In this issue of Blood, Reis et al1 identify a monoclonal antibody, INCA033989, that selectively targets mutant calreticulin (mutCALR) in myeloproliferative neoplasms (MPNs), inhibiting its oncogenic activity without affecting normal hematopoiesis.
MPNs are blood cancers that arise after the acquisition of a mutation in a hematopoietic stem cell (HSC). Three mutations underpin almost all cases, affecting the gene encoding Janus kinase 2 (JAK2),1 the thrombopoietin receptor (TPO-R), or CALR.2-5 All 3 mutations lead to constitutive activation of JAK-STAT signaling and uncontrolled proliferation of myeloid cells and their progenitors. The discovery of mutCALR as the pathogenic lesion in the majority of JAK2V617F-negative essential thrombocythemia and myelofibrosis cases4,5 came as a surprise, because a major role for CALR in hematopoiesis had not previously been described. The discovery sparked a decade of research unravelling the fascinating mechanism by which this chaperone protein, normally resident in the endoplasmic reticulum (ER), causes the activation of JAK-STAT signaling specifically in HSCs and megakaryocyte lineage cells. Both type I (52–base pair [bp] deletion) and type II (5-bp insertion) lesions lead to the same mutant C terminus of the protein, inducing binding to nascent forms of TPO-R in the ER and loss of the ER-retention signal. Binding to mutCALR induces cytokine-independent receptor homodimerization and TPO-R activation.6 The expression of the mutCALR–TPO-R complex on the cell surface of cancer stem cells and fibrosis-driving megakaryocytes creates a fortuitous opportunity for selective targeting and represents an exemplar target for anticancer immunotherapy.
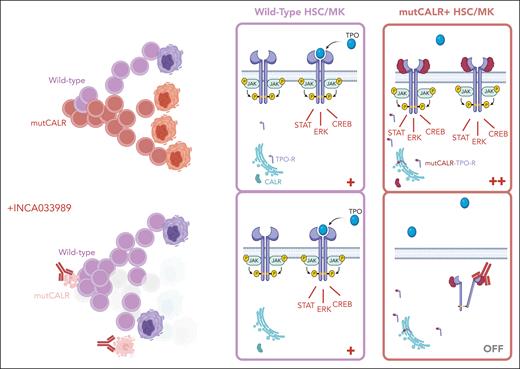
In their current work, Reis et al describe their approach to the discovery and validation of the first therapeutic antibody targeting mutCALR to reach the clinic. They show potent and selective inhibition of JAK-STAT signaling in cells coexpressing TPO-R and mutCALR, reducing the activation of downstream pSTAT3, pSTAT5, and pERK signaling and inhibiting cytokine-independent proliferation through dynamin-dependent endocytosis of the INCA033989/mutCALR/TPO-R complex. Importantly, wild-type and JAK2V617F-mutated hematopoietic cells were not affected by the antibody. In vivo, INCA033989 significantly reduced the proliferation of mutCALR-expressing cells in mouse models, lowering mutant cell counts in the blood and spleen while preserving normal hematopoiesis. Pharmacokinetic analyses indicated sustained therapeutic levels of the drug in plasma, with a favorable half-life. Critically, INCA033989 demonstrated the ability to target long-term repopulating HSCs in an MPN mouse model, which is key to impede disease persistence and clonal expansion. They also showed encouraging selective antagonism of mutCALR oncogenic signaling in human CD34+ cells and inhibition of pathogenic megakaryocyte differentiation (see figure).
In healthy cells, CALR is an ER-resident chaperone protein, and the TPO-R requires cytokine binding to trigger activation of downstream signaling. In cells bearing an oncogenic CALR mutation (mutCALR), mutCALR protein loses its ER retention signal and binds to nascent TPO-R protein, forming a complex that travels to the cell surface, causing cytokine-independent receptor signaling in HSCs and MK lineage cells, resulting in a competitive advantage for the mutant HSC and its progeny. INCA033989 binds to the unique C-terminal tail of mutCALR protein, displayed on the surface of TPO-R–expressing cells (HSCs and MKs), and drives receptor internalization and inhibition of TPO-R signaling. The aim is to switch off the overactive signaling, deplete the mutCALR clone, and enable healthy hematopoiesis to recolonize. Figure created with biorender.com.
In healthy cells, CALR is an ER-resident chaperone protein, and the TPO-R requires cytokine binding to trigger activation of downstream signaling. In cells bearing an oncogenic CALR mutation (mutCALR), mutCALR protein loses its ER retention signal and binds to nascent TPO-R protein, forming a complex that travels to the cell surface, causing cytokine-independent receptor signaling in HSCs and MK lineage cells, resulting in a competitive advantage for the mutant HSC and its progeny. INCA033989 binds to the unique C-terminal tail of mutCALR protein, displayed on the surface of TPO-R–expressing cells (HSCs and MKs), and drives receptor internalization and inhibition of TPO-R signaling. The aim is to switch off the overactive signaling, deplete the mutCALR clone, and enable healthy hematopoiesis to recolonize. Figure created with biorender.com.
We now have over a decade of experience with JAK inhibitors in MPNs and 4 approved agents: ruxolitinib, fedratinib, momelotinib, and pacritinib. Substantial data confirm the major clinical benefits of these agents, including for the control of splenomegaly, symptoms, and blood counts. However, currently approved JAK inhibitors are not selective for the mutant clone, and clonal remissions are not commonly observed in clinical practice. Interferon alfa, frequently used as cytoreductive agent in polycythemia vera and essential thrombocythemia, will induce a molecular response in over half of patients with JAK2V617F+ MPN,7,8 but molecular responses are less common in patients with CALR mutations.8 This study opens the door to a potentially transformative therapy, combining potent JAK-STAT inhibition with the ability to spare nonmutant hematopoiesis, potentially reversing the competitive advantage of the malignant clone and enabling healthy, wild-type hematopoiesis to regenerate.
Several important questions remain. First, will the antibody be effective in all molecular subtypes of mutCALR MPN and at all disease stages? In this study, the authors show preferential binding to cells bearing the CALRdel52 mutation over CALRins5 cells. Therefore higher-intensity dosing may be required to achieve the same efficacy in patients with type 2/type 2–like mutations vs type I. Similarly, whether MPNs with additional genetic lesions, particularly those in accelerated or blast phase, are still dependent on cytokine-independent TPO-R signaling for their competitive advantage is not known. Secondly, the myelofibrosis tumor microenvironment may present challenges to clinical efficacy. Although the therapeutic strategy of INCA033989 is not dependent on effector cell function, which may be inhibited in a megakaryocyte-dense, transforming growth factor β–rich environment, the presence of fibrosis and high expression of TPO-R on the plethora of megakaryocytes, compared with the rare HSCs, may impede access of the antibody to bone marrow-resident HSCs. Finally, we have clear evidence that MPN driver mutations are typically acquired many decades before the disease manifests clinically.9,10 Therefore, the time required for molecular responses with mutCALR–TPO-R signaling inhibition may be significant.
Nonetheless, the discovery of a mutCALR-specific inhibitory antibody marks an important breakthrough for the field, offering new hope for patients who currently have limited options beyond JAK inhibitors and other cytoreductive therapy. A phase 1 trial of INCA033989 has been initiated (https://clinicaltrials.gov/study/NCT06034002 and https://clinicaltrials.gov/study/NCT05936359), followed by a phase 1 study of a CD3-engaging bispecific antibody (https://clinicaltrials.gov/study/NCT06150157) hot on its heels. Together, these trials mark the beginning of a new era of targeted cancer therapy in myeloid neoplasms. The MPN community eagerly awaits the data from these pivotal trials.
Conflict-of-interest disclosure: B.P. is a cofounder and equity holder in Alethiomics Ltd, a spin out company from the University of Oxford and has received research funding from Alethiomics, Incyte, and Galecto, and honoraria for consulting and/or paid speaking engagements from Incyte, Constellation Therapeutics, Blueprint Medicines, Novartis, GlaxoSmithKline, and Bristol Myers Squibb. C.B. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal