Key Points
JAK2/mTOR inhibition decreases the differentiation of donor Th1 and Th17 cells after allogeneic hematopoietic cell transplantation.
Adding pacritinib (JAK2 inhibitor) to sirolimus and tacrolimus fails to improve graft-versus-host disease prevention in a phase 2 trial.
Visual Abstract
Our phase 1 graft-versus-host disease (GVHD) prevention trial of JAK2 inhibitor, pacritinib (PAC; recommended phase 2 dose: 100 mg orally twice a day on day 0 to +70) plus sirolimus and tacrolimus (SIR/TAC) demonstrated the regimen was safe and free of pan-JAK myelosuppression after allogeneic hematopoietic cell transplantation (alloHCT). PAC inhibits interleukin 6 (IL-6) receptor activity and pathogenic T helper cell 1 (Th1)/Th17 differentiation in preclinical models and the phase 1 trial. Herein, we report on our completed phase 2 trial of PAC/SIR/TAC after 8/8 human leukocyte antigen matched alloHCT. This single-arm phase 2 trial (NCT02891603) was powered to determine if PAC/SIR/TAC suppressed percentage phosphorylated STAT3 (pSTAT3)+ CD4+ T cells at day +21 (primary end point: percentage pSTAT3+ CD4+ T cells ≤ 35%) and estimated grade II to IV acute GVHD by day +100. The impact of PAC/SIR/TAC on T-cell subsets, CD28 (pS6 and pH3ser10), and IL-2 receptor (pSTAT5) signal transduction was also evaluated. Eligible patients (n = 28) received alloHCT for hematologic malignancies or myeloproliferative neoplasms. Reduced or myeloablative intensity conditioning was permitted. PAC/SIR/TAC met the primary end point, reducing percentage pSTAT3+ CD4+ T cells to 9.62% at day +21. Th1/Th17 cells were decreased at day +21, increasing the ratio of regulatory T cells to Th1 and Th17 cells with PAC/SIR/TAC at recommended phase 2 dose PAC compared with dose level 1 PAC. The cumulative incidence of grade II to IV acute GVHD by day +100 with PAC/SIR/TAC was similar to historic SIR/TAC values (46% vs 43%). Although PAC/SIR/TAC suppressed pSTAT3 and Th1/Th17 cells, the regimen did not improve acute GVHD prevention.
Introduction
Recent translational studies in graft-versus-host disease (GVHD) prevention have targeted interleukin 6 (IL-6), IL-12, and IL-23, their receptors, and downstream receptor signaling elements, like JAK2 and STAT3, to curtail host tissue damage by alloreactive T helper cell 1 (Th1) and Th17 cells.1 Early preclinical investigations first showed that MR-16-1, an anti–IL-6 receptor antibody, reduced acute GVHD in mice.2 IL-6 blockade improved regulatory T cell (Treg) induction from conventional T cells while suppressing Th1 and Th17 cells.2 Later, alloreactive donor T cells were identified as the source of IL-6 and confirmed that its neutralization reduced GVHD but preserved graft-versus-leukemia (GVL) in mice.3 The initial phase 1/2 trial of tocilizumab (anti–IL-6 receptor monoclonal antibody) with cyclosporine A/methotrexate (MTX) showed on-target suppression of phosphorylated STAT3 (pSTAT3) in monocytes and T cells, but no measurable changes in T-cell subsets.4 However, a favorable reduction in grade III to IV acute GVHD justified a subsequent randomized, controlled phase 3 trial.4 The phase 3 trial of tocilizumab with cyclosporine A/MTX failed to reduce the primary end point of grade II to IV acute GVHD or improve overall survival.5
Targeting the p40 subunit of IL-12 and IL-23 can achieve similar reductions in Th1 and Th17 responses after transplant.6 It was first shown that donor antigen-presenting cells orchestrated acute GVHD damage in the colon via IL-23.7 Later, preclinical studies ablating T-box expressed in T cells (T-bet) and retinoic acid–related orphan receptor gamma t (RORγt) in donor T cells resulted in dysfunctional Th1/Th17 differentiation and increased Tregs and Th2 cells.8 Moreover, transfer of T-bet/RORγt knockout (KO) donor T cells during allogeneic hematopoietic cell transplantation (alloHCT) reduced GVHD and preserved GVL in mice.8 A randomized, controlled phase 2 GVHD prevention trial of ustekinumab (anti–IL-12/IL-23p40 monoclonal antibody) plus sirolimus/tacrolimus (SIR/TAC) was performed in human leukocyte antigen (HLA)–matched alloHCT.6 The p40 cytokine blockade reduced interferon alfa (IFN-α) and IL-17 in the blood, indicating suppression of Th1 and Th17 responses.6 Ustekinumab plus SIR/TAC improved overall survival, chronic GVHD, chronic relapse-free survival compared with SIR/TAC controls.6 On the basis of these favorable survival results, ustekinumab is being studied as acute GVHD prevention following alloHCT with HLA-matched unrelated donors, but now paired with TAC/MTX (NCT04572815).
Our phase 1 trial of pacritinib (PAC)/SIR/TAC demonstrated that the regimen was safe and suggested preliminary efficacy in preventing grade II to IV acute GVHD after alloHCT.9 JAK2/STAT3 activity is required for IL-6, IL-12, and IL-23 receptor signaling and subsequent donor Th1 and Th17 differentiation.10,11 Patients treated with pacritinib, 100 mg twice a day, on the phase 1 trial exhibited low Th1 and Th17 numbers paired with stable amounts of immune-suppressive Tregs.9 Accompanying preclinical studies also demonstrated synergy by combining pacritinib with sirolimus for GVHD prevention in mice, yet donor T-cell antileukemia activity was preserved.9 Herein, we report on results from our completed phase 2 trial of PAC/SIR/TAC as acute GVHD prophylaxis after 8/8-HLA matched alloHCT.
Materials and methods
Overall trial design
The single-arm trial tested the JAK2-selective inhibitor, PAC, plus SIR/TAC as GVHD prophylaxis. CTI BioPharma Corp, a Sobi company, provided PAC. Eligible patients were treated on this institutional review board–approved phase 1/2 protocol (NCT02891603), enrolling at the Moffitt Cancer Center and the University of Minnesota. The phase 1 trial defined the recommended phase 2 dose (pacritinib, 100 mg twice daily).9 The phase 2 trial was powered to determine if PAC/SIR/TAC suppressed circulating STAT3+ CD4+ T cells at day +21 (primary end point) and reduced the cumulative incidence of grade II to IV acute GVHD by day +100 (secondary end point), and the trial investigated its impact on CD4 T-cell differentiation (Treg, Th1, and Th17) and CD28 and IL-2 receptor signal transduction (measuring S6 [mammalian target of rapamycin {mTOR}], H3 Ser10 [Aurora kinase], and STAT5 phosphorylation) in CD4+ T cells at days +21 and +100.
Included subjects
Included were subjects aged ≥18 years. HLA-A, HLA-B, HLA-C, and HLA-DRB1 matched-related or unrelated donors were allowed, and graft source was mobilized peripheral blood stem cells with a target of 5 × 106 to10 × 106 CD34+ cells/kg. Identical remission criteria from the phase 1 were required for acute leukemia, myelodysplastic syndrome, chronic myeloid leukemia, myeloproliferative neoplasms, and Hodgkin or non-Hodgkin lymphoma.9 Vital organ function parameters required were as follows: left ventricular ejection fraction ≥50%; forced expiratory volume, forced vital capacity, and adjusted diffusing capacity of the lungs for carbon monoxide ≥50% of predicted values; transaminases <2 times upper limit of normal values; creatinine clearance ≥50 mL/min, and Karnofsky performance status (≥80%). Those with uncontrolled infection, history of HIV or hepatitis B or C infection, hematopoietic cell transplantation–specific comorbidity index > 4,11 corrected QT interval, Fridericia’s formula (QTcF) > 450 ms, coagulation studies (prothrombin time, partial thromboplastin time, and thrombin time) >2× upper limit of normal values, grade 3 or higher cardiac or bleeding complications within the preceding 6 months, uncontrolled current myocardial infarction/angina, or class III to IV congestive heart failure were excluded. Conditioning regimens included myeloablative pharmacokinetic-targeted IV busulfan/fludarabine or a reduced-intensity fludarabine/melphalan regimen. Use of post-HCT cyclophosphamide, anti-thymocyte globulin, alemtuzumab, bortezomib, post-HCT tyrosine kinase inhibitors, or other JAK inhibitors was not permitted.
Treatment program
Pacritinib, 100 mg twice daily, was administered from day 0 onward, followed by treatment at 50% of original dose (100 mg daily) from day +70 through 83, then at 25% of original dose (100 mg every other day) from day +84 through 100, and then stopped. Concurrent strong cytochrome P450 3A4 (CYP3A4) inhibitors required a 50% dose reduction in pacritinib. Specific drug interruption and discontinuation rules were based on QTcF prolongation, left ventricular systolic dysfunction, diarrhea, and any other grade 3 or higher adverse events deemed at least possibly related to pacritinib therapy. Management of sirolimus (therapeutic target range of 5-14 ng/mL) and tacrolimus (target range of 3-7 ng/mL) followed program standards, and included guidelines for management of drug interactions and thrombotic microangiopathy (TMA). The protocol did not mandate the duration of tacrolimus or sirolimus administration after alloHCT.
Trial outcomes and power calculation
The primary objective of the phase 2 trial was to determine if pacritinib significantly reduced circulating pSTAT3+ CD4+ T cells at day +21 post-HCT, and was powered accordingly. Methods for pSTAT3 assessment have been published.9 Preliminary data from SIR/TAC-treated subjects at Moffitt Cancer Center (N = 18 subjects, received SIR/TAC without pacritinib) suggested that mean pSTAT3+ CD4+ T cells were 50% (SD, 25%), regardless of acute GVHD severity. With a sample size of 24 subjects on the phase 2 trial and 2-sided significance level of 0.05, there was 80% power to detect a decrease of 15% (effect size of 0.6) when SD is assumed 25%. The study would have 94% power under SD of 20% with 15% reduction (effect size of 0.75).
The secondary objective of the phase 2 trial was to determine whether the incidence of grade II to IV acute GVHD by day +100 was reduced compared with the historical benchmark using SIR/TAC alone at Moffitt Cancer Center. Acute GVHD was staged and graded according to consensus criteria. We hypothesized that SIR/TAC/pacritinib would reduce this outcome from 43% to 23% and used a Simon 2-stage phase 2 design with 10% 1-sided significance level and 90% power. If ≤9 of the initial 24 subjects developed grade II to IV acute GVHD, an expansion would permit an additional 14 patients to be treated, and the overall phase 2 acute GVHD outcome would be considered favorable if ≤12 of 38 total (31.6%) developed grade II to IV acute GVHD.
Other evaluated clinical outcomes included engraftment (time to neutrophil [absolute neutrophil count >500] and platelet recovery [platelets >20 without transfusion]), donor chimerism of bone marrow and sorted CD3 and CD33 blood populations, acute and chronic GVHD per consensus criteria,12,13 GVHD therapy, TMA,14 hepatic veno-occlusive disease,15 discontinuation of immune suppression, infectious complications, relapse, nonrelapse morality, overall survival, and causes of death.
Flow cytometry
Peripheral blood mononuclear cells were immunophenotyped by flow cytometry pretransplant, at day +21, and at day +100. T-cell subsets were characterized as follows: Tregs (CD4+, CD127-, CD25+, Foxp3+), T helper 1 (CD4+, IFN-γ+), T helper 2 (CD4+, IL-4+), and T helper 17 (CD4+, IL-17+). CD4+ T-cell phosphorylation of STAT3, STAT5, S6, and H3 Ser10 was evaluated. Clinical pathology flow cytometry panels provided additional data on T cells, natural killer (NK) cells, and B cells.
Statistical analysis
Patient baseline characteristics were summarized using descriptive statistics. As the primary end point of the study, 1-sample t-test was used to examine if day +21 pSTAT3+ CD4+ T cells were decreased by 15% from a historic mean of 50%. A 2-sided P value of <.05 was considered statistically significant. The cumulative incidence of grade II to IV acute GVHD, chronic GVHD, and the discontinuation of TAC and SIR was estimated using the competing risk approach, where relapse and nonrelapse mortality are considered competing risks. Overall survival and relapse-free survival were analyzed using the Kaplan-Meier method.
Results
PAC/SIR/TAC suppresses STAT3 and enhances STAT5 phosphorylation in CD4+ T cells
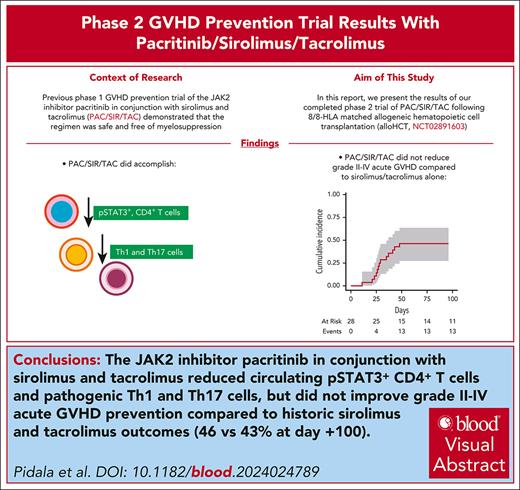
This phase 2 trial was powered to evaluate the biologic end point of inhibiting the frequency of pSTAT3+ CD4+ T cells at day +21. Consistent with the phase 1 trial, pacritinib, 100 mg twice a day, reduced the mean frequency of STAT3 phosphorylated CD4+ T cells to 9.62% (Figure 1A-B). Therefore, PAC/SIR/TAC successfully met the primary end point of this trial (eg, percentage pSTAT3+ CD4+ T cells ≤ 35% at day +21). The day +21 frequency of pSTAT3+ CD4+ T cells was significantly reduced with PAC/SIR/TAC compared with values at baseline pretransplant or the biologically inactive dose level 1 concentration of pacritinib (100 mg daily) at day +21. Moreover, pacritinib dosing interruptions for diarrhea early after transplant did not unfavorably impact pSTAT3 suppression at day +21 (Figure 1C). PAC/SIR/TAC significantly improved CD4+ T-cell STAT5 phosphorylation at day +21, polarizing the ratio of pSTAT5 to pSTAT3 CD4+ T cells (Figure 1D-F). Furthermore, pharmacokinetic dosing of SIR, targeting 5 to 14 ng/mL, significantly reduced CD4+ T-cell S6 phosphorylation, the downstream signaling element of mTOR (Figure 1G-H). The frequency of pH3 Ser10+ CD4+ T cells at day +21 with PAC/SIR/TAC was similar to pretransplant levels, but significantly reduced compared with dose level 1 values from the phase 1 trial (Figure 1I-J). Despite this reduction in pH3 Ser10, >70% of CD4+ T cells at day +21 still demonstrated intact Aurora kinase A activity, an mTOR-independent CD28 signaling pathway (Figure 1J).16,17
PAC/SIR/TAC suppresses STAT3 and enhances STAT5 phosphorylation in CD4+ T cells. (A) Percentage (%) of pSTAT3+ CD4+ T cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (B) %pSTAT3+ CD4+ T cells at day +21 among patients treated with PAC/SIR/TAC at dose level 1 with ineffective JAK2 inhibition vs PAC/SIR/TAC at the recommended phase 2 dose (RP2D) of PAC (primary end point: %pSTAT3+ CD4+ T cells ≤ 35%). (C) %pSTAT3+ CD4+ T at days +21 and +100 based on pacritinib dosing. (D) %pSTAT5+ CD4+ T (IL-2 receptor signaling element) cells pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (E) %pSTAT5+ CD4+ T cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (F) Graph demonstrating the ratio of pSTAT5+ to pSTAT3+ CD4+ T cells at day +21 based on dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (G) %pS6+ CD4+ T cells (CD28 signaling element) pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (H) %pS6+ CD4+ T cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (I) %pH3 Ser10+ CD4+ T cells (CD28 signaling element) pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (J) %pH3 Ser10+ CD4+ T cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. ∗P < .05, ∗∗P = .01-.001, ∗∗∗P = .001-.0001, ∗∗∗∗P < .0001. NS, not significant.
PAC/SIR/TAC suppresses STAT3 and enhances STAT5 phosphorylation in CD4+ T cells. (A) Percentage (%) of pSTAT3+ CD4+ T cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (B) %pSTAT3+ CD4+ T cells at day +21 among patients treated with PAC/SIR/TAC at dose level 1 with ineffective JAK2 inhibition vs PAC/SIR/TAC at the recommended phase 2 dose (RP2D) of PAC (primary end point: %pSTAT3+ CD4+ T cells ≤ 35%). (C) %pSTAT3+ CD4+ T at days +21 and +100 based on pacritinib dosing. (D) %pSTAT5+ CD4+ T (IL-2 receptor signaling element) cells pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (E) %pSTAT5+ CD4+ T cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (F) Graph demonstrating the ratio of pSTAT5+ to pSTAT3+ CD4+ T cells at day +21 based on dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (G) %pS6+ CD4+ T cells (CD28 signaling element) pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (H) %pS6+ CD4+ T cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (I) %pH3 Ser10+ CD4+ T cells (CD28 signaling element) pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (J) %pH3 Ser10+ CD4+ T cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. ∗P < .05, ∗∗P = .01-.001, ∗∗∗P = .001-.0001, ∗∗∗∗P < .0001. NS, not significant.
PAC/SIR/TAC inhibits inflammatory Th1 and Th17 cells, while increasing Th2 cells
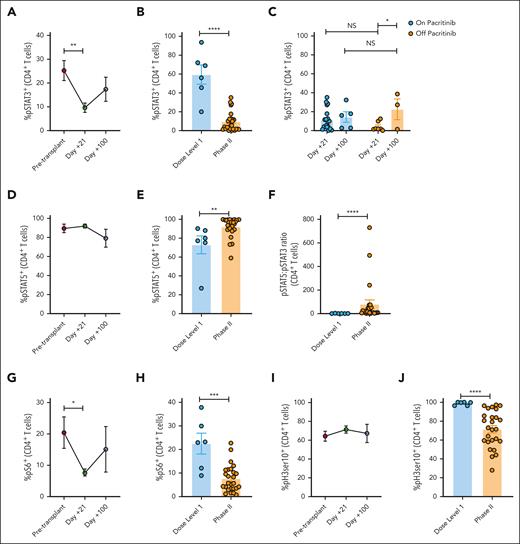
We and others have demonstrated that JAK inhibition impairs Th1 and Th17 cells, both implicated in acute GVHD pathogenesis.10,18,19 Neutralizing IL-6 or the p40 cytokines, IL-12 and IL-23, with monoclonal antibodies suppresses Th1 and Th17 responses after alloHCT.2,4-7 Consistent with these lines of work, PAC/SIR/TAC significantly reduces the frequency and absolute number of Th1 and Th17 cells at day +21 compared with pretransplant values or among those treated at dose level 1 concentrations of pacritinib (Figure 2A-F). We previously demonstrated that JAK2 KO donor T cells are polarized to a Th2 differentiation program, and that PAC increases Th2 cells in mice as well.18 We confirm that PAC/SIR/TAC significantly increases Th2 cells at day +21 compared with controls (Figure 2G-I).
PAC/SIR/TAC inhibits inflammatory Th1 and Th17 cells, while increasing Th2 cells. (A) %CD4+ Th1 cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (B-C) Percentage (B) and absolute number (C) of CD4+ Th1 cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs recommended phase 2 dose (RP2D) PAC/SIR/TAC. (D) %CD4+ Th17 cells pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (E-F) Percentage (E) and absolute number (F) of CD4+ Th17 cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (G) %CD4+ Th2 cells pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (H-I) Percentage (H) and absolute number (I) of CD4+ Th2 cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. ∗P < .05, ∗∗P = .01-.001, ∗∗∗∗P < .0001.
PAC/SIR/TAC inhibits inflammatory Th1 and Th17 cells, while increasing Th2 cells. (A) %CD4+ Th1 cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (B-C) Percentage (B) and absolute number (C) of CD4+ Th1 cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs recommended phase 2 dose (RP2D) PAC/SIR/TAC. (D) %CD4+ Th17 cells pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (E-F) Percentage (E) and absolute number (F) of CD4+ Th17 cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (G) %CD4+ Th2 cells pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (H-I) Percentage (H) and absolute number (I) of CD4+ Th2 cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. ∗P < .05, ∗∗P = .01-.001, ∗∗∗∗P < .0001.
PAC/SIR/TAC increases the ratio of treg to Th1 and Th17 cells
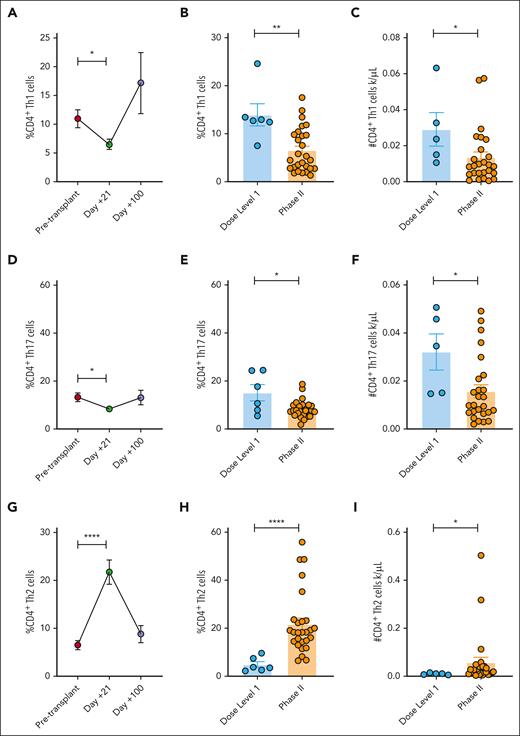
Although data from preclinical studies investigating donor JAK2 KO T cells in mice suggested that JAK2 inhibition would increase Treg numbers overall, this effect was not observed in the prior phase 1 trial of PAC/SIR/TAC.9,18 The data from the phase 2 PAC/SIR/TAC trial shows that although the day +21 Treg frequency is significantly increased from pretransplant values, we did not see a substantial increase in Treg frequency or absolute numbers compared with dose level 1 values (Figure 3A-C). However, the ratio of Treg:Th1 + Th17 at day +21 was significantly increased compared with pretransplant or day +21 values from dose level 1 treated patients (Figure 3D-E). Using in vitro studies, we confirmed the intensity of tacrolimus dosing (eg, 3 or 7 ng/mL) did not impact PAC/SIR/TAC effects on T helper subsets (supplemental Figure 1, available on the Blood website). In allogeneic mixed leukocyte reactions, PAC/SIR/TAC did not suppress in vitro T-cell production of the common γ chain cytokines, IL-2 or IL-15, but did significantly reduce the proinflammatory cytokines, granulocyte-macrophage colony-stimulating factor, IL-6, IFN-γ, and tumor necrosis factor-α (supplemental Figure 2).
PAC/SIR/TAC increases the ratio of Treg to Th1 and Th17 cells. (A) %CD4+ Tregs pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (B-C) Percentage (B) and absolute number (C) of CD4+ Tregs at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs recommended phase 2 dose (RP2D) PAC/SIR/TAC. (D) Graph showing the ratio of Tregs to Th1 and Th17 cells pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (E) Graph demonstrating the ratio of Tregs to Th1 and Th17 cells at day +21 based on dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. ∗P < .05. NS, not significant.
PAC/SIR/TAC increases the ratio of Treg to Th1 and Th17 cells. (A) %CD4+ Tregs pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (B-C) Percentage (B) and absolute number (C) of CD4+ Tregs at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs recommended phase 2 dose (RP2D) PAC/SIR/TAC. (D) Graph showing the ratio of Tregs to Th1 and Th17 cells pretransplant and at days +21 and +100 on phase 2 PAC/SIR/TAC. (E) Graph demonstrating the ratio of Tregs to Th1 and Th17 cells at day +21 based on dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. ∗P < .05. NS, not significant.
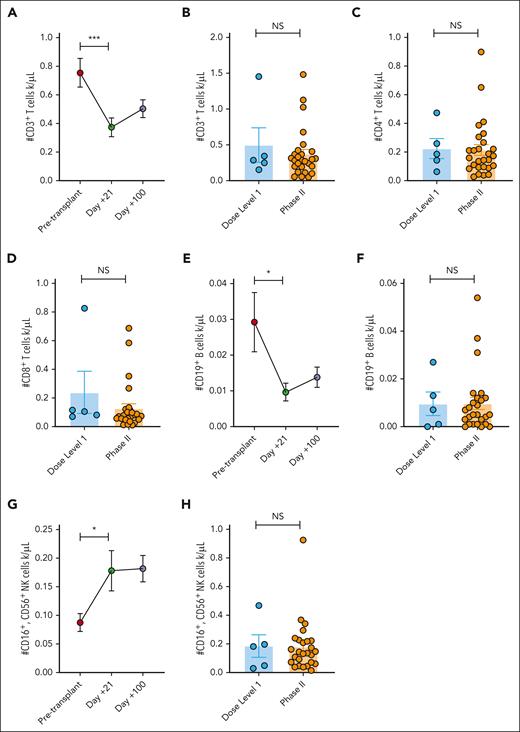
PAC/SIR/TAC maintains T, B, and NK cell engraftment
PAC/SIR/TAC significantly reduced the absolute number of T cells at day +21 compared with pretransplant values. However, the absolute numbers of total CD3+, CD4+, and CD8+ T cells at day +21 were similar among PAC/SIR/TAC patients regardless of the pacritinib dose (Figure 4A-D). Total CD19+ B cells followed a similar pattern (Figure 4E-F). Although the absolute numbers of NK cells were increased at day +21 with PAC/SIR/TAC when compared with pretransplant values, there was no difference when compared with dose level 1 data at day +21 (Figure 4G-H). T-cell apoptosis generated by immune suppression can inform clinical activity. Using in vitro co-culture experiments of human T cells and cytokine-matured, allogeneic dendritic cells, we investigated the impact of PAC/SIR/TAC on T-cell apoptosis. Overall, pacritinib exposure significantly increased T-cell apoptosis compared with dimethyl sulfoxide or SIR/TAC controls (supplemental Figure 3). However, CD4+ T-cell apoptosis was significantly greater when exposed to the combination of PAC/SIR/TAC (supplemental Figure 3).
PAC/SIR/TAC maintains T, B, and NK cell engraftment. (A) Number of CD3+ T cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (B-D) Number of CD3+ (B), CD4+ (C), and CD8+ (D) T cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs recommended phase 2 dose (RP2D) PAC/SIR/TAC. (E) Number of CD19+ B cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (F) Number of CD19+ B cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (G) Number of CD16+, CD56+ NK cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (H) Number of CD16+, CD56+ NK cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. ∗P < .05 and ∗∗∗P = .001-.0001. NS, not significant.
PAC/SIR/TAC maintains T, B, and NK cell engraftment. (A) Number of CD3+ T cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (B-D) Number of CD3+ (B), CD4+ (C), and CD8+ (D) T cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs recommended phase 2 dose (RP2D) PAC/SIR/TAC. (E) Number of CD19+ B cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (F) Number of CD19+ B cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. (G) Number of CD16+, CD56+ NK cells pretransplant and at days +21 and +100 among patients treated on phase 2 PAC/SIR/TAC. (H) Number of CD16+, CD56+ NK cells at day +21 among patients treated with dose level 1 PAC/SIR/TAC vs RP2D PAC/SIR/TAC. ∗P < .05 and ∗∗∗P = .001-.0001. NS, not significant.
PAC/SIR/TAC did not improve acute GVHD incidence vs historical benchmarks with SIR/TAC alone
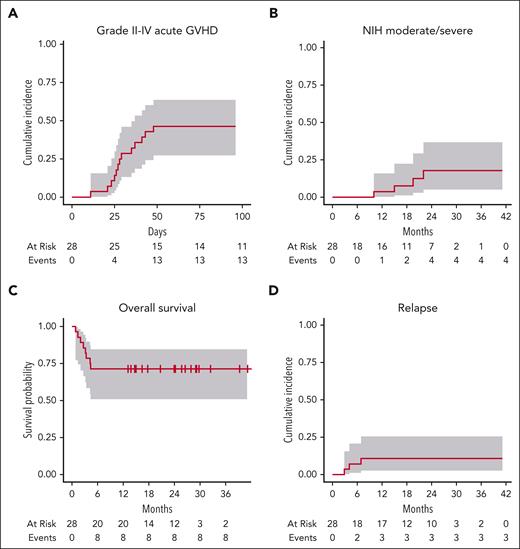
A total of 28 subjects were treated on the phase 2 trial. The observed acute GVHD incidence within the first stage of the 2-stage phase 2 design permitted enrollment in stage 2, yet the total enrollment to stage 2 was halted early because of futility in the observed acute GVHD incidence. Baseline characteristics of treated subjects are in Table 1. Most received fludarabine/melphalan conditioning and had matched unrelated donor peripheral blood stem cell grafts. The median duration of PAC therapy was 76 days (range, 7-100 days). PAC treatment duration and association with clinical outcomes are presented in supplemental data (supplemental Table 1; supplemental Figure 4). Median time to absolute neutrophil count (15 days; range, 11-23 days) and platelet (10 days; range, 0-26 days) engraftment, and donor chimerism (supplemental Table 2) did not differ from historic benchmarks for SIR/TAC alone. Grade 3 and above adverse events viewed possibly related to PAC are in supplemental data (supplemental Table 3). Grade II to IV acute GVHD was 46% (95% confidence interval [CI], 27%-64%), and grade III to IV acute GVHD was 18% (95% CI, 6.3%-34%) by day 100 post-HCT (Figure 5A). Acute GVHD organ staging is presented in supplemental data (supplemental Table 4). Overall grade III to IV acute GVHD was driven by stage 4 skin (N = 1), stage 4 skin/stage 3 gastrointestinal (GI) (N = 1), or stage 3 to 4 GI (N = 3). Among 13 subjects with overall grade II to IV acute GVHD, 11 received systemic corticosteroids (range of 0.5-2 mg/kg per day dosing per clinician discretion, not mandated by protocol). A total of 6 had corticosteroid-refractory disease and went on to second-line therapy (ruxolitinib, N = 6; extracorporeal photopheresis, N = 4; mycophenolate mofetil [MMF], N = 1). Ruxolitinib-specific response was not fully evaluable, as it was coadministered with extracorporeal photopheresis in 4 cases and MMF in another. However, steroid-refractory–acute GVHD outcomes were poor; 5 died from GVHD-associated complications, including infection and multiorgan failure. Separately, 2 subjects with overall grade I (skin stage 1, overall grade I) acute GVHD were successfully treated with 0.5 mg/kg per day prednisone. National Institutes of Health moderate/severe chronic GVHD was 3.6% (95% CI, 0.24%-16%) by 12 months, and 18% (95% CI, 5.1%-37%) by 24 months (Figure 5B), whereas any chronic GVHD was 25% (95% CI, 11%-42%) by 12 months and 45% (95% CI, 23%-65%) by 24 months. Two late acute GVHD cases were also observed (1 skin stage 1, 1 GI stage 1). With additional long-term follow-up (8 months after the final analysis), 1 additional patient developed chronic GVHD 14.5 months after complete immune suppression discontinuation.
Baseline characteristics of subjects treated in the phase 2 trial
| Characteristic . | Value (N = 28) . |
|---|---|
| Patient age, y | 67 (40-76) |
| Donor age, y | 25 (19-69) |
| Patient gender | |
| F | 12 (43) |
| M | 16 (57) |
| Race | |
| White | 28 (100) |
| Ethnicity | |
| Hispanic | 1 (4) |
| Non-Hispanic | 27 (96) |
| Disease | |
| AML | 13 (46) |
| CMML | 4 (14) |
| MDS | 9 (32) |
| MF | 2 (7) |
| KPS | |
| 80 | 9 (32) |
| 90 | 18 (64) |
| 100 | 1 (4) |
| HCT-CI | |
| 0-2 | 17 (61) |
| 3-4 | 11 (39) |
| Conditioning | |
| Bu(5300)/Flu | 7 (25) |
| Flu/Mel | 21 (75) |
| Donor type | |
| MRD | 7 (25) |
| MUD | 21 (75) |
| CMV (donor/recipient) | |
| Neg/neg | 4 (14) |
| Neg/pos | 8 (29) |
| Pos/neg | 6 (21) |
| Pos/pos | 10 (36) |
| Characteristic . | Value (N = 28) . |
|---|---|
| Patient age, y | 67 (40-76) |
| Donor age, y | 25 (19-69) |
| Patient gender | |
| F | 12 (43) |
| M | 16 (57) |
| Race | |
| White | 28 (100) |
| Ethnicity | |
| Hispanic | 1 (4) |
| Non-Hispanic | 27 (96) |
| Disease | |
| AML | 13 (46) |
| CMML | 4 (14) |
| MDS | 9 (32) |
| MF | 2 (7) |
| KPS | |
| 80 | 9 (32) |
| 90 | 18 (64) |
| 100 | 1 (4) |
| HCT-CI | |
| 0-2 | 17 (61) |
| 3-4 | 11 (39) |
| Conditioning | |
| Bu(5300)/Flu | 7 (25) |
| Flu/Mel | 21 (75) |
| Donor type | |
| MRD | 7 (25) |
| MUD | 21 (75) |
| CMV (donor/recipient) | |
| Neg/neg | 4 (14) |
| Neg/pos | 8 (29) |
| Pos/neg | 6 (21) |
| Pos/pos | 10 (36) |
Data are given as median (range) or number (percentage).
AML, acute myelogenous leukemia; Bu, busulfan; CMML, chronic myelomonocytic leukemia; CMV, cytomegalovirus; F, female; Flu, fludarabine; HCT-CI, transplant comorbidity index; KPS, Karnofsky performance status; M, male; MDS, myelodysplastic syndrome; Mel, melphalan; MF, myelofibrosis; MRD, matched related donor; MUD, matched unrelated donor; Neg, negative; Pos, positive.
Clinical outcomes following PAC/SIR/TAC therapy. (A) Cumulative incidence of grade II to IV acute GVHD. (B) Cumulative incidence of National Institutes of Health (NIH) moderate/severe chronic GVHD. (C) Overall survival. (D) Cumulative incidence of malignancy relapse post-HCT.
Clinical outcomes following PAC/SIR/TAC therapy. (A) Cumulative incidence of grade II to IV acute GVHD. (B) Cumulative incidence of National Institutes of Health (NIH) moderate/severe chronic GVHD. (C) Overall survival. (D) Cumulative incidence of malignancy relapse post-HCT.
By 24 months, 43% (95% CI, 24%-61%) had discontinued tacrolimus and 36% (95% CI, 16%-57%) had completed sirolimus, where discontinuation events were categorized as such only for intentional taper and discontinuation (not for toxicity or relapse). Overall survival was 71% (95% CI, 51%-85%) (Figure 5C). The cumulative incidence of relapse by 12 months was 11% (95% CI, 2.6%-25%) (Figure 5D), and nonrelapse mortality by 12 months was 29% (95% CI, 13%-46%). Four TMA cases occurred on the trial (grade 1, N = 1; grade 2, N = 3). Hepatic veno-occlusive disease occurred in 2 cases (1 moderate, 1 severe). A total of 3 subjects had cytomegalovirus reactivation post-HCT (viremia, N = 2; colitis, N = 1); no subjects on the trial received letermovir prophylaxis. There was no evidence for increased infectious complications (infection density; supplemental data table; supplemental Table 5). A total of 3 subjects discontinued PAC early in the setting of QTcF prolongation (supplemental Table 6). No significant prolongation of coagulation studies (supplemental Table 7) or reduction in cardiac ejection fraction (supplemental Table 8) was observed on therapy.
Acute GVHD did not correlate with duration of PAC therapy, depth of pSTAT3 inhibition, or T-cell subsets in blood
No correlation was seen between duration of PAC therapy and incidence of acute GVHD; most acute GVHD cases occurred during PAC therapy. As well, most chronic GVHD events occurred in subjects with the full intended duration of PAC therapy (supplemental Figure 4). Overall, PAC/SIR/TAC achieved low and uniform pSTAT3+ CD4+ T-cell values at day +21. There was no statistical difference in day +21 percentage pSTAT3+ CD4+ T cells when considering no GVHD vs any grade II to IV acute GVHD, the onset of acute GVHD, or the need for systemic immune suppression (Figure 6A-C). The day +21 frequency of Th1, Th2, Th17, or Tregs, as well as the ratio of Treg:Th1 + Th17 cells, were similar among PAC/SIR/TAC treated patients regardless of acute GVHD occurrence (Figure 6D-H).
Acute GVHD did not correlate with duration of PAC therapy, depth of pSTAT3 inhibition, or T-cell subsets. (A-C) Percentage (%) of pSTAT3+ CD4+ T cells at day +21 among patients treated on phase 2 PAC/SIR/TAC based on presence or absence of grade II to IV acute GVHD by day +100 (A), grade II to IV acute GVHD by day +30 (B), or use of systemic immune suppression (IS) beyond PAC/SIR/TAC to treat grade II to IV acute GVHD (C). (D-G) %CD4+ Th1 (D), Th2 (E), Th17 (F), or Treg (G) cells at day +21 among patients treated on phase 2 PAC/SIR/TAC based on presence of absence of grade II to IV acute GVHD by day +100. (H) Treg:Th1 + Th17 ratio at day +21 among patients without or with grade II to IV acute GVHD. NS, not significant.
Acute GVHD did not correlate with duration of PAC therapy, depth of pSTAT3 inhibition, or T-cell subsets. (A-C) Percentage (%) of pSTAT3+ CD4+ T cells at day +21 among patients treated on phase 2 PAC/SIR/TAC based on presence or absence of grade II to IV acute GVHD by day +100 (A), grade II to IV acute GVHD by day +30 (B), or use of systemic immune suppression (IS) beyond PAC/SIR/TAC to treat grade II to IV acute GVHD (C). (D-G) %CD4+ Th1 (D), Th2 (E), Th17 (F), or Treg (G) cells at day +21 among patients treated on phase 2 PAC/SIR/TAC based on presence of absence of grade II to IV acute GVHD by day +100. (H) Treg:Th1 + Th17 ratio at day +21 among patients without or with grade II to IV acute GVHD. NS, not significant.
PAC therapy was not associated with skewing of tissue-resident T-cell populations in GVHD target organs
We examined tissue-resident CD4+ T-cell subsets for subjects (N = 13 subjects, total of 23 individual organ site biopsies, including skin, stomach, small intestine, and rectum) with available GVHD target organ biopsies and compared these with biopsies from off-protocol, SIR/TAC alone matched control subjects (N = 13 subjects). Each case/control pair was matched for GVHD target organ site, clinical GVHD staging per organ site, and pathologic grade. Treg, Th1, Th2, and Th17 cells were quantified from tissue sections by immunofluorescence. Th2 appeared greater in the stomach and skin for cases vs controls, yet not statistically significant. No other significant differences were seen across case/control comparison (supplemental Figures 5-8).
Discussion
Our pharmacodynamic results show potent and uniform suppression of circulating pSTAT3+ CD4+ T cells with PAC/SIR/TAC, meeting the primary end point of the trial. Despite a presumably favorable increased ratio of Tregs to Th1/Th17 cells, PAC/SIR/TAC did not improve GVHD prevention after matched related or unrelated alloHCT. We found no clinical, immunologic, or medication adherence factor that correlated with acute GVHD breakthrough on PAC/SIR/TAC. Early posttransplant pacritinib treatment interruptions, such as for diarrhea, did not impact adequate suppression of pSTAT3+ CD4+ T cells. Immune metrics, like CD4+ T-cell protein phosphorylation and T-cell subsets, reverted to pretransplant values by day +100. This occurred following taper and complete discontinuation of pacritinib starting on day +70. The PAC/SIR/TAC data are consistent with a recent phase 3 trial that targeted IL-6 with tocilizumab in GVHD prevention,5 where JAK2 receptor signaling is critically involved. Completed clinical trials testing tocilizumab, and now PAC, reveal a biologic disconnect between effective IL-6/JAK2/pSTAT3 axis blockade and a disappointing lack of clinical improvement in acute GVHD prevention.5
Our experience with PAC/SIR/TAC is distinct from the recent success established with posttransplant cyclophosphamide (PTCy)-based GVHD prevention in the multicenter, randomized phase 3 1703 Blood and Marrow Transplant Clinical Trials Network (BMTCTN) trial, where life-threatening grade III to IV acute GVHD was reduced to <10% among those receiving reduced-intensity conditioning.20 It appears that the benefits of PTCy are not conditioning specific, as recipients of myeloablative HLA-matched or 7/8-mismatched allografts also experienced low rates of grade III to IV acute GVHD with PTCy/TAC/MMF in 2 single-center phase 2 trials.21,22 Interestingly, PTCy is active against all potentially alloreactive conventional T cells and does not selectively impact Th1 or Th17 cells.23 However, PTCy is thought to spare Tregs via an aldehyde dehydrogenase–dependent mechanism.23 This suggests effective immune tolerance posttransplant need only to maintain Tregs and suppress alloreactive T cells, and that specific inhibition of Th1 or Th17 cells are less clinically relevant.
Unlike PAC, ruxolitinib inhibits JAK1 and JAK2, which may provide a higher intensity of immune suppression.18,19,24,25 Although ruxolitinib is indicated in the treatment of refractory acute and chronic GVHD, it remains experimental in the setting of GVHD prophylaxis.26 Interim results from a phase 2 trial of ruxolitinib plus tacrolimus and methotrexate in alloHCT for myelofibrosis exhibit a favorable impact on overall survival with low rates of acute and chronic GVHD.27 Additionally, other JAK1/2 inhibitors, like baricitinib, increase the ratio of Tregs to conventional T cells and show preclinical activity in GVHD prevention.28 Compared with PAC, the more global suppressive effect of JAK1/2 inhibitors could be advantageous in the setting of GVHD prophylaxis.
Although our phase 2 trial of PAC/SIR/TAC did not improve acute GVHD prophylaxis, the regimen successfully reduced pSTAT3, preserved pSTAT5, and suppressed Th1 and Th17 cells. This immunologic profile echoes the results achieved with the rho associated coiled-coil containing protein kinase 2 (ROCK2) inhibitor, belumosudil.29 Belumosudil is US Food and Drug Administration approved for the treatment of refractory chronic GVHD.30 PAC is being investigated as therapy for chronic GVHD (NCT05531786) at the National Institutes of Health Clinical Center. The role for PAC in chronic GVHD therapy is further bolstered by its suppression of colony stimulating factor 1 receptor (CSF1R), given axatilimab, an anti–CSF-1R IgG4 antibody, reduces cutaneous profibrotic macrophages.31,32 We are eager to see how PAC performs in treating chronic GVHD. Although rigorous targeting of Th1 and Th17 cells has yet to consistently yield clinical benefit in acute GVHD prevention,5,6 this approach does appear relevant in treating chronic GVHD.29,33,34 Th1 cells are impaired by belumosudil, and Th17 cells are suppressed by either ROCK inhibition or the Bruton tyrosine kinase/IL-2 inducible T-cell kinase (BTK/ITK) inhibitor ibrutinib.29,33,34 These clinical observations may highlight important biologic differences between preclinical murine modeling of GVHD and human pathology.
Our experience with PAC/SIR/TAC will inform translational development of GVHD prevention. In particular, we observed high levels of Aurora kinase A activity in donor T cells during PAC/SIR/TAC prophylaxis, which along with mTOR is critically involved in CD28 signaling and implicated in acute GVHD.16,17 Furthermore, uncontrolled Aurora kinase A activity can serve as a pathway for escape alloreactivity despite adequate mTOR blockade.16,17 We are now conducting a phase 1/2 GVHD prevention trial of VIC-1911, a selective Aurora kinase A inhibitor, paired with PTCy and sirolimus (NCT05120570). Distinct from abatacept, a cytotoxic T lymphocyte antigen 4 (CTLA-4) Fc fusion protein approved in acute GVHD prevention, concurrent inhibition of mTOR and Aurora kinase A may allow for more comprehensive control over CD28 costimulation among alloreactive T cells.16,17,35 Moreover, the trial consists of 2 primary end points evaluating a reduction in both grade II to IV acute GVHD and relapse, as Aurora kinase A inhibitors have cytotoxic effects against leukemia and lymphoma.36,37 Given the exceptional results with PTCy-based GVHD prophylaxis,20-22 we expect that future novel GVHD prevention regimens should offer anticancer activity as well to truly improve posttransplant survival.
As limitations, we note the following. First, patient adherence to the full intended duration of PAC therapy was limited, with only 9 of 28 subjects completing 100 days of therapy without any treatment interruption (hold or discontinuation). Diarrhea (whether infectious or chemotherapy related) was a common source of treatment interruption, and most subjects on the trial received fludarabine/melphalan conditioning. Delivery of PAC or other investigational drugs may be better tolerated after conditioning therapy with lower expected rates of severe toxicity at baseline. However, multiple other toxicity events drove therapy interruption or cessation that had no relationship to diarrhea. Regardless of PAC treatment interruptions, patients still demonstrated a consistent and deep suppression of circulating day +21 pSTAT3+ CD4+ T cells. Second, the phase 2 was electively stopped early for observed futility in acute GVHD prevention. Thus, we cannot fully comment on the GVHD (especially chronic GVHD) incidence that would have been observed with a larger study population. We, however, note the CIs surrounding our estimates for acute and chronic GVHD in this trial suggest no major benefit over standard-of-care GVHD prevention approaches. Although transplant-related mortality was a function of acute GVHD, the 1-year overall survival was still 71%. No deaths or relapses occurred after 1 year at the current time of follow-up, with a low relapse rate of 11%. We performed in vitro studies to investigate the impact of PAC/SIR/TAC on T-cell exhaustion, where limiting CD8+ T-cell exhaustion has implications in disease control and GVL.38 PAC/SIR/TAC significantly reduced T-cell CD39, programmed death 1 (PD-1), and T-cell immunoglobulin and mucin domain 3 (TIM-3) expression (supplemental Figure 9). This suggests a potential favorable influence on disease control after alloHCT by PAC/SIR/TAC, despite its limitations in controlling GVHD.
Acknowledgment
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant R01 HL133823 (B.C.B.).
Authorship
Contribution: J.P. codesigned and led the phase 2 clinical trial at Moffitt Cancer Center, interpreted clinical/correlative data, and wrote the manuscript; S.G.H. led the phase 2 clinical trial at the University of Minnesota, interpreted clinical/correlative data, and edited the manuscript; K.W. and S.M. performed experiments and edited the manuscript; J.K. and B.C. performed statistical analyses and edited the manuscript; H.E., A.M., N.B., T.N., F.K., L.P., R.G.F., N.E.J., V.B., and M.L.D. enrolled patients, performed clinical analyses on the phase 2 clinical trial, and/or edited the manuscript; C.M.S. performed and analyzed immunofluorescence imaging; J.P., J.B., D.J.W., B.R.B., J.S.M., and C.A. participated in experimental design and edited the manuscript; and B.C.B. designed and performed experiments, codesigned the phase 2 clinical trial, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: J.P. reports research support from Novartis, Amgen, Takeda, Janssen, Johnson and Johnson, Pharmacyclics, AbbVie, CTI BioPharma Corp, a Sobi company, and Bristol Myers Squibb; and reports consulting and advisory board membership with Syndax, CTI BioPharma Corp, a Sobi company, Amgen, and Regeneron. S.G.H. reports other support from Incyte and VITRAC Therapeutics. S.G.H. is a clinical trial adjudicator for CSL Behr. B.C.B. reports research support from CTI BioPharma Corp, a Sobi company, VITRAC Therapeutics, and Incyte. B.C.B. holds a patent (WO2015120436A2) related to CD4+ T-cell phosphorylated STAT3 as a marker and therapeutic target of acute graft-versus-host disease (GVHD) and a provisional patent (WO2017058950A1) related to the use of JAK inhibitors for rejection and GVHD prevention. B.C.B. has a patent for WO2019165156 on CD83 chimeric antigen receptor (CAR) T, previously licensed to CRISPR Therapeutics. D.J.W. reports grants from Fate Therapeutics and Incyte outside the submitted work. J.S.M. consults for and holds stock in Fate Therapeutics, GT BioPharma, and Vycellix; and reports research support from Fate Therapeutics and GT Biopharma. J.S.M. serves on the Scientific Advisory Board of ONK Therapeutics, Wugan, and Sanofi. M.L.D. reports grants from CRISPR, Kite/Gilead, and Novartis; other support from Bellicum, Adicet, Adaptive Biotechnologies; and personal fees from Capstan and CARGO. M.L.D. has a patent for CD83 CAR previously licensed to CRISPR. B.R.B. reports research funding from BlueRock Therapeutics and Carisma Therapeutics and consulting fees from BlueRock Therapeutics, Editas Medicine, Janssen Oncology, Sandoz, Legend Biotech, GentiBio Inc, and Magenta Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Brian C. Betts, Roswell Park Comprehensive Cancer Center, 665 Elm St, Buffalo, NY 14203; email: brian.betts@roswellpark.org.
References
Author notes
Requests for deidentified participant data will be considered with an appropriate institutional material transfer agreement. Proposals for access consideration should be sent to brian.betts@roswellpark.org.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal