In this issue of Blood, Rahman et al describe a new class of noncoding element that blocks inappropriate oncogene activation.1 Somatically acquired deletions of an intronic tether element within the FTO gene lead to ectopic IRX3 expression in T-cell acute lymphoblastic leukemia (T-ALL).
Regulation of gene expression is a complex yet fundamental process that underlies normal development and is frequently corrupted to drive oncogenesis. Central to gene-regulatory control is the appropriate activity of noncoding DNA elements called enhancers; these elements bind transcription factors and other transcriptional regulators to activate a target gene’s expression through direct physical contacts mediated by DNA looping.2 Thus, regulation of genome architecture is a major control point of proper gene regulation.
Chromatin is organized in structures called topologically associating domains (TADs) that are, on average, 1 Mb in length. Genomic regions within a TAD interact with each other more frequently than with genomic regions in a neighboring TAD, which helps constrain the range of contacts made by a given enhancer. Disruption of TAD boundaries, amplification of enhancer elements, and structural variations that physically relocate enhancers across the genome are all well-established mechanisms of oncogene activation.3
In their work, Rahman and colleagues explored additional mechanisms of oncogene activation in T-ALL, which frequently exhibits ectopic expression of lineage-specific transcription factors. Through analysis of published RNA-seq data, the authors identified that the Iroquois homeobox transcription factor gene IRX3 is aberrantly expressed in nearly half of T-ALL cases. IRX3 is normally not expressed in the T lineage and has no known role in hematopoiesis; rather, it is known to play roles in the development and function of the hypothalamus, heart, and adipose tissue.4 Using a method that maps all genomic contacts between active enhancers and promoters, the authors nominated 2 putative regulatory elements located within the same TAD as IRX3 as potential drivers of this aberrant expression: an unannotated region located downstream of IRX3 within intron 8 of the neighboring FTO gene and the CRNDE/IRX5 enhancer/promoter region located upstream of IRX3. Further analysis of patient and cell line data identified that the putative regulatory element within FTO intron 8 is recurrently deleted in a subset of patients with aberrant IRX3 expression (1.4% and 6.2% of adult and pediatric cases of T-ALL, respectively), nominating this region as playing a role in preventing inappropriate IRX3 activation.
One class of DNA regulatory element reminiscent of this gene activation–blocking function is an insulator, which is classically defined as blocking the ability of an enhancer to activate a target promoter when placed in between the 2 elements.5 Indeed, the minimally deleted region in FTO intron 8 is bound by CTCF, the predominant insulator protein. Genetic deletion of the CTCF site was sufficient to increase IRX3 expression, thus confirming that this element plays a role in blocking inappropriate IRX3 activation.
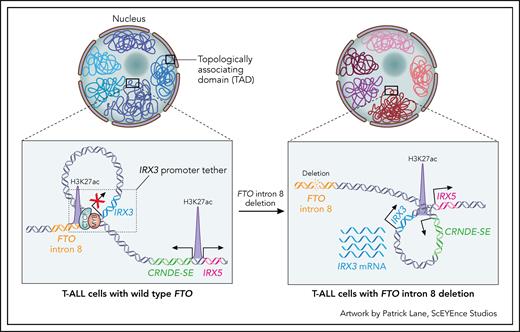
How this element maintains IRX3 in a transcriptionally repressed state is an important question. To address this, the authors analyzed IRX3 chromatin contacts after deletion of the CTCF site and observed that IRX3 subsequently formed increased chromatin contacts with the upstream CRNDE/IRX5 enhancer/promoter elements, which are active and expressed in early T-cell progenitors. Deletions of the CRNDE promoter led to a significant reduction in IRX3 expression, supporting a model whereby the FTO intron 8 element maintains IRX3 in a transcriptionally silent state by “tethering” it in a chromatin loop, such that it is blocked from interacting with the active upstream CRNDE/IRX5 elements (see figure). Although this promoter tether element may resemble a classical insulator, insulators are typically located between their target promoter and an enhancer,5 which is not the case here. Thus, the FTO intron 8 promoter tether may represent a new class of insulator-like noncoding element, as well as a new mechanism of oncogene activation that warrants further study, including the prevalence of promoter tethers in recurrently deleted regions across cancer genomes and the mechanism by which the tether element specifically sequesters a putative oncogene.
Proposed mechanism of IRX3 oncogenic activation in T-ALL due to the loss of a “promoter tether.” IRX3 resides in a TAD that contains 2 relevant regulatory elements: 1 within intron 8 of FTO and 1 within CRNDE. In cells with wild-type FTO (left panel), the IRX3 promoter is sequestered to a relatively inert region of FTO intron 8, yielding minimal transcriptional activity. This trapping is facilitated by CTCF binding at the FTO intron, which creates an IRX3 “promoter tether.” In T-ALL cells with focal deletions of FTO intron 8 (right panel), the loss of CTCF binding unleashes the IRX3 promoter, allowing it to be hijacked by the distal highly active developmental superenhancer of CRNDE, resulting in oncogenic IRX3 transcription.
Proposed mechanism of IRX3 oncogenic activation in T-ALL due to the loss of a “promoter tether.” IRX3 resides in a TAD that contains 2 relevant regulatory elements: 1 within intron 8 of FTO and 1 within CRNDE. In cells with wild-type FTO (left panel), the IRX3 promoter is sequestered to a relatively inert region of FTO intron 8, yielding minimal transcriptional activity. This trapping is facilitated by CTCF binding at the FTO intron, which creates an IRX3 “promoter tether.” In T-ALL cells with focal deletions of FTO intron 8 (right panel), the loss of CTCF binding unleashes the IRX3 promoter, allowing it to be hijacked by the distal highly active developmental superenhancer of CRNDE, resulting in oncogenic IRX3 transcription.
Deletions within FTO do not explain all cases with IRX3 expression. Although higher IRX3 expression was observed in CTCF mutated samples compared with wild-type samples, knockdown of CTCF in cell lines suggested a complex regulatory mechanism influenced by the genomic context. Interestingly, IRX3 is also expressed in acute myeloid leukemia through a different mechanism mediated by long noncoding transcripts arising from FTO.6 Moreover, the FTO locus contains additional well-characterized enhancer elements that specifically regulate IRX3 expression in cell types relevant for metabolic homeostasis,4 underscoring the complexity of this genomic region.
Rahman et al expand the list of somatically acquired mutations activating oncogene expression in T-ALL and highlight the importance of genomic configuration analysis to reveal mechanisms deregulating oncogene expression. This is specifically relevant to T-ALL, where noncoding alterations often promote enhancer hijacking, neoenhancer formation at oncogene loci, and aberrant activation of oncogene-associated enhancers.7-9 In a recent genomic analysis of more than 1300 children with T-ALL, over 50% of cases harbored driver noncoding alterations, many of which were shown to impact enhancer regulation in a subtype-specific manner, underscoring the need of whole genome sequencing to detect drivers of disease in T-ALL.9
Unlike mutations that alter protein sequence, inappropriately expressed genes do not display tumor-specific putative drug binding sites. However, as highlighted by the authors, tumor-specific aberrant enhancer activity is the driver of ectopic oncogene expression, raising the question of whether this activity itself can be therapeutically targeted. Is there an Achilles heel of these elements that, if elucidated, may lead to precise and stable repression of oncogenes? Chromatin-modulating drugs, which inhibit the activities of DNA methyltransferases, histone deacetylases, and histone acetyltransferases, may reverse impaired gene regulation.10 Importantly, bromodomain inhibitors that specifically target active chromatin have shown some clinical utility. However, these drugs impact gene regulation globally and are often toxic or not effective long term. As the compendium of enhancer-driven mechanisms of oncogene deregulation expands, it will become critical to deeply interrogate these mechanisms and determine whether there are unique vulnerabilities that distinguish them from normal enhancer regulation, and which may be therapeutically exploited.
Conflict-of-interest disclosure: I.I. has received consultation honoraria from Arima Genomics, travel expenses reimbursed by Mission Bio for invited talk, and honoraria from MD Education. L.E.M. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal