Key Points
ELN 2017 and 2022 prognostic classifiers did not adequately stratify OS outcomes for patients with AML treated with venetoclax-azacitidine.
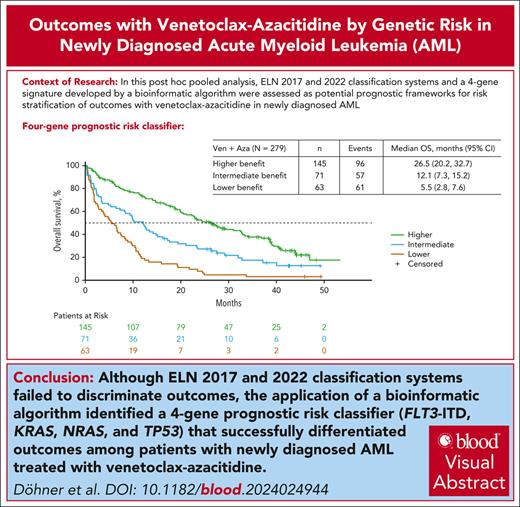
A 4-gene prognostic model (TP53, FLT3-ITD, NRAS, and KRAS) stratified OS outcomes with venetoclax-azacitidine treatment in AML.
Visual Abstract
The European LeukemiaNet (ELN) acute myeloid leukemia (AML) genetic risk classification systems are based on response to intensive chemotherapy; their ability to discriminate outcomes in older patients treated with venetoclax-azacitidine may be suboptimal. This pooled analysis of the phase 3 VIALE-A trial (NCT02993523) and phase 1b study (NCT02203773) examined prognostic stratification according to the 2017 and 2022 ELN risk classifications and derived new molecular signatures differentiating venetoclax-azacitidine–treated patients based on overall survival (OS). Overall, 279 patients treated with venetoclax-azacitidine and 113 patients treated with placebo-azacitidine were analyzed. The ELN 2017 or 2022 prognostic criteria classified most patients as adverse-risk AML (60.2% and 72.8% for venetoclax-azacitidine and 65.5% and 75.2% for placebo-azacitidine, respectively). Although outcomes with venetoclax-azacitidine improved across all ELN risk groups compared with placebo-azacitidine, ELN classification systems poorly discriminated venetoclax-azacitidine outcomes. By applying a bioinformatic algorithm, new molecular signatures were derived differentiating OS outcomes with venetoclax-azacitidine. The mutational status of TP53, FLT3 internal tandem duplication (FLT3-ITD), NRAS, and KRAS categorized patients into higher-, intermediate-, and lower-benefit groups (52%, 25%, and 23% of patients, respectively), each associated with a distinct median OS (26.5 months [95% confidence interval (CI), 20.2-32.7]; 12.1 months [95% CI, 7.3-15.2]; and 5.5 months [95% CI, 2.8-7.6], respectively). ELN prognostic classifiers did not provide clinically meaningful risk stratification of OS outcomes in patients treated with venetoclax-azacitidine. TP53, FLT3-ITD, NRAS, and KRAS mutation status allows the classification of these patients into 3 risk groups with distinct differences in median OS. These trials were registered at www.clinicaltrials.gov as #NCT02993523 and #NCT02203773.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2271.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning objectives
Upon completion of this activity, participants will:
Describe classifications and outcomes using European Leukemia Net (ELN) 2022 prognostic criteria, based on a pooled analysis of patients with newly diagnosed acute myeloid leukemia (AML) in the phase 3 VIALE-A trial (NCT02993523) and phase 1b study (NCT02203773)
Determine the prognostic significance of different genetic factors among subgroups of patients distinguished by overall survival benefit with venetoclax-azacitidine and classifications and outcomes using bioinformatic algorithm–derived new molecular signatures, based on a pooled analysis of patients with newly diagnosed AML in the phase 3 VIALE-A trial (NCT02993523) and phase 1b study (NCT02203773)
Identify clinical implications of the prognostic utility for overall survival of the 2022 ELN risk classifications compared with that of bioinformatic algorithm–derived new molecular signatures, based on a pooled analysis of patients with newly diagnosed AML in the phase 3 VIALE-A trial (NCT02993523) and phase 1b study (NCT02203773)
Release date: November 21, 2024; Expiration date: November 21, 2025
Introduction
Venetoclax, a highly selective oral B-cell leukemia/lymphoma 2 inhibitor, in combination with hypomethylating agents such as azacitidine, has been approved for the treatment of newly diagnosed acute myeloid leukemia (AML) in patients who are ineligible for intensive chemotherapy. An open-label, nonrandomized phase 1b trial (NCT02203773) and the confirmatory phase 3 VIALE-A randomized trial (NCT02993523) demonstrated that venetoclax-azacitidine led to high rates of complete remission (CR) and long overall survival (OS).1,2 In the VIALE-A trial, the median OS was 14.7 months with venetoclax-azacitidine and 9.6 months with placebo-azacitidine.3
Outcomes for patients with AML vary according to the commonly occurring cytogenetic and molecular features.4,5 The European LeukemiaNet (ELN) 2017 and 2022 recommendations for the diagnosis and management of AML include 3 prognostic groups (favorable, intermediate, and adverse) based on cytogenetic and molecular disease characteristics. The patients who were used to derive these models were younger and were treated with traditional intensive chemotherapy regimens with or without allogeneic stem cell transplantation.6,7 Therefore, the ELN risk classification may not be applicable to patients receiving venetoclax-azacitidine.
The objective of this exploratory post hoc pooled analysis was to assess the ability of the ELN 2017 and 2022 classification systems to risk-stratify newly diagnosed patients with AML who were ineligible for intensive chemotherapy and who were treated with venetoclax-azacitidine. Owing to the suboptimal performance of the ELN risk classifiers in the setting of venetoclax-azacitidine therapy, we endeavored to develop a more discriminatory prognostic framework based on molecular features to improve the risk stratification of OS outcomes for patients receiving venetoclax-azacitidine.
Methods
Patients and study design
This pooled analysis included patients from an open-label, nonrandomized phase 1b trial (NCT02203773) and the VIALE-A phase 3 randomized trial (NCT02993523). The study designs and eligibility criteria have been previously described.1,2 All patients received a standard dose of azacitidine (75 mg/m2 administered either subcutaneously or IV on days 1-7 every 28-day cycle). Venetoclax (400 mg) or placebo was administered orally, once daily, for 28-day cycles. Detailed information on the venetoclax dose has been previously reported.1,2 In both trials, enrolled patients had a confirmed diagnosis of AML per the World Health Organization 2008 criteria8 and were ineligible for intensive chemotherapy due to age ≥75 years or the presence of comorbidities. Patients with favorable-risk cytogenetics, such as t(8;21), inv(16), t(16;16), or t(15;17), per the National Comprehensive Cancer Network (NCCN) Guidelines version 2.2016, were excluded.
Both studies were approved by the local ethics committees and conducted in accordance with the International Conference on Harmonization, Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Outcomes
Response (CR or CR with incomplete hematologic remission [CRi]) was assessed at the end of cycle 1 and every 3 cycles thereafter, and evaluated per the modified International Working Group criteria for AML.9 OS was defined as the time from randomization in the VIALE-A study or the number of days from the first dose of the study drug in the phase 1b study to the date of death from any cause. The safety analyses included patients who received ≥1 dose of azacitidine or venetoclax. Adverse events (AEs) were graded based on the National Cancer Institute Common Terminology Criteria for AEs version 4.03. Measurable residual disease (MRD) response was defined as patients who achieved CR or CRi and demonstrated bone marrow MRD assessment with <1 residual blast per 1000 leukocytes (<10–3 or 0.1% as centrally assessed by multiparameter flow cytometry); rates were reported based on intention-to-treat populations.1
Molecular assessments
The baseline cytogenetic risk was determined locally and evaluated using the NCCN criteria (version 2.2016). DNA isolated from the bone marrow aspirates was collected before the first study drug dose. Mutations were analyzed centrally using MyAML (next-generation sequencing; Invivoscribe, San Diego, CA), TruSight Myeloid panel (Illumina, San Diego, CA), or Foundation One Heme panel (Foundation Medicine, Cambridge, MA). Patients with missing specimens or inconclusive results (specimens were not required for patients enrolled in China, which included 24 patients treated with venetoclax-azacitidine and 13 patients treated with placebo-azacitidine; no specimen was received for 16 patients in the venetoclax-azacitidine cohort and 5 patients in the placebo-azacitidine cohort; insufficient quantity of DNA was isolated for testing for 33 patients treated with venetoclax-azacitidine and 13 patients treated with placebo-azacitidine; and the specimen was mishandled by the central laboratory, 1 patient in each cohort) were excluded from the analysis. The inclusion of central molecular data allowed the reclassification of patients according to the ELN 2017 and 2022 recommendations.
Alternative prognostic model
Sequential multivariate extension of bootstrapping and aggregation of thresholds from trees (BATTing10) was used to derive molecular signatures able to differentiate survival outcomes in patients treated with venetoclax-azacitidine. Sequential BATTing is a subgroup identification method that identifies a desired subgroup based on P values for comparing signature-positive to signature-negative groups constructed using prespecified predictors. When constructing the benefit signature in this study, the sequential BATTing method was applied twice: first to identify the higher-benefit groups and separately, and subsequently, to identify the lower-benefit groups. Patients who did not belong to either the high- or low-benefit groups were assigned to the intermediate-benefit group. The sequence was as follows: (1) Identify the subgroup with the longest OS, (2) Identify the subgroup with the shortest OS, and (3) Place patients who do not belong to either group into an intermediate group. This method distinguishes subgroups by minimizing the hazard ratio (HR) P value for the selected groups compared with the remaining population. Although 31 genetic markers included in ELN 2022 recommendations and/or genes with prevalence ≥10% in the analysis population of venetoclax-azacitidine–treated patients were considered, only 29 were present in >1 patient and were included as candidate predictors to define prognostic risk molecular signatures for each OS benefit subgroup (supplemental Table 1, available on the Blood website). The candidate predictors were classified as either present (mutation detected or yes) or absent (wild-type [WT] or no).
Statistical analysis
The remission rates were summarized by descriptive statistics. OS was evaluated using Kaplan-Meier methodology, and HRs between treatment groups were estimated using the Cox proportional-hazards model adjusted for age, AML type, and cytogenetic risk (when evaluating benefit groups). The 95% confidence intervals (CIs) are reported for HRs and OS.
Results
Patients
This pooled analysis included 279 patients treated with venetoclax-azacitidine and 113 patients treated with placebo-azacitidine (supplemental Figure 1). The data cutoff was 1 December 2021 for VIALE-A and 19 July 2019 for the phase 1b study. The median duration of follow-up for patients in the pooled analysis was 42.7 months (95% CI, 40.8-44.2).
Risk reclassification by ELN recommendations
For the trials, patients were enrolled and classified according to the NCCN Guidelines version 2.2016 for risk stratification: 175 (62.7%) and 70 (61.9%) patients had intermediate-risk AML, and 104 (37.3%) and 43 (38.1%) patients had poor-risk AML in the venetoclax-azacitidine and placebo-azacitidine groups, respectively.
Reclassification according to the ELN 2017 and 2022 recommendations placed most patients into the adverse-risk category (supplemental Figure 2). According to ELN 2017, in the venetoclax-azacitidine and placebo-azacitidine groups, 46 (16.5%) and 20 (17.7%) patients had favorable-risk AML, 65 (23.3%) and 19 (16.8%) patients had intermediate-risk AML, and 168 (60.2%) and 74 (65.5%) patients had adverse-risk AML, respectively. According to ELN 2022, in the venetoclax-azacitidine and placebo-azacitidine groups, 35 (12.5%) and 13 (11.5%) patients had favorable-risk AML, 41 (14.7%) and 15 (13.3%) had intermediate-risk AML, and 203 (72.8%) and 85 (75.2%) had adverse-risk AML, respectively.
Venetoclax-azacitidine outcomes according to ELN 2017 and 2022 risk categories
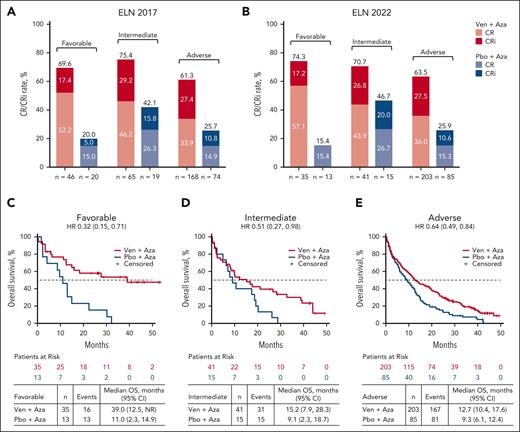
Response rates were improved with venetoclax-azacitidine vs placebo-azacitidine across all ELN prognostic risk groups. Using the ELN 2022 response criteria, CR/CRi with venetoclax-azacitidine vs placebo-azacitidine was achieved in 26 of 35 patients (74.3%) vs 2 of 13 patients (15.4%) in the favorable-risk group, 29 of 41 (70.7%) vs 7 of 15 (46.7%) in the intermediate-risk group, and 129 of 203 (63.5%) vs 22 of 85 (25.9%) in the adverse-risk group. Response rates were similarly improved with venetoclax-azacitidine vs placebo-azacitidine in the risk groups defined by the ELN in 2017 (Figure 1A-B).
Response rates with Ven-Aza or Pbo-Aza in favorable-, intermediate-, and adverse-risk groups. CR/CRi rate in patients treated with Ven-Aza or Pbo-Aza by (A) ELN 2017 and (B) ELN 2022. OS in patients treated with Ven-Aza or Pbo-Aza in the ELN 2022 (C) favorable-, (D) intermediate-, and (E) adverse-risk groups. Aza, azacitidine; NR, not reached; Pbo, placebo; Ven, venetoclax.
Response rates with Ven-Aza or Pbo-Aza in favorable-, intermediate-, and adverse-risk groups. CR/CRi rate in patients treated with Ven-Aza or Pbo-Aza by (A) ELN 2017 and (B) ELN 2022. OS in patients treated with Ven-Aza or Pbo-Aza in the ELN 2022 (C) favorable-, (D) intermediate-, and (E) adverse-risk groups. Aza, azacitidine; NR, not reached; Pbo, placebo; Ven, venetoclax.
OS outcomes also improved with venetoclax-azacitidine vs placebo-azacitidine across all ELN risk groups. Using ELN 2022 criteria, the median OS with venetoclax-azacitidine vs placebo-azacitidine was 39.0 vs 11.0 months (HR, 0.32; 95% CI, 0.15-0.71) in the favorable-risk group, 15.2 vs 9.1 months (HR, 0.51; 95% CI, 0.27-0.98) in the intermediate-risk group, and 12.7 vs 9.3 months (HR, 0.64; 95% CI, 0.49-0.84) in the adverse-risk group (Figure 1C-E). Similarly, the median OS was improved with venetoclax-azacitidine vs placebo-azacitidine across all risk groups per ELN 2017 (supplemental Figure 3).
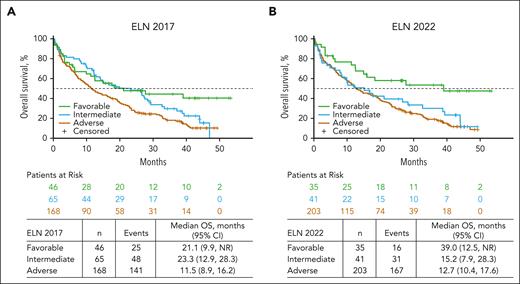
However, when analyzing only patients who received venetoclax-azacitidine, OS outcomes were similar for patients with favorable- and intermediate-risk AML by ELN 2017 (median OS, 21.1 months [95% CI, 9.9 to not reached] and 23.3 months [95% CI, 12.9-28.3], respectively). Additionally, per ELN 2022, OS outcomes were similar for patients treated with venetoclax-azacitidine who had intermediate- and adverse-risk AML (median OS, 15.2 months [95% CI, 7.9-28.3] and 12.7 months [95% CI, 10.4-17.6], respectively; Figure 2). These findings suggest that neither the ELN 2017 nor 2022 risk criteria satisfactorily distinguished outcomes among patients with AML treated with venetoclax-azacitidine.
OS in patients treated with Ven-Aza in favorable-, intermediate-, and adverse-risk groups. OS in patients treated with Ven-Aza by risk group per (A) ELN 2017 and (B) ELN 2022.
OS in patients treated with Ven-Aza in favorable-, intermediate-, and adverse-risk groups. OS in patients treated with Ven-Aza by risk group per (A) ELN 2017 and (B) ELN 2022.
Identification of genetic signatures for response to venetoclax-azacitidine
Subgroups of patients distinguished by OS benefit with venetoclax-azacitidine were analyzed to evaluate the prognostic significance of different genetic factors. Candidate predictors included in this analysis were genetic markers in the ELN 2022 recommendations and additional genes (TET2, DNMT3A, IDH1, IDH2, FLT3-TKD, and pooling of KRAS/NRAS) that were detected in ≥10% of the pooled cohort (supplemental Table 1).
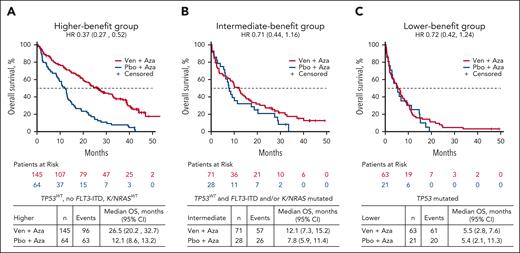
Prognostic outcome after venetoclax-azacitidine could be defined by the mutational status of only 4 genes in patients receiving venetoclax-azacitidine, demarcating patients into 3 distinct benefit groups. Patients in the higher-benefit group had WT TP53 and KRAS/NRAS and lacked FLT3 internal tandem duplication (FLT3-ITD); the intermediate-benefit group had WT TP53 but had FLT3-ITD and/or KRAS/NRAS mutation; and the lower-benefit group was positive for TP53 mutation (Table 1). Sensitivity analysis was conducted to determine the impact of the TP53 variant allele frequency (VAF) on the prognostic signature for the lower-benefit group. The analysis revealed no significant differences in the median OS between patients with TP53 VAF of ≥10% and those with TP53 VAF of <10% (supplemental Figure 4).
Characteristics, mutational status, and cytogenetics of patients treated with venetoclax-azacitidine or placebo-azacitidine in the higher-, intermediate-, and lower-benefit groups
| . | Ven + Aza . | Pbo + Aza . | ||||
|---|---|---|---|---|---|---|
| Higher benefit (n = 145) . | Intermediate benefit (n = 71) . | Lower benefit (n = 63) . | Higher benefit (n = 64) . | Intermediate benefit (n = 28) . | Lower benefit (n = 21) . | |
| Baseline characteristics | ||||||
| Median age, y | 77 | 76 | 77 | 77 | 75 | 75 |
| Age group, n (%) | ||||||
| <75 y | 58 (40) | 27 (38) | 24 (38.1) | 20 (31.2) | 14 (50) | 11 (52.4) |
| ≥75 y | 87 (60) | 44 (62) | 39 (61.9) | 44 (68.8) | 14 (50) | 10 (47.6) |
| AML type, n (%) | ||||||
| Primary | 105 (72.4) | 53 (74.6) | 46 (73) | 45 (70.3) | 24 (85.7) | 15 (71.4) |
| Secondary | 40 (27.6) | 18 (25.4) | 17 (27) | 19 (29.7) | 4 (14.3) | 6 (28.6) |
| ECOG PS, n (%) | ||||||
| <2 | 90 (62.1) | 37 (52.1) | 36 (57.1) | 44 (68.8) | 13 (46.4) | 12 (57.1) |
| ≥2 | 55 (37.9) | 34 (47.9) | 27 (42.9) | 20 (31.2) | 15 (53.6) | 9 (42.9) |
| % Blasts, n (%) | ||||||
| <30% | 37 (25.5) | 15 (21.1) | 28 (44.4) | 18 (28.1) | 4 (14.3) | 10 (47.6) |
| ≥30% to <50% | 38 (26.2) | 10 (14.1) | 13 (20.6) | 15 (23.4) | 6 (21.4) | 5 (23.8) |
| ≥50% | 70 (48.3) | 46 (64.8) | 22 (34.9) | 31 (48.4) | 18 (64.3) | 6 (28.6) |
| Mutations, n (%) | ||||||
| FLT3-ITD | 0 | 39 (54.9) | 4 (6.3) | 0 | 21 (75) | 0 |
| NRAS | 0 | 28 (39.4) | 5 (7.9) | 0 | 7 (25) | 1 (4.8) |
| KRAS | 0 | 11 (15.5) | 2 (3.2) | 0 | 2 (7.1) | 0 |
| TP53 | 0 | 0 | 63 (100) | 0 | 0 | 21 (100) |
| NPM1 | 22 (15.2) | 23 (32.4) | 1 (1.6) | 13 (20.3) | 7 (25) | 0 |
| NPM1/FLT3-ITD negative | 22 (15.2) | 7 (9.9) | 1 (1.6) | 13 (20.3) | 0 | 0 |
| NPM1/FLT3-ITD positive | 0 | 16 (22.5) | 0 | 0 | 7 (25) | 0 |
| IDH1/2 | 53 (36.6) | 21 (29.6) | 3 (4.8) | 19 (29.7) | 4 (14.3) | 0 |
| IDH1 | 22 (15.2) | 9 (12.7) | 1 (1.6) | 7 (10.9) | 2 (7.1) | 0 |
| IDH2 | 32 (22.1) | 13 (18.3) | 2 (3.2) | 13 (20.3) | 2 (7.1) | 0 |
| FLT3-TKD | 12 (8.3) | 3 (4.2) | 1 (1.6) | 9 (14.1) | 2 (7.1) | 1 (4.8) |
| DNMT3A | 40 (27.6) | 25 (35.2) | 7 (11.1) | 15 (23.4) | 13 (46.4) | 4 (19) |
| TET2 | 48 (33.1) | 29 (40.8) | 4 (6.3) | 24 (37.5) | 8 (28.6) | 3 (14.3) |
| ASXL1 | 18 (12.4) | 12 (16.9) | 5 (7.9) | 18 (28.1) | 7 (25) | 2 (9.5) |
| BCOR | 15 (10.3) | 13 (18.3) | 1 (1.6) | 9 (14.1) | 5 (17.9) | 1 (4.8) |
| EZH2 | 8 (5.5) | 6 (8.5) | 2 (3.2) | 6 (9.4) | 0 | 0 |
| RUNX1 | 43 (29.7) | 25 (35.2) | 2 (3.2) | 17 (26.6) | 9 (32.1) | 4 (19) |
| SF3B1 | 16 (11.0) | 6 (8.5) | 1 (1.6) | 5 (7.8) | 3 (10.7) | 1 (4.8) |
| SRSF2 | 42 (29.0) | 16 (22.5) | 4 (6.3) | 22 (34.4) | 8 (28.6) | 4 (19) |
| STAG2 | 21 (14.5) | 11 (15.5) | 2 (3.2) | 13 (20.3) | 7 (25) | 4 (19) |
| U2AF1 | 16 (11.0) | 7 (9.9) | 3 (4.8) | 3 (4.7) | 3 (10.7) | 0 |
| CEBPA | 8 (5.5) | 5 (7.0) | 0 | 7 (10.9) | 4 (14.3) | 1 (4.8) |
| ZRSR2 | 4 (2.8) | 2 (2.8) | 0 | 2 (3.1) | 0 | 0 |
| CEBPA-bZip | 4 (2.8) | 0 | 0 | 2 (3.1) | 1 (3.6) | 0 |
| Cytogenetics, n (%) | ||||||
| Complex karyotype | 19 (13.1) | 3 (4.2) | 50 (79.4) | 6 (9.4) | 1 (3.6) | 18 (85.7) |
| del(5) | 9 (6.2) | 0 | 40 (63.5) | 4 (6.2) | 0 | 15 (71.4) |
| del(7) | 20 (13.9) | 6 (8.5) | 22 (34.9) | 11 (17.2) | 2 (7.1) | 8 (38.1) |
| del(17) | 2 (1.4) | 0 | 13 (20.6) | 0 | 1 (3.6) | 4 (19) |
| t(v;11q23) | 2 (1.4) | 0 | 5 (7.9) | 2 (3.1) | 0 | 1 (4.8) |
| . | Ven + Aza . | Pbo + Aza . | ||||
|---|---|---|---|---|---|---|
| Higher benefit (n = 145) . | Intermediate benefit (n = 71) . | Lower benefit (n = 63) . | Higher benefit (n = 64) . | Intermediate benefit (n = 28) . | Lower benefit (n = 21) . | |
| Baseline characteristics | ||||||
| Median age, y | 77 | 76 | 77 | 77 | 75 | 75 |
| Age group, n (%) | ||||||
| <75 y | 58 (40) | 27 (38) | 24 (38.1) | 20 (31.2) | 14 (50) | 11 (52.4) |
| ≥75 y | 87 (60) | 44 (62) | 39 (61.9) | 44 (68.8) | 14 (50) | 10 (47.6) |
| AML type, n (%) | ||||||
| Primary | 105 (72.4) | 53 (74.6) | 46 (73) | 45 (70.3) | 24 (85.7) | 15 (71.4) |
| Secondary | 40 (27.6) | 18 (25.4) | 17 (27) | 19 (29.7) | 4 (14.3) | 6 (28.6) |
| ECOG PS, n (%) | ||||||
| <2 | 90 (62.1) | 37 (52.1) | 36 (57.1) | 44 (68.8) | 13 (46.4) | 12 (57.1) |
| ≥2 | 55 (37.9) | 34 (47.9) | 27 (42.9) | 20 (31.2) | 15 (53.6) | 9 (42.9) |
| % Blasts, n (%) | ||||||
| <30% | 37 (25.5) | 15 (21.1) | 28 (44.4) | 18 (28.1) | 4 (14.3) | 10 (47.6) |
| ≥30% to <50% | 38 (26.2) | 10 (14.1) | 13 (20.6) | 15 (23.4) | 6 (21.4) | 5 (23.8) |
| ≥50% | 70 (48.3) | 46 (64.8) | 22 (34.9) | 31 (48.4) | 18 (64.3) | 6 (28.6) |
| Mutations, n (%) | ||||||
| FLT3-ITD | 0 | 39 (54.9) | 4 (6.3) | 0 | 21 (75) | 0 |
| NRAS | 0 | 28 (39.4) | 5 (7.9) | 0 | 7 (25) | 1 (4.8) |
| KRAS | 0 | 11 (15.5) | 2 (3.2) | 0 | 2 (7.1) | 0 |
| TP53 | 0 | 0 | 63 (100) | 0 | 0 | 21 (100) |
| NPM1 | 22 (15.2) | 23 (32.4) | 1 (1.6) | 13 (20.3) | 7 (25) | 0 |
| NPM1/FLT3-ITD negative | 22 (15.2) | 7 (9.9) | 1 (1.6) | 13 (20.3) | 0 | 0 |
| NPM1/FLT3-ITD positive | 0 | 16 (22.5) | 0 | 0 | 7 (25) | 0 |
| IDH1/2 | 53 (36.6) | 21 (29.6) | 3 (4.8) | 19 (29.7) | 4 (14.3) | 0 |
| IDH1 | 22 (15.2) | 9 (12.7) | 1 (1.6) | 7 (10.9) | 2 (7.1) | 0 |
| IDH2 | 32 (22.1) | 13 (18.3) | 2 (3.2) | 13 (20.3) | 2 (7.1) | 0 |
| FLT3-TKD | 12 (8.3) | 3 (4.2) | 1 (1.6) | 9 (14.1) | 2 (7.1) | 1 (4.8) |
| DNMT3A | 40 (27.6) | 25 (35.2) | 7 (11.1) | 15 (23.4) | 13 (46.4) | 4 (19) |
| TET2 | 48 (33.1) | 29 (40.8) | 4 (6.3) | 24 (37.5) | 8 (28.6) | 3 (14.3) |
| ASXL1 | 18 (12.4) | 12 (16.9) | 5 (7.9) | 18 (28.1) | 7 (25) | 2 (9.5) |
| BCOR | 15 (10.3) | 13 (18.3) | 1 (1.6) | 9 (14.1) | 5 (17.9) | 1 (4.8) |
| EZH2 | 8 (5.5) | 6 (8.5) | 2 (3.2) | 6 (9.4) | 0 | 0 |
| RUNX1 | 43 (29.7) | 25 (35.2) | 2 (3.2) | 17 (26.6) | 9 (32.1) | 4 (19) |
| SF3B1 | 16 (11.0) | 6 (8.5) | 1 (1.6) | 5 (7.8) | 3 (10.7) | 1 (4.8) |
| SRSF2 | 42 (29.0) | 16 (22.5) | 4 (6.3) | 22 (34.4) | 8 (28.6) | 4 (19) |
| STAG2 | 21 (14.5) | 11 (15.5) | 2 (3.2) | 13 (20.3) | 7 (25) | 4 (19) |
| U2AF1 | 16 (11.0) | 7 (9.9) | 3 (4.8) | 3 (4.7) | 3 (10.7) | 0 |
| CEBPA | 8 (5.5) | 5 (7.0) | 0 | 7 (10.9) | 4 (14.3) | 1 (4.8) |
| ZRSR2 | 4 (2.8) | 2 (2.8) | 0 | 2 (3.1) | 0 | 0 |
| CEBPA-bZip | 4 (2.8) | 0 | 0 | 2 (3.1) | 1 (3.6) | 0 |
| Cytogenetics, n (%) | ||||||
| Complex karyotype | 19 (13.1) | 3 (4.2) | 50 (79.4) | 6 (9.4) | 1 (3.6) | 18 (85.7) |
| del(5) | 9 (6.2) | 0 | 40 (63.5) | 4 (6.2) | 0 | 15 (71.4) |
| del(7) | 20 (13.9) | 6 (8.5) | 22 (34.9) | 11 (17.2) | 2 (7.1) | 8 (38.1) |
| del(17) | 2 (1.4) | 0 | 13 (20.6) | 0 | 1 (3.6) | 4 (19) |
| t(v;11q23) | 2 (1.4) | 0 | 5 (7.9) | 2 (3.1) | 0 | 1 (4.8) |
Aza, azacitidine; ECOG PS, Eastern Cooperative Oncology Group performance status; Pbo, placebo; TKD, tyrosine kinase domain; Ven, venetoclax.
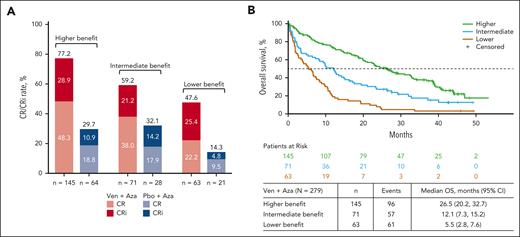
Most patients (52.0% [145/279]) in the venetoclax-azacitidine group were in the higher-benefit group, whereas the remainder were distributed uniformly between the intermediate- and lower-benefit groups (25.4% [71/279] and 22.6% [63/279], respectively).
CR + CRi rates with venetoclax-azacitidine were 77.2% in the higher-benefit group, 59.2% in the intermediate-benefit group, and 47.6% in the lower-benefit subgroup (Figure 3A). The time to first CR/CRi with venetoclax-azacitidine was 1.3 months, 1.2 months, and 1.4 months in the higher-, intermediate-, and lower-benefit groups, respectively. The median OS after venetoclax-azacitidine was 26.5 (95% CI, 20.2-32.7), 12.1 (95% CI, 7.3-15.2), and 5.5 months (95% CI, 2.8-7.6) in the higher-, intermediate-, and lower-benefit groups, respectively (Figure 3B).
Response in patients treated with Ven-Aza or Pbo-Aza by risk stratification. (A) Remission rates in patients treated with Ven-Aza or Pbo-Aza in the higher-, intermediate-, and lower-benefit groups. (B) OS in patients treated with Ven-Aza in the higher-, intermediate-, and lower-benefit groups.
Response in patients treated with Ven-Aza or Pbo-Aza by risk stratification. (A) Remission rates in patients treated with Ven-Aza or Pbo-Aza in the higher-, intermediate-, and lower-benefit groups. (B) OS in patients treated with Ven-Aza in the higher-, intermediate-, and lower-benefit groups.
Hematologic remission rates were higher with venetoclax-azacitidine than with placebo-azacitidine across all 3 benefit groups (Figure 3A). The median OS was notably longer with venetoclax-azacitidine than with placebo-azacitidine in the higher- and intermediate-benefit groups, whereas it was similar for venetoclax-azacitidine and placebo-azacitidine in the TP53-mutated lower-benefit group (Figure 4). A higher MRD response rate was achieved with venetoclax-azacitidine than with placebo-azacitidine across all 3 benefit groups (supplemental Table 2).
OS in patients treated with Ven-Aza or Pbo-Aza by risk stratification. (A) Higher-, (B) intermediate-, and (C) lower-benefit groups.
OS in patients treated with Ven-Aza or Pbo-Aza by risk stratification. (A) Higher-, (B) intermediate-, and (C) lower-benefit groups.
The lower-benefit TP53 mutation group had fewer co-occurring recurrent AML-associated mutations and more frequent adverse-risk cytogenetic abnormalities (complex karyotype, del[5], del[7], and del[17]) than the higher- and intermediate-benefit groups (Table 1; supplemental Figures 5-7). In contrast, patients in the higher- and intermediate-benefit groups had a diversity of biological drivers of AML, as classified by the International Consensus Classification (ICC) (supplemental Figures 5 and 6). In both groups, the number of AML with myelodysplasia-related gene mutations was the largest, followed by AML with NPM1 mutations as the second largest. All 4 patients with CEBPA-bZip mutations were in the higher-benefit group. Nearly all patients in the lower-benefit group were designated as having AML with TP53 mutation by the ICC convention (supplemental Table 3).
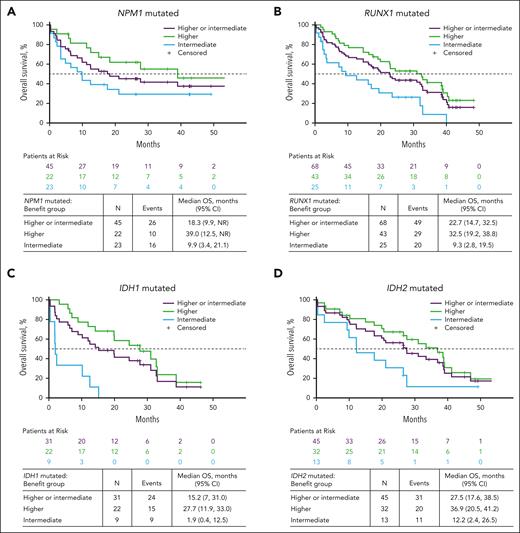
Given recent reports of particularly favorable outcomes with venetoclax-based therapies for patients with NPM1, RUNX1, IDH2, and IDH1 mutations,11 we evaluated the outcomes based on the presence or absence of these 4 recurrent mutations. Notably, all 4 mutations were identified in all 3 benefit groups. For patients with an IDH2 mutation, those in the higher-benefit group had a median OS of 36.9 months (95% CI, 20.5-41.2) vs 12.2 months (95% CI, 2.4-26.5) in the intermediate-benefit group. Similar differences were observed in patients with either a RUNX1 or NPM1 mutation: patients with a RUNX1 mutation in the higher-benefit group had a median OS of 32.5 months (95% CI, 19.2-38.8) vs 9.3 months (95% CI, 2.8-19.5) in the intermediate-benefit group, and patients with an NPM1 mutation in the higher-benefit group had a median OS of 39.0 months (95% CI, 12.5 to not reached) vs 9.9 months (95% CI, 3.4-21.1) in the intermediate-benefit group. Lower median OS was observed for patients with an IDH1 mutation: the median OS of the higher-benefit group was 27.7 months (95% CI, 11.9-33.0) vs 1.9 months (95% CI, 0.4-12.5) for patients in the intermediate-benefit group (Figure 5A-D; supplemental Figure 8). For patients with myelodysplasia-related gene mutations, the median OS was 22.9 months (95% CI, 19.2-31.0) in the higher-benefit group and 12.9 months (95% CI, 7.3-23.3) in the intermediate-benefit group (supplemental Figure 8). CR + CRi rate differences were also observed between the higher- and intermediate-benefit groups with IDH2, IDH1/2, RUNX1, NPM1, and IDH1 mutations: 90.6%, 86.8%, 83.7%, 81.8%, and 81.8% vs 69.2%, 57.1%, 60.0%, 56.5%, and 33.3%, respectively (supplemental Figure 9).
OS in patients treated with Ven-Aza in the higher- and intermediate-benefit groups with recurrent mutations. Data are for patients with (A) NPM1 mutations, (B) RUNX1 mutations, (C) IDH1 mutations, and (D) IDH2 mutations.
OS in patients treated with Ven-Aza in the higher- and intermediate-benefit groups with recurrent mutations. Data are for patients with (A) NPM1 mutations, (B) RUNX1 mutations, (C) IDH1 mutations, and (D) IDH2 mutations.
In an analysis of clinical factors, patients treated with venetoclax-azacitidine in the higher-benefit group by age ≥75 vs <75 years appeared similar, with a median OS of 27.5 months (95% CI, 20.5-39.0) vs 23.4 months (95% CI, 15.0-32.5), respectively (supplemental Table 4). Males had a median OS of 26.6 months (95% CI, 19.2-32.7) compared with 22.9 months (95% CI, 18.7-39.0) for females. The median OS was similar for Eastern Cooperative Oncology Group performance status (ECOG PS) (26.5 months [95% CI, 19.8-34.1] vs 27.2 months [95% CI, 17.0-33.0] for ECOG PS <2 and ≥2, respectively) and % blasts (26.6 months [95% CI, 19.9-38.5] vs 26.5 months [95% CI, 16.4-33.0] for <50% blasts and ≥50% blasts, respectively). In a multivariable analysis including genetic (TP53, FLT3-ITD, and K/N RAS status) and clinical variables (age, AML type, baseline blast counts, and ECOG PS), the genetic variables identified in the prognostic model were statistically significant for OS, but the clinical features were not associated (supplemental Table 5).
Safety analysis
The safety analysis included 275 patients treated with venetoclax-azacitidine and 112 with placebo-azacitidine. Overall rates of grade ≥3 treatment-emergent AEs (TEAEs) and serious TEAEs were similar between treatment groups and across benefit groups. Higher rates of hematologic TEAEs, including grade ≥3 neutropenia and febrile neutropenia, were observed in the venetoclax-azacitidine vs placebo-azacitidine group, irrespective of the benefit group. Death (including non-TEAE causes) was the highest in the lower-benefit group and the lowest in the higher-benefit group in both treatment groups. Although the numbers were small, early death rates were similar on day 30 for venetoclax-azacitidine and placebo-azacitidine across all subgroups. On day 60, there was a numerically higher death rate for patients treated with venetoclax-azacitidine in the lower-benefit group than in the higher- and intermediate-benefit groups (supplemental Table 6).
Discussion
This pooled analysis retrospectively applied ELN risk categories to patients with newly diagnosed AML ineligible for intensive chemotherapy from the phase 1b and VIALE-A studies to determine whether these risk categories were prognostically relevant for patients receiving venetoclax-azacitidine therapy, a question that has gained interest since the approval of this combination. Several recent single-center/regional retrospective studies have aimed to address this question and have identified prognostic risk categories associated with venetoclax-azacitidine in patients with intensive chemotherapy–ineligible AML.12-14 Our study is the first, to our knowledge, to identify a prognostic risk classification in patients who were treated with venetoclax-azacitidine while enrolled in large, global clinical trials.
The response to therapy for patients with AML varies according to genetic and clinical variables, with genomic alterations explaining approximately two-thirds of survival outcome variation and the other third attributable to demographic, clinical, and treatment variables.4-6 The ELN 2017 recommendations stratified AML risk into 3 categories (favorable, intermediate, and adverse).6 In 2022, ELN recommendations for risk stratification were revised to include recurrent AML-defining genetic abnormalities, TP53-mutated AML, myelodysplasia-related gene mutations (BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2), or myelodysplasia-related cytogenetic abnormalities.
The widely used genetic risk stratification systems (such as ELN or NCCN systems) are largely based on outcomes observed after intensive chemotherapy, predominantly in younger patients.4,5 Venetoclax-azacitidine is considered a new standard of care for patients with AML who are ineligible for intensive chemotherapy across biological risk groups.7 In a recent study from the ASTRAL-1 trial, the 2017 and 2022 ELN genetic risk classifications were unable to discriminate outcomes in older, unfit patients treated with hypomethylating agent monotherapy.15 Another hypomethylating agent monotherapy study retrospectively applied ELN risk stratification to survival outcomes and reported that, although ELN 2022 was better able to stratify risk groups compared with ELN 2017, the only statistically significant difference among the ELN 2022 groups was between the favorable and adverse groups.16 Such risk stratification systems for predicting outcomes in patients ineligible for intensive chemotherapy and treated with venetoclax-azacitidine have recently been reported to be suboptimal.12,14
In this analysis, risk reclassification by the ELN 2017 or 2022 criteria resulted in most patients being recategorized from intermediate- to adverse-risk AML. The increase in the size of the adverse-risk group was largely driven by the myelodysplasia-related genetic signature genes RUNX1 and ASXL1 (ELN 2017) and BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, and ZRSR2 (ELN 2022). In comparison, patients with favorable risk by ELN 2022 were those with NPM1 mutations without concomitant presence of FLT3-ITD.
We observed that although venetoclax-azacitidine improved remission rates and OS compared with placebo-azacitidine in all genetic risk groups in both ELN 2017 and 2022, risk stratification based on ELN 2017 and 2022 was suboptimal for assessing outcomes after venetoclax-azacitidine. In particular, OS outcomes with venetoclax-azacitidine were similar for favorable- and intermediate-risk groups by ELN 2017 and for intermediate- and adverse-risk groups by ELN 2022, highlighting the need for a new risk classification for this therapy.
In this exploratory analysis of venetoclax-azacitidine–treated patients, we identified mutational signatures in 3 subgroups defined by OS benefit. Clinical factors did not appear to drive this prognostic model. The higher-benefit group represented slightly more than half of the enrolled patients and comprised all patients without TP53 mutations, signaling mutations (KRAS/NRAS), or FLT3-ITD. AML with myelodysplasia-related gene mutations was the largest component in this group (60%), followed by patients with NPM1 mutations without FLT3-ITD (15%). Among patients in the higher-benefit group, median OS outcomes were notable for NPM1 (39.0 months), IDH2 (36.9 months), RUNX1 (32.5 months), and IDH1 (27.7 months) mutated disease (Figure 5). Both NPM1 and IDH1/2 have previously been suggested to be sensitive to treatment with venetoclax-based regimens.17 Recently, Bataller et al retrospectively evaluated the outcomes of 159 patients treated with venetoclax plus hypomethylating agents at a single center and reported a similar lack of clear separation/prognostication of the ELN 2017 or 2022 criteria. Furthermore, the authors validated the proposed 4-gene predictive signature in their independent cohort.12
Our study provides further evidence that these mutations are associated with a favorable outcome in venetoclax-azacitidine–treated patients, with most benefit restricted to patients without concomitant signaling mutations, such as FLT3-ITD and/or NRAS/KRAS mutation (Figure 5). For example, NPM1-mutated AML without signaling mutations had a median survival of 39.0 months compared with only 9.9 months for NPM1-mutated AML with co-occurring FLT3-ITD or RAS mutations. Signaling mutations are known to confer resistance to venetoclax and likely explain why the NPM1 mutation, as a single gene, was not identified in the predictive signature.18
Although RUNX1 was identified as a poor prognostic marker in the ELN 2017 and ELN 2022 risk classifications,6,7 it was not a poor prognostic marker in the current study, as shown in Figure 5, consistent with other recent findings.19,20 The intermediate-benefit group comprised patients with WT TP53 but with the presence of FLT3-ITD and/or KRAS/NRAS mutations. Venetoclax-azacitidine did not enhance survival outcomes among patients with FLT3-ITD, as has been reported elsewhere,21 and appears less favorable in patients with KRAS/NRAS mutations, possibly due to enhanced expression of prosurvival partners such as MCL1.21,22 Patients in the lower-benefit group comprised those with TP53 mutation in whom venetoclax-azacitidine improved response rates compared with placebo-azacitidine but did not prolong survival.
AML with myelodysplasia-related gene mutations in both the higher- and intermediate-benefit groups represented the largest subset of AML categories per the ICC. This is remarkable given the fact that these mutations have been associated with poor outcomes in the context of intensive chemotherapy. These benefits of venetoclax have also recently been suggested by a study evaluating the impact of AML ontogeny on outcomes with venetoclax.23
Outcomes with venetoclax-azacitidine in patients with TP53 mutation were previously studied in this pooled group of patients as well as in other studies.24 Similar to the findings reported here, patients with TP53 mutation treated with venetoclax-azacitidine had improved remission rates but no prolonged duration of response or OS benefit compared with hypomethylating agent monotherapy.
A limitation of this study was that 10 of the candidate predictor genetic markers used to evaluate prognostic subgroups had low (<10%) prevalence among patients treated with venetoclax-azacitidine and thus may have been too rare to identify a signal in this patient population. Additionally, this study excluded patients with prior myeloproliferative neoplasms or prior hypomethylating agent therapy for myelodysplastic syndrome.
Thus, although this 4-gene signature for venetoclax-azacitidine–treated patients provided excellent outcome stratification into 3 distinct benefit groups, this classification warrants further validation in independent data sets. This especially applies to the high-risk patient subset, given the treatment challenges with this group and the current lack of any superior treatment approach. Treatment with venetoclax-azacitidine may still be appropriate despite the lack of OS benefit due to the improved initial composite remission rates, potentially improving quality of life and/or allowing consolidation with allogeneic transplantation in selected high-risk patients.
In conclusion, the 2017 and 2022 ELN genetic risk groups did not provide clinically meaningful stratification of OS for treatment-naive patients with AML ineligible for intensive chemotherapy treated with venetoclax-azacitidine. A novel alternative mutational model was developed based on 3 prognostic risk signatures, defined by the mutational status of just 4 genes (FLT3-ITD, KRAS, NRAS, and TP53), corresponding with higher benefit, intermediate benefit, and lower benefit with venetoclax-azacitidine. The major advantage of this risk model is that it is simple and thus widely applicable to patients in the community setting. These findings lay the groundwork for identifying prognostic indicators of response to venetoclax-azacitidine and will require validation in a larger independent data set.
Acknowledgments
AbbVie and the authors thank all the trial investigators and the patients who participated in this clinical trial.
Medical writing support was provided by Theodora Szasz and Rohina Rubicz of Bio Connections LLC, and funded by AbbVie.
Venetoclax is being developed in collaboration between AbbVie and Genentech. AbbVie and Genentech sponsored the study; contributed to the analysis and interpretation of the data; and participated in the writing, review, and approval of the publication. No honoraria or payments were made for authorship.
Authorship
Contribution: H.D., C.D.D., Y.S., M.D., B.C., and D.A.P. conceptualized and designed the study; H.D., K.W.P., C.D.D., A.H.W., B.A.J., V.A.P., M.J.T., A.C.S., B.C., and D.A.P. made provision for study materials or patients; B.C. collected and assembled the data; H.D., K.W.P., C.D.D., A.H.W., B.A.J., V.A.P., M.J.T., C.R., A.C.S., X.L., G.K., Z.L., Y.S., M.D., B.C., and D.A.P. contributed to data analysis and interpretation; H.D., K.W.P., C.D.D., A.H.W., B.A.J., V.A.P., M.J.T., C.R., A.C.S., G.K., M.D., B.C., and D.A.P. wrote the manuscript ; H.D., G.K., and B.C. were accountable for all aspects of the work; and all authors had access to the relevant data and participated in the drafting, reviewing, and providing the final approval for the manuscript.
Conflict-of-interest disclosure: H.D. reports advisory role for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, Bristol Myers Squibb (BMS), Celgene, GEMoaB, Gilead Sciences, Janssen, Jazz Pharmaceuticals, Novartis, and Syndax and research funding from AbbVie, Agios, Amgen, Astellas, BMS, Jazz Pharmaceuticals, Kronos Bio, and Novartis. K.W.P. reports research funding from AbbVie, Agios, Daiichi Sankyo, and Millennium and advisory board membership for AbbVie, Astellas, AstraZeneca, Boston Biomedical, BMS, Celgene, Novartis, Jazz Pharmaceuticals, and Servier. C.D.D. reports research funding from AbbVie, Astex, BeiGene, BMS, Cleave, Foghorn, Jazz Pharmaceuticals, Loxo, and Servier and advisory role for AbbVie, Astellas, BMS, GlaxoSmithKline, Genmab, Genentech, Jazz Pharmaceuticals, Loxo, Servier, and Schrodinger. A.H.W. has served on advisory boards for Novartis, AstraZeneca, Astellas, Janssen, Jazz Pharmaceuticals, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead Sciences, BMS, and BeiGene; has consulted for AbbVie, Servier, Novartis, Shoreline, and Aculeus; receives research funding to institution from Novartis, AbbVie, Servier, BMS, Syndax, Astex, AstraZeneca, and Amgen; serves on speaker’s bureaus for AbbVie, Novartis, BMS, Servier, and Astellas; is an employee of the Walter and Eliza Hall Institute (WEHI; WEHI receives milestone and royalty payments related to the development of venetoclax, and current and past employees of WEHI may be eligible for financial benefits related to these payments); and receives such financial benefit. B.A.J. reports consultancy/advisory role for AbbVie, BMS, Daiichi Sankyo, Genentech, Gilead Sciences, GlycoMimetics, Kymera, Kura, Rigel, Schrodinger, Syndax, and Treadwell; protocol steering committee membership for GlycoMimetics; data monitoring committee membership for Gilead Sciences; travel reimbursement/support from Rigel; and research funding to institution from AbbVie, Amgen, AROG, Aptose, Biomea Fusion, BMS, Celgene, F. Hoffmann-La Roche, Forma, Forty Seven, Genentech/Roche, Gilead Sciences, GlycoMimetics, Hanmi, Immune-Onc, Incyte, Jazz, Kymera, Loxo, Pfizer, Pharmacyclics, and Treadwell. V.A.P. reports consultancy fees and honoraria from AbbVie, Jazz Pharmaceuticals, Novartis, and Rigel. M.J.T. reports research funding from AbbVie, Gilead Sciences, Janssen, Merck, Pharmacyclics, Syndax, and TG Therapeutics and consultancy fees from AbbVie, AstraZeneca, Celgene, Janssen, Pharmacyclics, and Roche/Genentech. C.R. reports research funding from AbbVie, BMS, Jazz Pharmaceuticals, Astellas, and IQVIA and advisory role for AbbVie, Jazz Pharmaceuticals, Astellas, Novartis, BMS, Takeda, and Servier. A.C.S. reports clinical trial funding from AbbVie, Amgen, AstraZeneca, BMS, GlycoMimetics, Kite/Gilead Sciences, Loxo, Novartis, Pfizer, Servier, Syndax, and Syros and advisory board membership for AbbVie, Amgen, Astellas, BMS, Jazz Pharmaceuticals, Novartis, Pfizer, and Teva. G.K. and M.D. report employment by Genentech Inc and may own stock/options in Roche. X.L., Z.L., Y.S., J.P., and B.C. report employment by AbbVie and may own stock or other options. D.A.P. reports research funding from AbbVie, Teva, Karyopharm, and BMS; consultancy or advisory board membership for LINK, Daiichi Sankyo, Aptevo, Rigel, Novartis, Sumitomo, Adicet, AbbVie, Syros, Qihan, Seres, Gilead Sciences, OncoVerity, BMS, Boehringer Ingelheim, Sanofi, Karyopharm, MEI Pharma, Inc, and Syndax; and support from the Leukemia and Lymphoma Society Scholar in Clinical Research and the Robert H. Allen MD Chair in Hematology research. S.B. declares no competing financial interests.
Correspondence: Hartmut Döhner, Department of Internal Medicine III, Ulm University Hospital, Albert-Einstein-Allee 23, 89081 Ulm, Germany; email: hartmut.doehner@uniklinik-ulm.de.
References
Author notes
Presented in part in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 to 13 December 2022.
AbbVie is committed to responsible data sharing regarding the clinical trials sponsored by the company. This includes access to anonymized, individual, and trial-level data (analysis data sets) as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research and will be provided after review and approval of a research proposal, statistical analysis plan, and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the link https://vivli.org/ourmember/abbvie/ and then select “Home.”
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal