-1 peptide–pulsed (1nM) T2 cells (C) or primary HA-1+ AML (D) in 4-hour chromium release assay by CD4 (light blue bars) and CD8 (dark blue bars) cell infusion products (effector:target = 20:1). (E-F) Expression of T-cell differentiation markers (left), activation markers (middle), and activation/inhibition markers (right) in CD4 (n = 11) (E) and CD8 (n = 12) (F) infusion products. Horizontal line represents the median. Each color represents an individual patient. (G) Percentage of CD4 HA-1 TCR-T (in CD4 infusion products, n = 8) or CD8 TCR-T (in CD8 [n = 12] or CD4 infusion products [n = 8]) secreting interferon (IFN)γ (red), IL-2 (blue), tumor necrosis factor (TNF)α (green), individually (unfilled bars) or combinations of cytokines (filled bars) in response to HA-1 peptide–pulsed (1nM) T2 cells by intracellular cytokine staining. Error bars indicate standard error of the mean. Statistics determined by an unpaired t test (2-sided P value: ∗P <. 05, ∗∗P < .01; ∗∗∗P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/10/10.1182_blood.2024024105/2/m_blood_bld-2024-024105-gr2.jpeg?Expires=1767702144&Signature=Q~wVtkzhAqbvCBwPB-fbbAvIo~HU4SCpVRIKJQsAcZsbyaZJ2SabHOuDagSdmVmG1ufW85s5X1mN5T7yvx1yy6VFGnu8n426xVw1Vo75p5ks8896iLIk4gKYN5gdy8IkNbDlYpoOl27m0oQreslLEcN1SGAGMGj16u5QX1agNO6gN~xOapsRK09prhZYfRli46DfQnNHnosDHTxmh4o6iNj4BntapQEXUN2RJ99PY0dCEmNdmcu1IbTYADPH8MZhL~aFMo3Puys1QHjW6~FoYePzik0oTHd5IE7zh-384jFnFvKOodRL~5S~qfLG-skO0GrH6uXtlgAGrVZp4ddyCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal