Key Points
Successful pregnancies are possible among female alloHCT recipients, although birth rate is >6 times lower than in the general population.
Key factors increasing the chance of pregnancy were younger age at HCT, nonmalignant disease, no TBI or dose <8 Gy and RIC/NMA conditioning
Visual Abstract
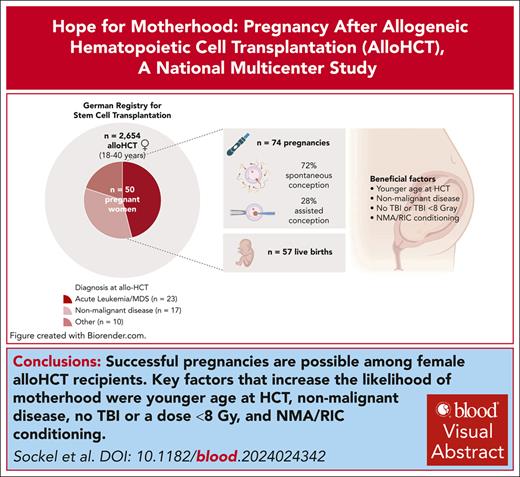
Improved long-term survival rates after allogeneic hematopoietic cell transplantation (alloHCT) make family planning for young adult cancer survivors an important topic. However, treatment-related infertility risk poses challenges. To assess pregnancy and birth rates in a contemporary cohort, we conducted a national multicenter study using data from the German Transplant Registry, focusing on adult women aged 18 to 40 years who underwent alloHCT between 2003 and 2018. Of 2654 women who underwent transplantation, 50 women experienced 74 pregnancies, occurring at a median of 4.7 years after transplant. Fifty-seven of these resulted in live births (77%). The annual first birth rate among HCT recipients was 0.45%, which is >6 times lower than in the general population. The probability of a live birth 10 years after HCT was 3.4%. Factors associated with an increased likelihood of pregnancy were younger age at alloHCT, nonmalignant transplant indications, no total body irradiation or a cumulative dose of <8 Gy, and nonmyeloablative/reduced-intensity conditioning. Notably, 72% of pregnancies occurred spontaneously, with assisted reproductive technologies used in the remaining cases. Preterm delivery and low birth weight were more common than in the general population. This study represents the largest data set reporting pregnancies in a cohort of adult female alloHCT recipients. Our findings underscore a meaningful chance of pregnancy in alloHCT recipients. Assisted reproductive technologies techniques are important and funding should be made available. However, the potential for spontaneous pregnancies should not be underestimated, and patients should be informed of the possibility of unexpected pregnancy despite reduced fertility. Further research is warranted to understand the impact of conditioning decisions on fertility preservation.
Introduction
Improved procedures for allogeneic hematopoietic cell transplantation (alloHCT) and optimized supportive therapies have led to an increasing number of long-term survivors in recent years.1 For these patients achieving a normal health and social life represents a major goal. Particularly for adolescents and young adults (<40 years), this often includes the desire to have children.2
However, gonadal dysfunction and permanent loss of fertility owing to precedent intensive chemotherapy such as high-dose alkylating agents or total body irradiation (TBI),3-6 transplant-related morbidity, and long-term medication represent relevant limitations for pregnancies in female transplant recipients.
Current data on pregnancy rates after alloHCT are limited. Previous studies had small sample sizes or encompassed both allogeneic and autologous transplant recipients, representing 2 entirely distinct treatment modalities.7-10 Furthermore, most of the published research did not focus on pregnancies among female transplant recipients but instead included pregnancies of healthy female partners from male transplant recipients. Therefore, the objective of this study was to conduct a systematic analysis of pregnancies in adult female transplant recipients who underwent alloHCT in Germany in a contemporary area (between 2003 and 2018) to estimate pregnancy and birth rates in this population and identify potential risk factors.
Methods
Data source
Data collection was divided into 2 parts:
Retrospective registry data collection: medical data were analyzed retrospectively from all adult women of childbearing age (between 18 and 40 years) who underwent first alloHCT in a German transplant center between 2003 and 2018. Data were retrieved from the German Registry for Stem Cell Transplantation database, which delivers aggregate German data sets to the European Society for Blood and Marrow Transplantation (EBMT) registry. Patients had given an informed consent for the use of their data in research projects. Baseline data on demographics, underlying disease, donor characteristics, and the transplant procedure were exported from the registry database. Transplant-specific follow-up data and information on reported pregnancies and live births after alloHCT were collected during annual registry follow-ups. To ensure data validity and integrity, transplant centers were requested to validate the registered data.
Detailed information collected by interviews with the pregnant women: subsequently, women with documented pregnancies were asked by their treating local physician to participate in an interview to collect detailed information about conception, pregnancy course, and child development. All participants provided a separate written informed consent for this data collection. The study was approved by the ethical committee of the Technical University Dresden (EK 305082018).
Definitions
Myeloablative and nonmyeloablative/reduced-intensity conditioning (NMA/RIC) regimen were categorized according to the recommendations published by Bacigalupo et al.11
Statistical analysis
Competing risks models were used to calculate pregnancy and first live birth rates per person-year after alloHCT. The initial state was the time point “transplantation.” Events were first pregnancies for the annual pregnancy rate and first live births for the annual first birth rate. Competing events were “death without prior live birth” for calculation of the live birth rate and “death without prior pregnancy” for the pregnancy rate. Further states, such as second live birth or second pregnancy, were not considered in the respective model. The cumulative incidence function was estimated with the Aalen-Johansen estimator.12 We calculated the annual pregnancy rate/first live birth rate after alloHCT by dividing the total number of reported pregnancies/first live births within 10 years after transplantation by the sum of follow-up times in years for all patients during that time.13 We compared the annual first live birth rate after alloHCT with the annual first birth rate of women aged 18 to 40 years in Germany in 2019 obtained from German Bureau of Statistics.14
Given the relatively low number of first events, we used Poisson regression models for multivariate analysis to assess the incidence rate's dependency on covariates. In a sensitivity analysis, we compared these results with those obtained from a Cox regression model. In addition, to account for acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD), we conducted a landmark Poisson regression with a 2-year landmark.15 For interpretation, we used a significance level of 5%. Analyses were performed by using R statistical software version 4.3.2 (R Core Team, Vienna, Austria)
Results
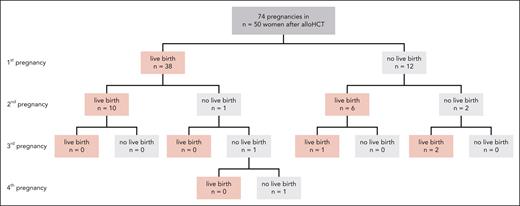
Between 2003 and 2018, a total of 2654 adult women of childbearing age (18-40 years) underwent transplantation in Germany (Table 1 for detailed patient characteristics). At least 1 pregnancy was reported for 50 women, and some women became pregnant several times after alloHCT (Figure 1), resulting in 74 pregnancies. Altogether 57 pregnancies (77%) resulted in live births. The median time between transplantation and first reported pregnancy was 4.7 years (range, 0.7-14.8; interquartile range, 4.3). No woman was pregnant at the time point of alloHCT.
Patient characteristics of the whole cohort (N = 2654) and of 50 patients with at least 1 reported pregnancy after alloHCT
| Variable . | All patients (N = 2654) . | Patients with pregnancies (n = 50) . |
|---|---|---|
| Patient age at transplantation, y | ||
| 18-25 | 662 (25%) | 31 (62%) |
| >25-30 | 553 (21%) | 14 (28%) |
| >30-35 | 586 (22%) | 5 (10%) |
| >35-40 | 853 (32%) | — |
| Diagnosis | ||
| Acute leukemia/MDS | 1945 (73%) | 23 (46%) |
| Acquired BM failure, hemoglobinopathies | 180 (7%) | 17 (34%) |
| Other | 529 (20%) | 10 (20%) |
| Karnofsky performance score | ||
| 80-100 | 1909 (72%) | 40 (80%) |
| <80 | 110 (4%) | — |
| Missing | 635 (24%) | 10 (20%) |
| Donor type | ||
| Identical sibling | 709 (27%) | 20 (40%) |
| Alternative donors∗ | 1945 (73%) | 30 (60%) |
| Conditioning† | ||
| Myeloablative | 1354 (51%) | 10 (20%) |
| RIC/NMA | 1300 (49%) | 40 (80%) |
| Cyclophosphamide-based conditioning | ||
| No | 1278 (48%) | 27 (54%) |
| Yes | 1376 (52%) | 23 (46%) |
| TBI | ||
| No | 1228 (46%) | 36 (72%) |
| <8 Gy | 293 (11%) | 8 (15%) |
| ≥8 Gy | 877 (33%) | 4 (8%) |
| Missing | 256 (10%) | 2 (4%) |
| aGVHD until day 100 | ||
| No/grade 1 | 1788 (67%) | 38 (76%) |
| >grade 1 | 742 (28%) | 11 (22%) |
| Missing | 124 (5%) | 1 (2%) |
| cGVHD until year 2‡ | ||
| Yes | 731 (32%) | 15 (30%) |
| No | 888 (39%) | 35 (70%) |
| Missing | 633 (28%) | — |
| Variable . | All patients (N = 2654) . | Patients with pregnancies (n = 50) . |
|---|---|---|
| Patient age at transplantation, y | ||
| 18-25 | 662 (25%) | 31 (62%) |
| >25-30 | 553 (21%) | 14 (28%) |
| >30-35 | 586 (22%) | 5 (10%) |
| >35-40 | 853 (32%) | — |
| Diagnosis | ||
| Acute leukemia/MDS | 1945 (73%) | 23 (46%) |
| Acquired BM failure, hemoglobinopathies | 180 (7%) | 17 (34%) |
| Other | 529 (20%) | 10 (20%) |
| Karnofsky performance score | ||
| 80-100 | 1909 (72%) | 40 (80%) |
| <80 | 110 (4%) | — |
| Missing | 635 (24%) | 10 (20%) |
| Donor type | ||
| Identical sibling | 709 (27%) | 20 (40%) |
| Alternative donors∗ | 1945 (73%) | 30 (60%) |
| Conditioning† | ||
| Myeloablative | 1354 (51%) | 10 (20%) |
| RIC/NMA | 1300 (49%) | 40 (80%) |
| Cyclophosphamide-based conditioning | ||
| No | 1278 (48%) | 27 (54%) |
| Yes | 1376 (52%) | 23 (46%) |
| TBI | ||
| No | 1228 (46%) | 36 (72%) |
| <8 Gy | 293 (11%) | 8 (15%) |
| ≥8 Gy | 877 (33%) | 4 (8%) |
| Missing | 256 (10%) | 2 (4%) |
| aGVHD until day 100 | ||
| No/grade 1 | 1788 (67%) | 38 (76%) |
| >grade 1 | 742 (28%) | 11 (22%) |
| Missing | 124 (5%) | 1 (2%) |
| cGVHD until year 2‡ | ||
| Yes | 731 (32%) | 15 (30%) |
| No | 888 (39%) | 35 (70%) |
| Missing | 633 (28%) | — |
BM, bone marrow; MDS, myelodysplastic syndrome.
Unrelated donor or haploidentical donor.
See supplemental Table 1 for details regarding the conditioning regimen.
Only patients with a follow-up beyond day 100 were included (n = 2252).
Outcomes of 74 pregnancies reported from 50 women out of 2654 adult women who underwent alloHCT in Germany between 2003 and 2018 at childbearing age (18-40 years).
Outcomes of 74 pregnancies reported from 50 women out of 2654 adult women who underwent alloHCT in Germany between 2003 and 2018 at childbearing age (18-40 years).
Patient and transplant characteristics of women who became pregnant
Pregnancies were most frequently observed in younger women between 18 and 25 years of age at the time of alloHCT. No pregnancies were reported in women who underwent alloHCT at an age older than 35 years. The median age at which women became pregnant after alloHCT was 29.6 years (range, 21.7-39.3; interquartile range, 6.8). The underlying diseases were acute leukemia/myelodysplastic neoplasia in 23 patients (with acute myeloid leukemia in 17 patients, acute lymphatic leukemia in 4 patients, and myelodysplastic neoplasm in 2 patients), acquired bone marrow failure syndrome (mainly aplastic anemia [n = 13] and paroxysmal nocturnal hemoglobinuria [n = 3]) and hemoglobinopathies in 17 patients, and others (including 6 patients with chronic myeloid leukemia and 4 with Hodgkin lymphoma).
Twenty percent of women underwent myeloablative conditioning (MAC), whereas 80% underwent NMA/RIC according to Bacigalupo.11 Details among the conditioning regimens are presented in supplemental Table 1, available on the Blood website. TBI was part of the conditioning regimen in 12 of 50 patients, with 4 women receiving a cumulative dose of ≥8 Gy. Eleven of 50 women (22%) who got pregnant later had antecedent relevant (grade ≥2) aGVHD. Notably, cGVHD had also been reported in 15 of these women (30%) at any time point after HCT.
Pregnancy and birth rates
Pregnancy rate
The annual rate of a first reported pregnancy after alloHCT was 0.53% (95% confidence interval [CI], 0.31-0.59).
Birth rate and comparison with the age-matched general population
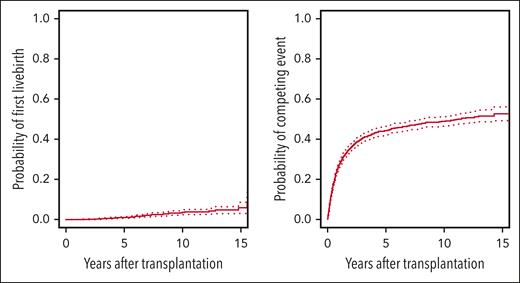
The cumulative incidence curves for first live birth and the competing event death are presented in Figure 2. At 10 years after HCT, the cumulative incidence of a first live birth was 3.4% (95% CI, 2.3-4.5). The annual first live birth was 0.45% (95% CI, 0.31-0.59) and seemed to remain constant throughout the observation period (hazards not shown). In contrast, there was no clear plateau observed regarding the cumulative hazard of the competing event “death” and most events occurred within the first 2 years after alloHCT.
Cumulative incidences for first live birth and its competing event. The figure shows Aalen-Johansen estimates for the cumulative incidences of first live birth (left panel) and its competing risk death without previous first live birth (right panel). 95% CIs are displayed by dotted lines.
Cumulative incidences for first live birth and its competing event. The figure shows Aalen-Johansen estimates for the cumulative incidences of first live birth (left panel) and its competing risk death without previous first live birth (right panel). 95% CIs are displayed by dotted lines.
Next, we compared the birth rate of female alloHCT recipients with that of women in the general German population aged 18 to 40 years. Based on German population data,14 the live birth rate for women in 2019 was 6.43%. Approximately 47% of all live births in Germany were first births,16 resulting in a first birth rate of 3.02% for women of that age in Germany. Therefore, the first birth rate in the general German population was more than 6 times higher than the annual first birth rate of 0.45% among women after alloHCT.
Birth rates in particular subgroups
We performed distinct analyses for particular subgroups in which we expected higher birth rates. Patients with bone marrow failure or hemoglobinopathies showed a higher annual first birth rate of 2.15% (95% CI, 0.98-3.31) vs 0.32% (95% CI, 0.19-0.44) for patients with malignancies (acute leukemia, myelodysplastic syndrome, other). Among women with hematologic malignancies, those who received MAC with cumulative TBI doses of ≥8 Gy had an annual first live birth rate of 0.08% (95% CI, 0.00-0.19). In contrast, those who did not receive MAC with TBI ≥8 Gy had an annual first live birth rate of 0.42% (95% CI, 0.25-0.60).
Women who were alive 2 years after HCT and had no live births by that time were included in the 2-year landmark analysis. The annual rate of a first live birth in these women was 0.73% (95% CI, 0.49-0.96). This represents a 66% higher likelihood of live birth for women who have survived the first 2 years than to the entire baseline cohort of women.
Risk factor analyses
Risk factor analyses for pregnancy and live birth were performed using multivariate Poisson regression. Incidence rate ratios (RRs) from the Poisson regression model can be interpreted similarly to hazard ratios of a Cox regression model. A value greater than 1 indicates a greater chance for a first live birth; a value less than 1 indicates a lower chance. Factors associated with a higher likelihood of pregnancy were NMA/RIC (RR, 2.78 [95% CI, 1.26-6.59]; P = .01) and nonmalignant transplant indications such as hemoglobinopathy/bone marrow failure syndrome (RR, 2.65 [95% CI, 1.27-5.51]; P = .01; supplemental Table 2). Higher age at HCT (RR, 0.36 [95% CI, 0.25-0.50]; P < .001) and the use of TBI ≥8 Gy (RR, 0.29 [95% CI, 0.08-0.83]; P = .03) were associated with a lower likelihood of a pregnancy after alloHCT (supplemental Table 2). Results for the alternative competing risk scenario, considering first live birth and death as events of interest, are presented in Table 2 and indicate similar outcomes.
Factors associated with first live birth and its competing event: total population and 2-year landmark population
| Time period . | 0-10 y . | P value . | 2-10 y . | P value . | 0-10 y . | P value . | 2-10 y . | P value . |
|---|---|---|---|---|---|---|---|---|
| Event . | First live birth RR (95%CI) . | First live birth RR (95%CI) . | Competing event RR (95%CI) . | Competing event RR (95%CI) . | ||||
| Intercept (baseline hazard) | 0.02 (0.006-0.04) | <.001 | 0.02 (0.006-0.07) | <.001 | 0.09 (0.07-0.11) | <.001 | 0.02 (0.008-0.03) | <.001 |
| Patient age, reference: 18-25 y | ||||||||
| Increase by 1 category | 0.36 (0.24-0.51) | <.001 | 0.37 (0.23-0.54) | <.001 | 1.02 (0.97-1.1) | .41 | 1.01 (0.87-1.16) | .94 |
| TBI, reference: no TBI | ||||||||
| TBI <8 Gy | 0.63 (0.23-1.50) | .33 | 0.78 (0.26-2.11) | .64 | 1.06 (0.84-1.34) | .61 | 0.92 (0.45-1.75) | .80 |
| TBI ≥8 Gy | 0.31 (0.09-0.88) | .04 | 0.20 (0.03-0.80) | .04 | 0.91 (0.78-1.06) | .24 | 0.81 (0.53-1.22) | .32 |
| Missing information | 0.21 (0.03-0.73) | .03 | 0.12 (0.006-0.60) | .04 | 0.75 (0.59-0.93) | .01 | 0.50 (0.25-0.95) | .04 |
| Donor type, reference: identical sibling | ||||||||
| Alternative donor | 0.59 (0.33-1.11) | .09 | 0.75 (0.36-1.61) | .44 | 1.48 (1.28-1.71) | <.001 | 0.99 (0.70-1.44) | .99 |
| Conditioning with cyclophosphamide, reference: other | ||||||||
| Cyclophosphamide | 0.70 (0.35-1.43) | .33 | 0.58 (0.23-1.38) | .22 | 0.96 (0.84-1.11) | .612 | 1.03 (0.70-1.49) | .90 |
| Conditioning intensity, reference: myeloablative | ||||||||
| RIC/NMA | 2.42 (1.07-5.89) | .04 | 2.08 (0.77-6.14) | .16 | 1.15 (0.98-1.35) | .09 | 1.26 (0.81-1.94) | .30 |
| Diagnosis, reference: AML or MDS | ||||||||
| BM failure/hemoglobinopathy | 2.45 (1.09-5.42) | .03 | 3.24 (1.19-8.88) | .02 | 0.52 (0.37-0.70) | <.001 | 0.11 (0.006-0.51) | .03 |
| Diagnosis other | 1.00 (0.43-2.20) | .99 | 1.02 (0.37-2.58) | .97 | 0.83 (0.71-0.97) | .02 | 0.84 (0.55-1.25) | .40 |
| aGVHD, reference: none/grade 1 | ||||||||
| aGVHD grade 2-4 | — | — | 1.32 (0.50-3.12) | .55 | — | — | 1.35 (0.94-1.92) | .10 |
| aGVHD missing | — | — | 2.87 (0.16-15.10) | .32 | — | — | 0 | .97 |
| cGVHD, reference: none | ||||||||
| cGVHD yes | — | — | 1.04 (0.49-2.20) | .92 | — | — | 2.63 (1.77-4.00) | <.001 |
| Time period . | 0-10 y . | P value . | 2-10 y . | P value . | 0-10 y . | P value . | 2-10 y . | P value . |
|---|---|---|---|---|---|---|---|---|
| Event . | First live birth RR (95%CI) . | First live birth RR (95%CI) . | Competing event RR (95%CI) . | Competing event RR (95%CI) . | ||||
| Intercept (baseline hazard) | 0.02 (0.006-0.04) | <.001 | 0.02 (0.006-0.07) | <.001 | 0.09 (0.07-0.11) | <.001 | 0.02 (0.008-0.03) | <.001 |
| Patient age, reference: 18-25 y | ||||||||
| Increase by 1 category | 0.36 (0.24-0.51) | <.001 | 0.37 (0.23-0.54) | <.001 | 1.02 (0.97-1.1) | .41 | 1.01 (0.87-1.16) | .94 |
| TBI, reference: no TBI | ||||||||
| TBI <8 Gy | 0.63 (0.23-1.50) | .33 | 0.78 (0.26-2.11) | .64 | 1.06 (0.84-1.34) | .61 | 0.92 (0.45-1.75) | .80 |
| TBI ≥8 Gy | 0.31 (0.09-0.88) | .04 | 0.20 (0.03-0.80) | .04 | 0.91 (0.78-1.06) | .24 | 0.81 (0.53-1.22) | .32 |
| Missing information | 0.21 (0.03-0.73) | .03 | 0.12 (0.006-0.60) | .04 | 0.75 (0.59-0.93) | .01 | 0.50 (0.25-0.95) | .04 |
| Donor type, reference: identical sibling | ||||||||
| Alternative donor | 0.59 (0.33-1.11) | .09 | 0.75 (0.36-1.61) | .44 | 1.48 (1.28-1.71) | <.001 | 0.99 (0.70-1.44) | .99 |
| Conditioning with cyclophosphamide, reference: other | ||||||||
| Cyclophosphamide | 0.70 (0.35-1.43) | .33 | 0.58 (0.23-1.38) | .22 | 0.96 (0.84-1.11) | .612 | 1.03 (0.70-1.49) | .90 |
| Conditioning intensity, reference: myeloablative | ||||||||
| RIC/NMA | 2.42 (1.07-5.89) | .04 | 2.08 (0.77-6.14) | .16 | 1.15 (0.98-1.35) | .09 | 1.26 (0.81-1.94) | .30 |
| Diagnosis, reference: AML or MDS | ||||||||
| BM failure/hemoglobinopathy | 2.45 (1.09-5.42) | .03 | 3.24 (1.19-8.88) | .02 | 0.52 (0.37-0.70) | <.001 | 0.11 (0.006-0.51) | .03 |
| Diagnosis other | 1.00 (0.43-2.20) | .99 | 1.02 (0.37-2.58) | .97 | 0.83 (0.71-0.97) | .02 | 0.84 (0.55-1.25) | .40 |
| aGVHD, reference: none/grade 1 | ||||||||
| aGVHD grade 2-4 | — | — | 1.32 (0.50-3.12) | .55 | — | — | 1.35 (0.94-1.92) | .10 |
| aGVHD missing | — | — | 2.87 (0.16-15.10) | .32 | — | — | 0 | .97 |
| cGVHD, reference: none | ||||||||
| cGVHD yes | — | — | 1.04 (0.49-2.20) | .92 | — | — | 2.63 (1.77-4.00) | <.001 |
Results from Poisson regression modeling for the competing risk setting: first live birth and its competing event death without previous first live birth. We show incidence RRs. Values >1 indicate an increased chance/risk for this event; values <1 indicate a reduced chance/risk for this event. Age categories (18-25 years, 25-30 years, 30-35 years, and 35-40 years) were evaluated as continuous covariates. Results indicate changes in event rates from 1 category to the next higher category.
Boldface P values represent statistical signficance at a level <5%. Italicized text represents the calculations for the 2-year landmark analysis.
AML, acute myeloid leukemia; BM, bone marrow; CI, confidence interval; MDS, myelodysplastic syndrome.
A 2-year landmark analysis was conducted to evaluate the impact of aGVHD and cGVHD. Assuming an event-free status (alive and no live birth) at to 2 years after transplantation, aGVHD and cGVHD had no influence on the incidence rate of live births. In contrast, cGVHD increased the incidence rate of the competing risk “death.”
Maternal outcomes of pregnant female alloHCT recipients
With a median follow-up of 8.9 years since alloHCT and 3.7 years since first pregnancy, all 50 patients who became pregnant after alloHCT remained alive. Only 1 relapse was observed. The woman who experienced a relapse underwent transplantation for acute myeloid leukemia. Her first pregnancy 8 years after alloHCT resulted in an abortion, followed by a live birth 9 years after transplantation. This patient experienced relapse 1 year after she had given birth to her child. After intensive salvage chemotherapy and donor lymphocyte infusion, she was alive and in remission 5 years after relapse.
Detailed information on conception, pregnancy complications and fetal outcomes
Detailed information on conception and outcomes were obtained from 32 women with 54 pregnancies.
Concerning the type of conception, 39 of 54 pregnancies (72%) occurred spontaneously, whereas methods of assisted reproductive technologies (ARTs) and/or fertility preservation were used by the remaining women in 15 of 54 pregnancies (28%).
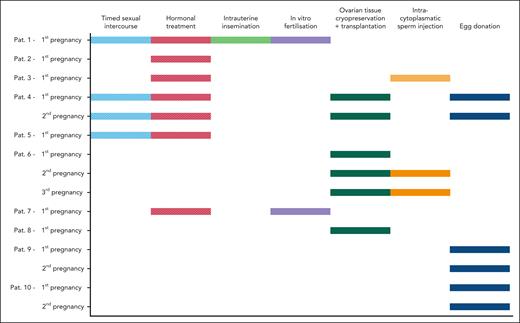
Techniques that led to successful pregnancies included in vitro fertilization (n = 2), ovarian tissue cryopreservation and retransplantation (n = 6), and oocyte donation (n = 6). Several women used multiple methods sequentially to achieve pregnancy. The different methods are presented in Figure 3.
Information on assisted reproductive technologies and fertility preservation methods. Detailed information on conception for 15 pregnancies of 10 patients. Several women used multiple methods sequentially. Dashed bars indicate unsuccessful pregnancies and solid bars represent successful pregnancies.
Information on assisted reproductive technologies and fertility preservation methods. Detailed information on conception for 15 pregnancies of 10 patients. Several women used multiple methods sequentially. Dashed bars indicate unsuccessful pregnancies and solid bars represent successful pregnancies.
Maternal complications during pregnancy and delivery
Maternal complications during pregnancy were documented in 25 of 52 pregnancies, with no information available for 2 patients. In some cases, multiple complications occurred within a single pregnancy (Table 3). The most frequent complications were vascular related in 16 pregnancies (preeclampsia, pregnancy-induced hypertension, edema, placental insufficiency, and oligohydramnios), uterovaginal complications in 6 cases (cervical insufficiency, placenta previa, and preterm labor), and 6 cases of other complications (medication-related issues and typical pregnancy discomforts).
Detailed information on maternal complications during pregnancy
| Maternal complications during pregnancy . | n = 52 . |
|---|---|
| Vascular associated complications | 16 (31%) |
| Pregnancy-induced hypertension | 6 |
| Preeclampsia | 1 |
| Edema | 3 |
| Placental insufficiency | 2 |
| Oligohydramnios | 4 |
| Endocrinological diseases | 4 (8%) |
| Gestational diabetes | 3 |
| Thyroid disease | 1 |
| Uterovaginal complications | 6 (12%) |
| Cervical insufficiency | 3 |
| Placenta previa | 1 |
| Preterm labor | 2 |
| Infections | 4 (8%) |
| Vaginal infection | 2 |
| Hand-foot-and-mouth disease | 1 |
| Urogenital infection | 1 |
| Vaginal bleeding | 4 (8%) |
| Renal diseases | 2 (4%) |
| Renal dysfunction | 2 |
| Pulmonary diseases | 1 (2%) |
| Pleural effusion | 1 |
| Other | 7 (14%) |
| Drug-associated complications | 3 |
| Typical pregnancy symptoms | 3 |
| Psychological stress | 1 |
| Maternal complications during pregnancy . | n = 52 . |
|---|---|
| Vascular associated complications | 16 (31%) |
| Pregnancy-induced hypertension | 6 |
| Preeclampsia | 1 |
| Edema | 3 |
| Placental insufficiency | 2 |
| Oligohydramnios | 4 |
| Endocrinological diseases | 4 (8%) |
| Gestational diabetes | 3 |
| Thyroid disease | 1 |
| Uterovaginal complications | 6 (12%) |
| Cervical insufficiency | 3 |
| Placenta previa | 1 |
| Preterm labor | 2 |
| Infections | 4 (8%) |
| Vaginal infection | 2 |
| Hand-foot-and-mouth disease | 1 |
| Urogenital infection | 1 |
| Vaginal bleeding | 4 (8%) |
| Renal diseases | 2 (4%) |
| Renal dysfunction | 2 |
| Pulmonary diseases | 1 (2%) |
| Pleural effusion | 1 |
| Other | 7 (14%) |
| Drug-associated complications | 3 |
| Typical pregnancy symptoms | 3 |
| Psychological stress | 1 |
Spontaneous vaginal delivery was reported for 29 pregnancies (54%) and 15 women (28%) underwent cesarean delivery, whereas detailed information was missing for 10 pregnancies. Among the cesarean deliveries, 11 were planned for various reasons, including eclampsia (n = 1), premature birth (n = 2), birth canal obstruction owing to mother’s hip prothesis associated with previous therapy (n = 2), birth obstruction owing to fetal malposition (n = 2), previous cesarean delivery (n = 2), and placental insufficiency (n = 2).
Fetal outcomes
Information on fetal outcomes were obtained from 44 of 54 pregnancies. Preterm deliveries, defined as birth before 37 weeks of pregnancy, were reported for 10 of 44 pregnancies (23%). Two neonates were born between the 28th and 32nd gestation weeks whereas the other 8 neonates were born between the 32nd and 37th gestation weeks. Normal birth weight (>2500 g) was documented in 37 of 44 newborns (84%), whereas 6 of 44 (14%) had a low birth weight (1500-2500 g) and only 1 of 44 (2%) a very low birth weight (<1500 g). All newborns survived.
In addition, the survey collected information about diagnosed health conditions of the children. One chromosomal abnormality was diagnosed: Ullrich-Turner syndrome (45X), which involved aortic stenosis that required surgical correction when the child was 3 days old. At the time of the survey, this child was 11 years old and the mother had reported normal physical and mental development. Most frequently reported comorbidities were related to atopic diseases (n = 10). Furthermore, 1 child had a tic disorder, another child had myopia, and 1 child had a hemangioma.
The median age of all children at the time of the survey was 13 years. The vast majority of mothers in this study assessed the physical and mental development of their children as age appropriate. However, 3 children were assessed by their mothers as having a physical developmental delay. Those 3 children had experienced complications during or after pregnancy: One of the 3 children was born prematurely and required ventilation in a neonatal intensive care unit after birth. At the time of the developmental delay assessment, the child was 12 years old. Another child was delivered at term but with low birth weight owing to placental insufficiency and intrauterine growth restriction. This child was 7 years old at the time when the developmental disorder was reported. The third child was delivered at term with a normal weight, but during pregnancy the mother had a Coxsackie virus infection, leading to oligohydramnios. This child was 4 years old at the time of the developmental disorder assessment.
Discussion
Long-term survivorship after alloHCT has increased during recent years and return to a normal social life, including family planning, has emerged as a relevant topic for younger patients. In this large national multicenter study evaluating pregnancy and live birth rates after alloHCT, we demonstrate that female HCT recipients can achieve successful and safe pregnancies, despite facing various fertility risks during treatment. Although the annual first birth rate after alloHCT is more than 6 times lower than in the general German population, our findings suggest a realistic chance for pregnancy.
To our knowledge, this is the largest analysis on birth and pregnancy rates among a homogenous cohort of adult female alloHCT recipients, contrasting with previous studies7-10 that included heterogeneous patient cohorts (with both allogeneic and autologous transplant recipients, as well as healthy female partners of male HCT recipients).
The median time from transplantation to first pregnancy in our cohort was 4.7 years. It is generally advised to avoid pregnancy within the first year after HCT owing to heightened infection risk, increased risk of relapse, and GVHD. However, 1 woman became pregnant within the first year after transplantation and successfully delivered.
Risk factor analysis revealed that older age at alloHCT (P < .001) and high doses of TBI ≥8 Gray (P = .03) were associated with decreased chances of pregnancy and childbirth. Notably, no woman older than 35 years at alloHCT became pregnant, which might be attributed to the well-documented decline in ovarian reserve with increasing age. However, it is important to note that we do not have information regarding the desire and attempts toward pregnancy for women who did not report pregnancies. Various psychosocial factors, such as already existing children, socioeconomic challenges from cancer treatment, partnership status, and the recommended timing between transplantation and pregnancy, could potentially lead to a low desire for pregnancy in women older than 35 years.17
NMA/RIC (P = .01) and nonmalignant transplant indications such as hemoglobinopathy/bone marrow failure syndrome (P = .01) were positive predictive factors for pregnancy and live birth. The birth rate for those with nonmalignant transplant indications was 2.15%, which closely aligns with the birth rate of the general German population aged 18 to 40 years, which is 3.02%.14 Benign diseases such as aplastic anemia or hemoglobinopathy showed a high incidence of fertility recovery after HCT.18,19 Typically, these patients did not undergo classical myeloablative regimens, except for high-dose cyclophosphamide 200 mg/kg in aplastic anemia. Of note, in our cohort, there was also a relevant proportion of women (n = 27) with underlying malignant diseases (acute leukemia or lymphoma) who had usually undergone previous treatment but still had the chance to get pregnant.
The high risk of infertility associated with MAC regimens, particularly those including high-dose alkylating agents (busulfan, cyclophosphamide, melphalan) and/or TBI, which can cause permanent damage to the ovaries and follicles,20-22 is well known and confirmed by our data. Nevertheless, in our cohort, 10 of 50 women got pregnant even after MAC. These findings suggest that MAC alone does not necessarily impede fertility; rather, additional factors such as cumulative previous therapies, the combination of alkylating agents with TBI, and patient age (depleted ovarian reserve) contribute to the high risk of ovarian failure, as previously described.23-25
Similar observations apply to TBI, which is known for inducing irreversible ovarian damage, resulting in fibrosis and the complete loss of follicular maturation.26 Furthermore, radiation has been proposed to cause uterine vascular damage, loss of elasticity resulting in restricted uterine expansion during pregnancy, and impaired endometrial receptivity,.27,28 The extent of radiation-induced damage varies based on factors such as irradiated volume, total dose, fractionation schedule, and patient age.29 Notably, one-third of women got pregnant after conditioning with TBI, including 3 patients exposed to high cumulative TBI doses exceeding 10 Gy. However, we observed a higher preterm delivery rate (23%) than the general population and TBI is one of the established risk factors for preterm deliveries.
Interestingly, studies have indicated that combining myeloablative TBI doses with ovarian shielding has the potential to preserve fertility after alloHCT by reducing the radiation dose to the ovaries to ∼2.4 Gy.30-32 Therefore, ovarian shielding might be considered in some women who undergo TBI at childbearing age, especially if they are in complete remission.
Moreover, additional data are required to determine optimal conditioning regimens that both preserve fertility and exert a strong antitumor effect. The application of ovarian shielding in TBI may be considered on a case-by-case basis. In addition, there is evidence supporting the use of treosulfan, which seems to be less detrimental to fertility compared with busulfan.33-35 However, its antineoplastic activity may not be sufficient for young women, although previous studies indicated that busulfan-based and treosulfan-based conditioning might result in similar overall survival and event-free survival.36-38 Further prospective studies are essential to address these issues and provide more evidence.
Prior GVHD, which can be associated with permanent organ dysfunction and previous immunosuppressive therapy, was not an obstacle for pregnancy in our study. Among the 50 women who became pregnant, half had previously experienced aGVHD, and 14 had cGVHD. However, detailed information on active GVHD or use of immunosuppressive therapy at the time of pregnancy was not available.
Pregnancy in cancer survivors may also pose risks to the mother. In our study, vascular problems were the most common maternal complications, further increasing the risk of fetal growth restriction and preterm delivery. Pre-eclampsia incidence was low (1 of 54 pregnancies), whereas hypertension was observed in 6 of 52 pregnancies, aligning with general population rates (4.6% for preeclampsia and 10% for hypertensive disorders in the general population).39 Nevertheless, long-term use of calcineurin inhibitors or corticosteroids may elevate individual risk owing to their vasoconstrictive effects. Contrary to expectations, infectious complications did not play a prominent role. However, regular screening for infections, especially asymptomatic urinary tract infections during pregnancy, is of course justified. Close interdisciplinary monitoring of pregnant women after HCT is recommended to reduce maternal complications.
Surprisingly, 39 of the 54 pregnancies (72%) occurred spontaneously. This is one of the most important and unexpected observations in our study, highlighting the need to educate women about potential fertility restoration after transplant to prevent unplanned or unwanted pregnancies. ART and usage of fertility preservation methods with cryopreservation of oocytes or ovarian tissue were reported only in a quarter of pregnancies. In some cases, fertility treatments were performed several times or different methods were used until pregnancy occurred. Interestingly oocyte donation, which is not permitted in Germany but is internationally a standard for women with severely reduced ovarian reserve, was also considered as an option to fulfill the desire for parenthood after alloHCT. Of note, some patients reported facing rejection of oocyte donation within their own familiar environment.
To discuss all these issues, fertility counseling should be offered to all young woman before transplantation, yet only 55% of transplant physicians refer patients to fertility specialists, owing to disease related factors, time constraints, or the assumption of pre-existing infertility owing to previous therapies.40 National and international collaborations as the network of centers for reproductive medicine (FertiPROTEKT) in Germany raise awareness for the aspect of cancer-related infertility.
Fetal outcomes were favorable with a live birth rate of 78%, comparable with the general population’s miscarriage risk estimates.41 Prospective register studies from Norway and Denmark indicate a comparable risk of miscarriage, ranging from 10% to 15% at age 25 years to 33% for women aged ≥40 years.41,42 However, our study noted significantly higher rates of preterm deliveries, defined as delivery before the completed 37th week (23%) and low birth weight <2500 g (16%), than general population data with preterm delivery rates of 8% to 11% and low birth weight rates of 1% to 2%.43,44 The rate of cesarean deliveries at 28% was similar to that in the general population (which ranges from 24% to 30% in Europe)45 and lower than those reported by Salooja et al7 for women with malignant diseases (50%) and aplastic anemia (33%).
No increased incidence of childhood illnesses was reported by the mothers compared with the general population. Nevertheless, 1 child was found to have a congenital abnormality (Ullrich-Turner syndrome), although its association with the preceding treatment and alloHCT cannot be proven. The occurrence of atopic diseases (including asthma, atopic dermatitis, and hay fever) reported in the study aligns with the prevalence observed in the general population at the age of 13 years (10% to 35%).46-48
Our study has limitations, mainly owing to its retrospective nature. Although we conducted prospective telephone interviews, it was challenging to collect comprehensive information from all pregnant women over the extensive 15-year study period. Retrospective data regarding the use of gonadotropin-releasing hormone (GnRH) analogues or the ovarian function before alloHCT, such as anti-Müllerian hormone levels, could not be obtained. This information would be valuable in selecting conditioning regimens, given that pregnancy may already be unlikely for some women before transplantation. In addition, relying on self-reported pregnancy and pregnancy outcomes poses challenges, especially when patients experience few post-transplant complications and attend follow-up visits infrequently, leading to potential underreporting of unsuccessful pregnancies. Therefore, we focused more on birth rates than pregnancy rates in this study. However, to address this issue adequately in future, structured questions on pregnancy desires and conception methods should be added to registry data for adolescents and young adults registries. Moreover, prospective observational studies that address this topic are warranted. Caution is needed when interpreting pregnancy and birth rates in comparison with the general German population, given that women with life-threatening diseases may have lower willingness to conceive. Particularly, psychosocial factors such as the knowledge of the life-threatening nature of the situation (as shown in Figure 2, which illustrates the risk of dying after transplantation) should be considered. Prospective studies are necessary to estimate how many women may not want to become pregnant in this situation and how many, despite their desire, cannot.
Finally, the collection of ARTs may be subject to bias because of the unavailability of certain techniques throughout the entire duration of the study. For instance, ovarian tissue retransplantation was introduced in Germany in 200849 and only became standard practice within the last 10 years.
In conclusion, pregnancies after alloHCT, whether natural or assisted with ARTs, are possible with generally favorable outcomes. Lower age at alloHCT (<35 years), NMA/RIC conditioning, lower TBI doses of <8 Gray, and nonmalignant transplant indications increase the chances for parenthood in female HCT recipients. Fertility counseling before HCT should be provided to every woman of childbearing age, not only to discuss fertility preservation techniques but also to inform patients about the potential for natural fertility restoration after HCT to prevent unexpected pregnancies. Collaboration between transplant clinicians and gynecologists is crucial for monitoring maternal risk during pregnancy. Finally, the coverage of ART methods, particularly cryopreservation of ovarian tissue or oocytes before gonadotoxic treatment, has been established in the statutory health insurance. However, legalizing egg donation in all countries would provide broader family planning options for women with treatment-related premature ovarian failure and increase support for these techniques. Prospective and detailed data collection is crucial to further advance the field, increase awareness of fertility aspects in women with HCT, and offer the fundament for tailored procedural choices for conditioning regimens with respect to fertility preservation.
Acknowledgments
The authors thank all data managers in the German transplant centers who participated in data collection and the German Registry for Stem Cell Transplantation coordinators for their continuous support during the whole project. The authors thank all patients who became pregnant and supported this project by providing detailed information on conception and pregnancy course.
S.S. was supported by the German Research Foundation (DFG; grant BE4500/4-1).
Authorship
Contribution: K.S., A.N., M.G., S.S., and J.S. designed research and wrote the manuscript; K.S., M.D., I.H., N.K., F.A.A., F.S., J.M.M., M.E., W.B., J.F., H.B., G.K., M.K., U.P., D.B., C.Schmid, M.v.B., K.E.-H., L.H., K.T.-G., R.T., G.B., J.T., A.F., M.F., D.W., T.L., J.W., K.S.-E., C.Scheid, U.H., S.K., I.W.B., A.B., G.W., J.H., R.S., S.K., C.J., F.W., and M.B. participated as study investigators and provided patient data; K.S., A.N., H.N., and C.T. participated in the collection and assembly of data; S.S., J.B., K.S., A.N., and J.S. contributed to data analysis; S.W. contributed to visual abstract design; and all authors participated in data interpretation and critical review and revision of this manuscript and provided approval for the manuscript submission.
Conflict-of-interest disclosure: I.H. is chairperson of the board of trustees of the German Foundation for Young Adults with Cancer; received research funding from the H.W. & J. Hector Foundation; and received honoraria from AbbVie, medac, Novartis, and Takeda. The remaining authors declare no competing financial interests.
A complete list of the members of the German Cooperative Transplant Study Group appears in the supplemental Appendix.
Correspondence: Katja Sockel, Department of Internal Medicine I, University Hospital Carl Gustav Carus, TU Dresden, Fetscherstr 74, 01307 Dresden, Germany; email: katja.sockel@ukdd.de.
References
Author notes
S.S. and J.S. contributed equally to this study.
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 12 December 2022.
All data can be provided on request from the corresponding author, Katja Sockel (katja.sockel@ukdd.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal