Issue Archive
Table of Contents
BLOOD COMMENTARIES
REVIEW ARTICLE
CLINICAL TRIALS AND OBSERVATIONS
JAK2/mTOR inhibition fails to prevent acute GVHD despite reduced Th1/Th17 cells: final phase 2 trial results
Clinical Trials & Observations
Preclinical data demonstrate that Janus kinase 2/signal transducer and activator of transcription (JAK2/STAT3) activity is integral to differentiation of alloreactive T cells, suggesting that JAK2 inhibition could prevent the development of graft-versus-host disease (GVHD). In a multicenter phase 2 trial, Pidala et al tested this hypothesis, reporting that a GVHD prevention regimen of pacritinib, an oral JAK2 inhibitor, in combination with sirolimus and tacrolimus successfully suppressed phosphorylated STAT3 signaling in CD4+ T cells but did not reduce acute GVHD in comparison to historical controls. While a negative trial, the correlative data inform future directions for GVHD prevention studies.
Diagnostic guidelines for familial hemophagocytic lymphohistiocytosis revisited
Clinical Trials & Observations
Clinical diagnostic criteria for primary (also known as familial) hemophagocytic lymphohistiocytosis (HLH) were codified in 2004 (HLH-2004), and this score has endured over 2 decades. Now, through case-control analysis of international pediatric trial databases, Henter and colleagues report on the updated criteria on behalf of the Histiocyte Society. Use of these criteria streamlines the clinical diagnosis, discriminates primary HLH from other inflammatory disorders, and provides guidance on genetic testing.
LYMPHOID NEOPLASIA
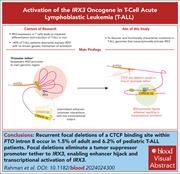
Focal deletions of a promoter tether activate the IRX3 oncogene in T-cell acute lymphoblastic leukemia
Regulation of gene expression is frequently corrupted to drive oncogenesis. Rahman and colleagues identify that the Iroquois-family homeobox transcription factor gene IRX3 is aberrantly expressed in nearly 50% of T-cell acute lymphoblastic leukemia cases, in some because of acquired deletions of an intronic tether within the FTO gene that would normally block inappropriate IRX3 activation. The authors’ work reveals a new class of noncoding regulatory elements and highlights how genomic configuration analysis can reveal mechanisms for deregulating oncogene expression.
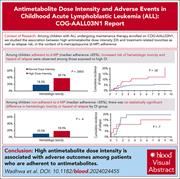
Antimetabolite dose intensity and adverse outcomes in children with acute lymphoblastic leukemia: a COG-AALL03N1 report
Maintenance therapy is a fundamental backbone of acute lymphoblastic leukemia treatment in children. This study by Wadhwa and colleagues addresses an important question regarding the risk and benefits of dose escalation of antimetabolite therapy to achieve a narrow range of myelosuppression in maintenance. Using data from a prospective trial, the authors identified that higher dose intensity does not benefit patients with good adherence, reinforcing how imperative assessment of adherence is when considering dose increases.
MYELOID NEOPLASIA
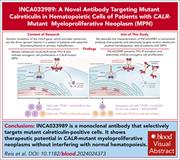
Selective targeting of mutated calreticulin by the monoclonal antibody INCA033989 inhibits oncogenic function of MPN
Mutations in calreticulin (mutCALR) drive most cases of Janus kinase 2 (JAK2)V617F–negative essential thrombocythemia and myelofibrosis by triggering inappropriate activation of JAK/signal transducer and activator of the transcription signaling, specifically in stem cells and megakaryocyte lineage cells. mutCALR binds the thrombopoietin receptor in the endoplasmic reticulum, and the complex is translocated to the cell surface. Reis and colleagues report generation and preclinical characterization of a monoclonal antibody INCA033989 that selectively targets mutCALR in myeloproliferative neoplasms inhibiting its oncogenic activity without affecting normal hematopoiesis. The authors’ data form the basis for ongoing clinical trials.
THROMBOSIS AND HEMOSTASIS
Bleeding events in patients with cancer: incidence, risk factors, and impact on prognosis in a prospective cohort study
Clinical Trials & Observations
Cancer-associated venous thromboembolism (VTE) in ambulatory patients is well recognized, but less well characterized is clinically relevant bleeding (CRB) in patients with cancer. In a prospective observational study, Englisch et al identified that ambulatory patients receiving systemic anticancer therapy were at a high risk of CRB. The authors report a 10% cumulative incidence of CRB, with a 5% incidence of major bleeding at 6 months in 791 cancer patients, including in those not receiving anticoagulation or antiplatelet agents at entry. Physicians need to be mindful of this when considering primary VTE prophylaxis; furthermore, there is an urgent need for the development of validated bleeding risk stratification tools to assist in decision making.
LETTER TO BLOOD
A weekly low-dose regimen of decitabine and venetoclax is efficacious and less myelotoxic in a racially diverse cohort
Clinical Trials & Observations
When an entirely new class of drug such as BCL2 inhibitors enters practice, optimizing benefits and reducing risks typically require exploration beyond registration trials. Goldfinger et al report on a prospective phase 2 clinical trial testing weekly dosing of venetoclax and decitabine in first-line therapy of patients with acute myeloid leukemia or high-risk myelodysplasia. The regimen is tolerable, with 90% of patients able to receive continuous therapy without dose reductions or delays. Preliminary efficacy is encouraging, but prospective randomized comparison to standard regimens is required to understand the potential place of this regimen.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Confocal microscopy image of Ba/F3-TPOR/CALRdel52 cells shows colocalization of the monoclonal antibody INCA033989 (magenta) which binds mutant calreticulin and lysosome-associated membrane protein 2 (green) upon cellular internalization of INCA033989. The nucleus is shown in blue. See the article by Reis et al on page 2336.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals