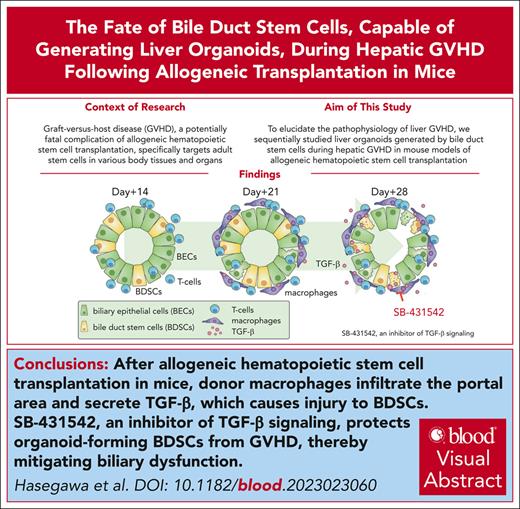
Key Points
GVHD targets organoid-forming bile duct stem cells in a TGF-β–dependent manner.
TGF-β inhibitor protects organoid-forming bile duct stem cells against GVHD and mitigates biliary dysfunction.
Visual Abstract
Graft-versus-host disease (GVHD) is a major life-threatening complication that occurs after allogeneic hematopoietic cell transplantation (HCT). Although adult tissue stem cells have been identified as targets of GVHD in the skin and gut, their role in hepatic GVHD is yet to be clarified. In the current study, we explored the fate of bile duct stem cells (BDSCs), capable of generating liver organoids in vitro, during hepatic GVHD after allogeneic HCT. We observed a significant expansion of biliary epithelial cells (BECs) on injury early after allogeneic HCT. Organoid-forming efficiency from the bile duct was also significantly increased early after allogeneic HCT. Subsequently, the organoid-forming efficiency from bile ducts was markedly decreased in association with the reduction of BECs and the elevation of plasma concentrations of bilirubin, suggesting that GVHD targets BDSCs and impairs the resilience of BECs. The growth of liver organoids in the presence of liver-infiltrating mononuclear cells from allogeneic recipients, but not from syngeneic recipients, was significantly reduced in a transforming growth factor-β (TGF-β)–dependent manner. Administration of SB-431542, an inhibitor of TGF-β signaling, from day 14 to day 28, protected organoid-forming BDSCs against GVHD and mitigated biliary dysfunction after allogeneic HCT, suggesting that BDSCs are a promising therapeutic target for hepatic GVHD.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment for hematologic malignancies, bone marrow failure syndromes, and inherited immunodeficiencies. Graft-versus-host disease (GVHD) is the major obstacle to performing allogeneic HCT, and the liver is one of the major target organs in GVHD. The most prominent pathologic features of hepatic GVHD include portal mononuclear cell infiltration, bile duct damage, and varying degrees of parenchymal damage.1 Although hepatocytes are highly regenerative, biliary epithelial cells (BECs) have limited regenerative capability, and severe ductal injury leads to vanishing bile duct syndrome.2 Emerging evidence indicates that tissue resilience is promoted by the regeneration from tissue-specific stem cells.3,4 Although we and others found that GVHD primarily targets tissue stem cells in the gut and skin,5-9 the fate and pathophysiological role of tissue stem or progenitor cells in the liver remain to be clarified. It has been shown that multipotent stem cells residing in bile duct epithelia produce liver organoids.10-12 In the current study, we explored the fate of bile duct stem cells (BDSCs) during GVHD and developed a novel treatment strategy targeting BDSCs in the hepatic GVHD using mouse models of allogeneic HCT.
Materials and methods
Mice
Female C57BL/6 (B6: H-2b), B6D2F1 (BDF1: H-2b/d), and BALB/c (H-2d/d) mice were purchased from CLEA Japan. B6.Cg-Gt(ROSA)-26Sortm14(CAG-tdTOMATO)Hze/J (B6-R26tdTomato) and B6.Cg-Tg(Lck-cre)548Jxm/J (B6-Lck-cre) mice were purchased from Jackson Laboratory. Lck-cre×R26tdTomato mice were generated by crossing B6-R26tdTomato mice with B6-Lck-cre mice. All animal experiments were performed under the auspices of the Institutional Animal Care and Research Advisory Committee (approval numbers 17-0026 and 23-0097). Experiments in this article were performed in a nonblinded manner.
Hematopoietic cell transplantation
BDF1 recipient mice at 8 to 12 weeks old received 11 Gy total body irradiation, split into 2 doses with a 4-hour interval, followed by IV injection of 5 × 106 splenocytes plus 5 × 106 bone marrow (BM) cells from allogeneic B6 donors or syngeneic BDF1 donors on day 0. In the experiments in which Lck-cre×R26tdTomato mice were used as donors, graft cells were infused into syngeneic B6 or allogeneic BDF1 recipients. In the indicated experiments, lethally irradiated (11 Gy) B6 recipients were transplanted with 5 × 106 splenocytes plus 5 × 106 BM cells from allogeneic BALB/c donors. Transplanted mice were kept in a specific pathogen-free condition with normal chow and autoclaved hyperchlorinated water. Survival of recipient mice was monitored daily, and GVHD severity was assessed weekly using clinical GVHD scores, weight loss, posture, activity, fur texture, and skin integrity, as previously described.13
Reagents
SB-431542 (LC Laboratories) was dissolved in 1% dimethyl sulfoxide (Sigma-Aldrich) mixed in phosphate-buffered saline at a concentration of 0.5 mg/mL and was intraperitoneally injected at 5 mg/kg daily from day 14 to day 28.
Flow cytometric analysis of the liver
After incubation with 0.4 mg/mL Collagenase type IV (Sigma-Aldrich) in RPMI 1640 (Sigma-Aldrich) containing 10% fetal calf serum (Biowest) for 1 hour at 37°C, the cells were harvested for further analysis. Liver mononuclear cells (LMCs) were recovered from the interface between 40% and 80% Percoll (GE Healthcare) after centrifugation at 2800 rpm for 20 minutes. Staining was performed after red blood cell lysis with lysing buffer (BD Biosciences). The antibodies used in flow cytometric analyses are listed in supplemental Table 1 (available on the Blood website). For intranuclear staining, cells were fixated and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) after staining of surface molecules. Samples were analyzed using FACSCanto II (BD Biosciences), FACSAria III (BD Biosciences), and FlowJo software (Tree Star, Inc).
Quantitative polymerase chain reaction
The total RNA from the liver, BECs, or organoids was extracted using ISOGEN II (Nippon Gene). cDNA was synthesized using a ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo). Polymerase chain reactions (PCRs) and analyses were performed with Applied Biosystems StepOnePlus (Thermo Fisher Scientific) using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific), and the primer/probe sets used are listed in supplemental Table 2. The relative amount of each messenger RNA was determined using the standard curve method and was normalized to the level of 18S rRNA in each sample.
Culture of liver organoids
Liver organoids were generated as previously described.10 Briefly, the right lobe of the liver was cut into small pieces and incubated in 10 mL of tissue dissociation cocktail consisting of advanced Dulbecco modified Eagle medium/F-12 (Gibco) supplemented with 0.125 mg/mL collagenase IV (Sigma-Aldrich), 0.125 U/mL dispase (Stemcell Technologies), and 11.25 mM HEPES (Nacalai Tesque), at 37°C for 20 minutes. Samples were vigorously pipetted and settled by gravity for 1 minute, and the supernatant was discarded. Samples were incubated for another 20 minutes in 10 mL of tissue dissociation cocktail and then pipetted vigorously, and the supernatant was pooled for further processing. This step was repeated 4 times, and pooled supernatant was filtered using a 70-μm cell strainer to a new 50-mL conical tube. Floating particles in the flow-through were collected using a 40-μm cell strainer and resuspended into 30 μL of Corning Matrigel growth factor reduced basement membrane matrix, phenol red-free, LDEV-free (Corning Life Sciences) and dispensed to a 24-well plate to form a dome (50 μL/well). After incubation at 37°C for 10 minutes to let domes solidify, 750 μL of HepatiCult Organoid Growth Medium (Stemcell Technologies) was supplemented with HepatiCult OGM Mouse Supplement (Stemcell Technologies). Organoids were enumerated 7 days later. We counted only large organoids (diameter > 200 μm), based on a previous article that suggested these large organoids were exclusively derived from BDSCs.14 To test the impacts of interferon gamma (IFN-γ), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) on organoid growth, organoids generated from the bile duct fragments of naïve liver were homogenized and seeded at a concentration of 200 fragments/well on the 24-well plate in the presence or absence of recombinant mouse TNF-α (rmTNF-α; R&D Systems), rmIFN-γ (BioLegend), and rmTGF-β (BioLegend). The concentration of rmTNF-α and rmIFN-γ ranged from 1 to 100 ng/mL, and that of rmTGF-β ranged from 0.01 to 1 ng/mL. The number of organoids was quantified 4 days later. The number of organoids exhibited some variation between experiments, even in the control arm, likely because of the varying extent of organoid dissociation at the initiation of the culture. Therefore, we presented the data as a fold change from the controls.
Coculture of liver organoids with liver-infiltrating mononuclear cells
Coculture of organoids and LMCs was performed in the 1:1 mixture of ALyS705 medium without interleukin-2 (Cell Science & Technology Institute) and HepatiCult OGM Medium. LMCs were collected on day 28 after allogeneic or syngeneic transplantation and cultured at a concentration of 1 × 105/well with organoids. The number of intact organoids was quantified 4 days later. In the indicated experiments, LMCs were separated into T cells and non-T cells using fluorescence-activated cell sorting with a FACSAria III (BD Biosciences), and then cocultured with liver organoids.
Results
Hepatic GVHD induces portal infiltration of donor T cells followed by macrophages
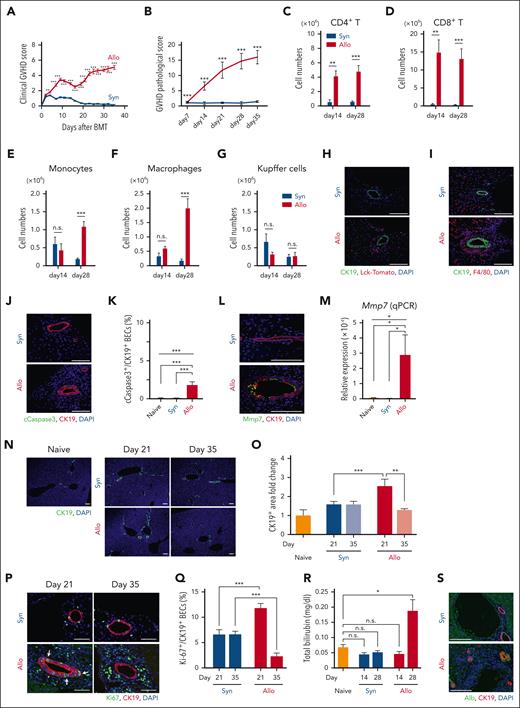
To explore the role of tissue stem cells in hepatic GVHD, we took advantage of a well-established mouse model of allogeneic HCT, in which lethally irradiated BDF1 recipients were transplanted with of 5 × 106 splenocytes plus 5 × 106 BM cells from major histocompatibility complex–mismatched B6 donors. Clinical GVHD scores were significantly elevated compared with syngeneic controls, and mortality occurred at a later time point (Figure 1A; supplemental Figure 1A). Pathologic examination of the liver on day 21 demonstrated portal infiltration of inflammatory cells, lymphoepithelial lesions in the biliary epithelia, and apoptosis and exfoliation of BECs in allogeneic animals, but not in syngeneic animals (supplemental Figure 1B). Pathologic GVHD scores were slightly but significantly elevated on day 7, and they became markedly elevated from the second week after allogeneic HCT, but not after syngeneic HCT (Figure 1B).
Hepatic GVHD induces apoptosis and transitory proliferation of BECs. (A-G) Lethally irradiated BDF1 mice were transplanted from allogeneic B6 (Allo) or syngeneic BDF1 (Syn) donors on day 0. Clinical GVHD scores (A; n = 11-12/group) and pathologic liver GVHD scores (B; n = 10/group) are shown. (C-G) Flow cytometric analyses of liver mononuclear cells were performed at indicated time points (n = 7-12/group). See also supplemental Figures 1 and 2. Absolute numbers of donor CD4+ T cells (C), CD8+ T cells (D), donor monocytes (E), infiltrating donor macrophages (F), and Kupffer cells (G) are shown. (H,I) Lethally irradiated B6 (Syn) or BDF1 (Allo) mice were transplanted with purified T cells from B6 Lck-cre×R26Tomato combined with B6 T-cell-depleted-BM cells on day 0. (H) Immunofluorescent staining of CK19 (green), tdTomato+ donor T cells (red) with DAPI (4′,6-diamidino-2-phenylindole; blue) staining on day 21 after HCT. (I) Immunofluorescent staining of CK19 (green), F4/80 (red) with DAPI (blue) staining on day 28 after HCT. (J-S) Lethally irradiated BDF1 mice were transplanted as in Figure 1A. Immunofluorescent staining of cleaved caspase-3 (cCaspase3; green) and CK19 (red) with DAPI (blue) staining on day 21 after HCT. Representative images (J) and the proportion (K) of cCaspase 3+ BECs (n = 4-5/group). (L) Immunofluorescent staining of Mmp7 (green) and CK19 (red) with DAPI (blue) staining on day 21. (M) Relative expression of Mmp7 in the liver on day 21 (n = 5/group). (N,O) Immunofluorescent staining of CK19 (green) with DAPI (blue) staining on days 21 and 35. Representative images (N) and proportion (O) of CK19+ area were shown as fold change from naive mice (n = 5/group). (P,Q) Immunofluorescent staining of Ki-67 (green) and CK19 (red) with DAPI (blue) staining on days 21 and 35. Representative images (P) and proportions (Q) of Ki-67+ cells among CK19+ BECs were shown (n = 5/group). White arrows indicate clusters of Ki-67+ BECs. (R) Plasma levels of total bilirubin from the recipients (n = 10/group) and naïve BDF1 (n = 4) were shown. (S) Immunofluorescent staining of albumin (Alb; green) and CK19 (red) with DAPI (blue) staining 70 days after HCT. (A-G, O, Q, R) Data from 2 independent experiments were combined and shown as mean ± standard error (SE). (K,M) Data from 1 of 2 independent experiments are shown as mean ± SE. Bar, 100 μm. ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Hepatic GVHD induces apoptosis and transitory proliferation of BECs. (A-G) Lethally irradiated BDF1 mice were transplanted from allogeneic B6 (Allo) or syngeneic BDF1 (Syn) donors on day 0. Clinical GVHD scores (A; n = 11-12/group) and pathologic liver GVHD scores (B; n = 10/group) are shown. (C-G) Flow cytometric analyses of liver mononuclear cells were performed at indicated time points (n = 7-12/group). See also supplemental Figures 1 and 2. Absolute numbers of donor CD4+ T cells (C), CD8+ T cells (D), donor monocytes (E), infiltrating donor macrophages (F), and Kupffer cells (G) are shown. (H,I) Lethally irradiated B6 (Syn) or BDF1 (Allo) mice were transplanted with purified T cells from B6 Lck-cre×R26Tomato combined with B6 T-cell-depleted-BM cells on day 0. (H) Immunofluorescent staining of CK19 (green), tdTomato+ donor T cells (red) with DAPI (4′,6-diamidino-2-phenylindole; blue) staining on day 21 after HCT. (I) Immunofluorescent staining of CK19 (green), F4/80 (red) with DAPI (blue) staining on day 28 after HCT. (J-S) Lethally irradiated BDF1 mice were transplanted as in Figure 1A. Immunofluorescent staining of cleaved caspase-3 (cCaspase3; green) and CK19 (red) with DAPI (blue) staining on day 21 after HCT. Representative images (J) and the proportion (K) of cCaspase 3+ BECs (n = 4-5/group). (L) Immunofluorescent staining of Mmp7 (green) and CK19 (red) with DAPI (blue) staining on day 21. (M) Relative expression of Mmp7 in the liver on day 21 (n = 5/group). (N,O) Immunofluorescent staining of CK19 (green) with DAPI (blue) staining on days 21 and 35. Representative images (N) and proportion (O) of CK19+ area were shown as fold change from naive mice (n = 5/group). (P,Q) Immunofluorescent staining of Ki-67 (green) and CK19 (red) with DAPI (blue) staining on days 21 and 35. Representative images (P) and proportions (Q) of Ki-67+ cells among CK19+ BECs were shown (n = 5/group). White arrows indicate clusters of Ki-67+ BECs. (R) Plasma levels of total bilirubin from the recipients (n = 10/group) and naïve BDF1 (n = 4) were shown. (S) Immunofluorescent staining of albumin (Alb; green) and CK19 (red) with DAPI (blue) staining 70 days after HCT. (A-G, O, Q, R) Data from 2 independent experiments were combined and shown as mean ± standard error (SE). (K,M) Data from 1 of 2 independent experiments are shown as mean ± SE. Bar, 100 μm. ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Flow cytometric analysis of LMCs demonstrated that donor CD4+ and CD8+ T cells infiltrated into the liver within 2 weeks after allogeneic HCT (Figure 1, C-D; supplemental Figure 1, C-D). Infiltration of monocytes and monocyte-derived inflammatory macrophages, identified as F4/80+ CD11bhigh cells, was not observed at 2 weeks but became prominent on day 28. Meanwhile, liver-resident Kupffer cells, identified as F4/80+ CD11blow cells, did not show a significant increase in allogeneic animals compared with the syngeneic controls (Figure 1, E-G; supplemental Figures 1E and 2). Immunofluorescence studies to evaluate localization of infiltrating donor T cells and macrophages in the liver using B6-Lck-cre×R26tdTomato mice as donors showed massive infiltration of tdTomato+ donor T cells and F4/80+ macrophages around cytokeratin 19 (CK19)+ bile ducts in the portal area of the liver (Figure 1, H-I). These portal infiltrating macrophages in allogeneic animals were morphologically distinct from Kupffer cells, with relatively elongated shape, residing on the surface of the sinusoidal wall of both syngeneic and allogeneic animals (Figure 1I).
Hepatic GVHD induces apoptosis of BECs in association with biliary dysfunction
To evaluate injury of BECs in hepatic GVHD, we performed immunofluorescence studies targeting cleaved caspase-3, a hallmark of apoptosis. BECs expressing cleaved caspase-3 were significantly increased in allogeneic mice compared with syngeneic or naïve controls on day 21 after HCT (Figure 1, J-K). BECs in the allogeneic mice expressed matrix metalloproteinase 7 (Mmp7), a specific marker of BEC injury on day 21 (Figure 1L).15,16 Quantitative PCR analysis confirmed elevation of Mmp7 in the liver after allogeneic HCT (Figure 1M). It has been shown that there is an expansion of BECs, known as ductular reaction, after many types of liver injury. Immunofluorescent analysis of CK19 demonstrated the injury of BECs was associated with transient expansion of BECs on day 21; however, these reactive BECs were reduced at day 35 after allogeneic HCT, indicating that hepatic GVHD also inhibits regenerative response of BECs (Figure 1, N-O). Consistent with these findings, the proportion of Ki-67+ proliferating BECs was significantly higher in allogeneic mice compared with the syngeneic controls on day 21, whereas it was lower than that of the syngeneic controls on day 35 (Figure 1, P-Q). Importantly, clusters of Ki-67+ BECs were observed on day 21 only in allogeneic mice, suggesting that BEC injury induced the proliferation of BEC progenitors residing in the biliary epithelia (Figure 1P). Along with the reduction in BECs, an elevation in plasma concentrations of bilirubin, a hallmark of hepatic GVHD, was observed at a later time point of allogeneic transplantation (Figure 1R). On day 70, the remaining CK19+ BECs in allogeneic animals demonstrated atypical expression of the hepatocyte-specific marker albumin, suggesting that BEC progenitors possess a multipotent differentiation capacity (Figure 1S). Taken together, these findings indicate that BEC injury in allogeneic mice induces the proliferation of BDSCs, and subsequently, GVHD targets these cells at a later time point.
GVHD targets organoid-forming BDSCs in a TGF-β–dependent manner
Because it has been shown that the reduction of tissue stem cells in intestinal GVHD results in a decrease in the efficiency of intestinal organoid generation,17 we next explored whether the organoid-forming efficiency could be altered in hepatic GVHD (Figure 2A). The number of liver organoids generated from the bile ducts of the liver right lobe from allogeneic animals was on an increasing trend from days 7 to 21, whereas it turned to a decreasing trend from days 28 to 42 after allogeneic HCT (Figure 2B). Strikingly, the number of organoids was profoundly reduced at later time points, days 28 to 42, compared with the combined average number of organoids from naïve mice and syngeneic controls during days 28 to 35. In contrast, the number of organoids generated from the syngeneic controls was comparable to those from naïve mice (Figure 2B-C; supplemental Figure 3A). Along with the reduction in numbers, the size of organoids was also reduced at later time points after allogeneic HCT (supplemental Figure 3, B-C).
GVHD targets organoid-forming BDSCs in a TGF-β–dependent manner. Lethally irradiated BDF1 mice were transplanted from BDF1 (Syn) or B6 (Allo) donors as in Figure 1A, and the liver right lobes were subjected to organoid-forming assay. A schematic overview (A) of the organoid-forming assay and the number of liver organoids derived from the right lobe of the livers at indicated time points are shown. (B; n = 5-15/group). Time trends from days 7 to 21 to days 28 to 42 within Allo group were significantly different. The average value from days 28 to 42 in Allo group was significantly less than the combined average of the naïve and syngeneic groups during the same period. (C) Representative images of liver organoids generated from recipients’ livers on day 35. Bar, 500 μm. Quantitative polymerase chain reaction (Q-PCR) targeting IFN-γ (D), TNF-α (E), and TGF-β (H) was performed using total RNA extracted from recipient’s liver on day 28 (n = 6-11/group). (F,G,I) Liver organoids produced from naïve BDF1 mice were dissociated into small pieces, seeded at a concentration of 200 fragments/well on the 24-well plate in the presence or absence of IFN-γ (F), TNF-α (G), or TGF-β (I). Organoids were enumerated 4 days later, and the fold changes from controls were calculated (n = 6/group). (J) Dissociated organoids were cultured in the presence or absence of TGF-β and an SMAD2/3 inhibitor, SB-431542, for 4 days. The fold changes from controls were calculated. (K) The total RNA extracted from BECs from naïve BDF1 mice, syngeneic or allogeneic recipients, or liver organoids generated from naïve BDF1 mice was subjected to Q-PCR targeting the TGF-β receptor (n = 6-10/group). (B,D-K) Data from 2 independent experiments were combine and shown as mean ± standard error. ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
GVHD targets organoid-forming BDSCs in a TGF-β–dependent manner. Lethally irradiated BDF1 mice were transplanted from BDF1 (Syn) or B6 (Allo) donors as in Figure 1A, and the liver right lobes were subjected to organoid-forming assay. A schematic overview (A) of the organoid-forming assay and the number of liver organoids derived from the right lobe of the livers at indicated time points are shown. (B; n = 5-15/group). Time trends from days 7 to 21 to days 28 to 42 within Allo group were significantly different. The average value from days 28 to 42 in Allo group was significantly less than the combined average of the naïve and syngeneic groups during the same period. (C) Representative images of liver organoids generated from recipients’ livers on day 35. Bar, 500 μm. Quantitative polymerase chain reaction (Q-PCR) targeting IFN-γ (D), TNF-α (E), and TGF-β (H) was performed using total RNA extracted from recipient’s liver on day 28 (n = 6-11/group). (F,G,I) Liver organoids produced from naïve BDF1 mice were dissociated into small pieces, seeded at a concentration of 200 fragments/well on the 24-well plate in the presence or absence of IFN-γ (F), TNF-α (G), or TGF-β (I). Organoids were enumerated 4 days later, and the fold changes from controls were calculated (n = 6/group). (J) Dissociated organoids were cultured in the presence or absence of TGF-β and an SMAD2/3 inhibitor, SB-431542, for 4 days. The fold changes from controls were calculated. (K) The total RNA extracted from BECs from naïve BDF1 mice, syngeneic or allogeneic recipients, or liver organoids generated from naïve BDF1 mice was subjected to Q-PCR targeting the TGF-β receptor (n = 6-10/group). (B,D-K) Data from 2 independent experiments were combine and shown as mean ± standard error. ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Next, we explored the mechanism by which GVHD reduces liver organoid formation in allogeneic mice. Quantitative PCR using the total RNA extracted from the liver on day 28 demonstrated that expression of both IFN-γ and TNF-α was significantly elevated in allogeneic recipients, but not in syngeneic controls (Figure 2, D-E). On the basis of the previous reports showing that proinflammatory cytokines, such as IFN-γ and TNF-α, induced apoptosis of intestinal organoids,17-19 we explored the impacts of these cytokines on growth of liver organoids derived from naïve BDF1 mice. Unlike intestinal organoids, neither of these cytokines, even at high concentrations, had an impact on the growth of liver organoids (Figure 2, F-G; supplemental Figure 4, A-D). Consistent with these findings, expression levels of IFN-γ receptor and TNF-α receptor were significantly less in BECs compared with intestinal epithelial cells (supplemental Figure 5). We found that the expression levels of TGF-β in the liver were significantly elevated in allogeneic animals compared with naïve and syngeneic controls (Figure 2H). When added to organoids generated from naïve mice, TGF-β significantly reduced organoid formation (Figure 2I; supplemental Figure 4, E-F). SB-431542, a selective SMAD2/3 inhibitor, restored organoid formation in a dose-dependent manner despite the presence of TGF-β, indicating that the canonical TGF-β pathway inhibits liver organoid formation20 (Figure 2J; supplemental Figure 6, A-B). Interestingly, TGF-β receptor expression in BECs was upregulated after allogeneic HCT, but not after syngeneic transplantation. TGF-β receptor expression was also upregulated in liver organoids generated from naïve mice to the same levels as in freshly harvested BECs from allogeneic recipients, explaining the susceptibility of these naïve mice-derived organoids to TGF-β (Figure 2K).
TGF-β–producing inflammatory macrophages reduce liver organoid formation
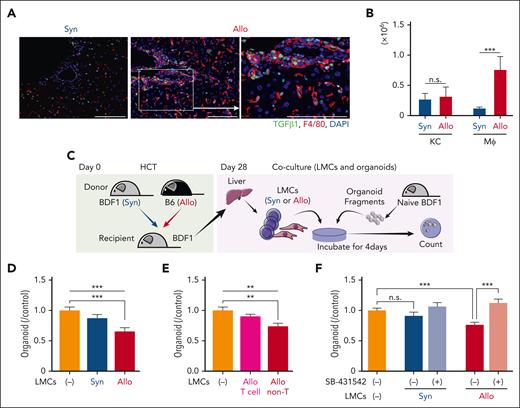
Next, we explored the producer cells of TGF-β in hepatic GVHD. Immunofluorescent study of F4/80 and TGF-β demonstrated that most (80.4% ± 5.8%) of portal infiltrating macrophages were TGF-β positive, as shown in cutaneous chronic GVHD21-23 (Figure 3A; supplemental Figure S6C). Flow cytometric analysis demonstrated that absolute numbers of TGF-β–producing inflammatory macrophages in the liver were significantly increased in allogeneic mice compared with syngeneic controls, whereas numbers of TGF-β–producing Kupffer cells were comparable between syngeneic and allogeneic recipients (Figure 3B). Next, we conducted coculture experiments to examine whether the LMCs from the recipients have an impact on the growth of liver organoids (Figure 3C). We found that LMCs from allogeneic recipients, but not from syngeneic recipients, significantly suppressed the growth of liver organoids derived from naïve mice (Figure 3D; supplemental Figure 6, D-E). When LMCs were divided into T cells and non-T cells, the latter consisting of 65% macrophages, only the coculture with non-T cells, not the purified T cells, significantly reduced the number of organoids, further confirming the major role of macrophages in TGF-β production (Figure 3E; supplemental Figure 6F). SB-431542 significantly restored the diminished organoid formation that was observed in the presence of LMCs from allogeneic recipients, highlighting the inhibitory role of TGF-β produced by these cells in liver organoid formation (Figure 3F; supplemental Figure 6G). Taken together, these findings indicate that the presence of TGF-β–producing inflammatory macrophages infiltrating the portal area of the liver is accountable for the observed decline in the capacity of liver organoid formation during hepatic GVHD.
Inflammatory macrophages reduce liver organoid formation in a TGF-β–dependent manner. (A) Immunofluorescent staining of the liver sections with TGF-β1 (green) and F4/80 (red) on day 28. Bar, 100 μm. (B) Intracellular staining of TGF-β was performed on day 28. Absolute numbers of TGF-β–producing Kupffer cells (KCs) and infiltrating macrophages (Mφs) were shown (n = 9-10/group). (C-D) Liver organoids derived from naïve BDF1 were cultured in the presence or absence of LMCs from syngeneic (Syn) or allogeneic (Allo) recipients. (D) Organoids were enumerated 4 days later, and the fold changes from controls were calculated (n = 7/group). (E) LMCs were separated into T cells and non-T cells, and then cocultured with liver organoids. Organoids were enumerated 4 days later (n = 8/group). (F) Organoids were cultured in the presence or absence of LMCs from syngeneic or allogeneic recipients with or without SB-431542 for 4 days. The fold changes from controls were calculated (n = 8-14/group). Data from 2 (B-E) or 3 (F) similar experiments were combined and shown as mean ± standard error.
Inflammatory macrophages reduce liver organoid formation in a TGF-β–dependent manner. (A) Immunofluorescent staining of the liver sections with TGF-β1 (green) and F4/80 (red) on day 28. Bar, 100 μm. (B) Intracellular staining of TGF-β was performed on day 28. Absolute numbers of TGF-β–producing Kupffer cells (KCs) and infiltrating macrophages (Mφs) were shown (n = 9-10/group). (C-D) Liver organoids derived from naïve BDF1 were cultured in the presence or absence of LMCs from syngeneic (Syn) or allogeneic (Allo) recipients. (D) Organoids were enumerated 4 days later, and the fold changes from controls were calculated (n = 7/group). (E) LMCs were separated into T cells and non-T cells, and then cocultured with liver organoids. Organoids were enumerated 4 days later (n = 8/group). (F) Organoids were cultured in the presence or absence of LMCs from syngeneic or allogeneic recipients with or without SB-431542 for 4 days. The fold changes from controls were calculated (n = 8-14/group). Data from 2 (B-E) or 3 (F) similar experiments were combined and shown as mean ± standard error.
Protection of BDSCs mitigated biliary dysfunction in hepatic GVHD
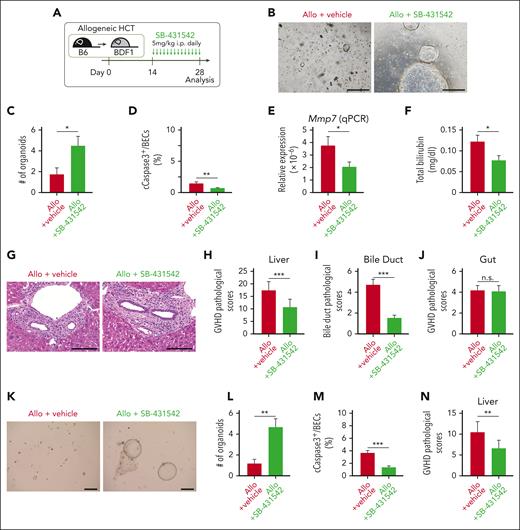
We next tested if TGF-β inhibition with SB-431542 could protect organoid-forming BDSCs against hepatic GVHD in vivo. Mice were daily administrated intraperitoneally with SB-431542 from day 14 after allogeneic HCT (Figure 4A). SB-431542 significantly restored the efficiency of organoid formation on day 28 after allogeneic HCT, indicating that TGF-β inhibition protected the organoid-forming BDSCs against GVHD (Figure 4, B-C; supplemental Figure 7). Furthermore, SB-431542 significantly reduced the proportion of apoptotic BECs, suppressed Mmp7 expression in the liver, and decreased plasma levels of bilirubin in allogeneic animals, suggesting that the protection of organoid-forming BDSCs through TGF-β inhibition resulted in the mitigation of biliary dysfunction in hepatic GVHD (Figure 4, D-F). The pathologic GVHD scores in the liver, particularly those related to bile duct damage, were significantly improved in SB-431542–treated allogeneic mice, whereas the gut pathologic GVHD scores were not improved (Figure 4, G-J). To test the impact of TGF-β inhibition on donor T-cell expansion and differentiation, we evaluated the numbers of donor CD4+ and CD8+ T cells and the expression of forkhead box protein p3 (Foxp3), granzyme B, and programmed cell death 1 (PD-1), as well as the production of IFN-γ and interleukin-17 in those cells on day 28. We found no effects of SB-431542 on these profiles (supplemental Figure 8). Therefore, we concluded that SMAD2/3 inhibition from day 14 after transplant did not affect donor T-cell expansion and function, whereas it directly protected BDSCs against TGF-β primarily produced by macrophages. Finally, we confirmed that SB-431542 protected BDSCs against GVHD and mitigated BEC injury and liver GVHD in another mouse model of acute GVHD, in which B6 recipients were transplanted from BALB/c donors, thereby excluding strain-specific effects of SMAD2/3 inhibition on BDSCs (Figure 4, K-N).
Protection and expansion of BDSCs mitigated biliary dysfunction in hepatic GVHD. (A-J) Lethally irradiated BDF1 mice were transplanted as in Figure 1A. Allogeneic (Allo) recipients were intraperitoneally (i.p.) injected with 5 mg/kg SB-431542 daily from day 14 to day 28 after allogeneic HCT. (A) A schematic overview of SB-431542 treatment. Representative images (B) and numbers (C) of organoids derived from the right lobe of the livers on day 28 after HCT (n = 8-10/group). Bar, 500 μm. (D) The proportion of cleaved caspase 3 (cCaspase3)+ BECs on day 21 (n = 7-8/group). (E) Relative expression of Mmp7 in the liver on day 28 (n = 13-15/group). (F) Plasma levels of total bilirubin on day 28 (n = 12-13/group). Representative hematoxylin and eosin images of the liver (G), pathologic liver GVHD scores (H; n = 15/group), bile duct pathologic scores (I: n = 15/group), and pathologic intestinal GVHD scores (J; n = 15/group) on day 28. Bar, 100 μm. (K-N) Lethally irradiated B6 recipients were transplanted from BALB/c donors, and i.p. injected with SB-431542 daily from day 14 to day 28 after HCT. Representative images (K) and numbers (L) of organoids derived from the right lobe of the liver on day 28 (n = 10/group), the proportion of cCaspase3+ BECs on day 21 (M; n = 10/group), and pathologic liver GVHD scores on day 28 (N; n = 10/group). Bar, 500 μm. Data from 2 (C-F, L-N) or 3 (H-J) independent experiments were combined and shown as mean ± standard error. ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Protection and expansion of BDSCs mitigated biliary dysfunction in hepatic GVHD. (A-J) Lethally irradiated BDF1 mice were transplanted as in Figure 1A. Allogeneic (Allo) recipients were intraperitoneally (i.p.) injected with 5 mg/kg SB-431542 daily from day 14 to day 28 after allogeneic HCT. (A) A schematic overview of SB-431542 treatment. Representative images (B) and numbers (C) of organoids derived from the right lobe of the livers on day 28 after HCT (n = 8-10/group). Bar, 500 μm. (D) The proportion of cleaved caspase 3 (cCaspase3)+ BECs on day 21 (n = 7-8/group). (E) Relative expression of Mmp7 in the liver on day 28 (n = 13-15/group). (F) Plasma levels of total bilirubin on day 28 (n = 12-13/group). Representative hematoxylin and eosin images of the liver (G), pathologic liver GVHD scores (H; n = 15/group), bile duct pathologic scores (I: n = 15/group), and pathologic intestinal GVHD scores (J; n = 15/group) on day 28. Bar, 100 μm. (K-N) Lethally irradiated B6 recipients were transplanted from BALB/c donors, and i.p. injected with SB-431542 daily from day 14 to day 28 after HCT. Representative images (K) and numbers (L) of organoids derived from the right lobe of the liver on day 28 (n = 10/group), the proportion of cCaspase3+ BECs on day 21 (M; n = 10/group), and pathologic liver GVHD scores on day 28 (N; n = 10/group). Bar, 500 μm. Data from 2 (C-F, L-N) or 3 (H-J) independent experiments were combined and shown as mean ± standard error. ∗P < .05, ∗∗P < .01, ∗∗∗P < .005.
Discussion
Emerging evidence indicates that GVHD targets tissue stem cells in addition to mature epithelial cells, disrupting tissue homeostasis and decreasing the resilience of GVHD target organs, such as the gut and skin.5,6,8 The liver is a unique organ endowed with high regenerative capacity throughout life.11 It has been shown that telomerase reverse transcriptase–expressing hepatocytes are distributed randomly in the liver and maintain local hepatocytes without a requirement for facultative stem cells.24,25 Hepatic GVHD primarily targets the BECs, which are another major epithelial component of the liver.26
Mouse and human BECs contain a putative stem or progenitor population that likely resides in the canals of Hering. This BDSC population is endowed with high regenerative capacity and multipotency, as it can differentiate not only into BECs but also into hepatocytes in cases of severe hepatocyte injury.11,12,27 Distinct from BDSCs, leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5)+ stem-like cells emerged in the periportal zone after liver injury and contributed to the regeneration of surrounding parenchymal cells.10,28 Although the close relationship between injury-induced Lgr5+ stem-like cells and BDSCs was initially suggested because of their shared expression of BEC markers, these Lgr5+ cells are now recognized as periportal hepatocytes and fibroblasts with progenitor potential.28 Consistent with this, we did not find Lgr5+ BECs during hepatic GVHD (data not shown). In the current study, we focused on the organoid-forming capacity of BECs, which likely represents the number of stem cells in the bile ducts, and for the first time, we found that GVHD reduced the organoid-forming efficiency of BECs. The phenotypic identification of organoid-forming BECs should be explored in future studies.
It has long been recognized that there is an expansion of reactive BECs forming luminal structures after various types of liver injury in a yes-associated protein (YAP)-dependent manner, a phenomenon termed as the ductular reaction.11,28,29 Recently, it has been shown that ductular reaction compensates bile-excreting function and plays a critical role in the reconstruction of the biliary tree structure.30 We found that the bile duct expanded early after allogeneic HCT. However, it reduced at the later time point when the reduction in the organoid-forming capacity of the BECs and the development of jaundice were observed. It is noteworthy that the absence of a prominent ductular reaction is 1 of the pathologic characteristics that support the diagnosis of liver GVHD in humans.31-33 Because liver biopsy is almost always performed after the development of clinical hepatic GVHD, which is indicated by the presence of jaundice, BDSCs are already damaged by GVHD and ductular reaction is suppressed at the time of the biopsy. Profound depletion of BDSCs could lead to ductopenia or vanishing bile duct, representative pathologic features of chronic hepatic GVHD.26
The phenotypical identification of BDSCs is an area of active research using both animal models and human samples. Although several markers were proposed to identify BDSCs, such as epithelial cell adhesion molecule (EpCAM), trophoblast cell surface antigen 2 (TROP2), sex determining region Y box protein 9 (SOX9), LGR4, and CD133, these markers are shared by other populations, such as mature BECs and pericentral hepatocytes, making the quantification of BDSCs using phenotypic markers difficult.12,25,34,35 Thus, we took advantage of the liver organoid culture system to assess the frequencies of BDSCs in the bile duct after HCT. Liver organoid-forming capacity was transiently increased after allogeneic HCT and depleted at later time points. The method to enumerate BDSCs using specific markers in liver GVHD needs to be established in future studies, which will enable the direct assessment of apoptosis in these cells during liver GVHD. Currently, we believe that the colony-forming assay is the best way to evaluate the stem cell function in the bile duct. Liver organoids could be differentiated into both BECs and hepatocytes in the context of culture conditions, indicating multipotency of organoid-forming stem cells.10,36 We also found emergence of BECs with intermediate phenotypes between BECs and hepatocytes at later time points, potentially indicating multipotent differentiation capacity of stem cells in the biliary ducts.37 It was reported that intermediate cells were also observed in the chronic human liver injury.37
We and others previously showed that IFN-γ signaling plays a critical role in depletion of intestinal stem cells in GVHD and apoptosis induction of intestinal organoids by allogeneic T cells, and ruxolitinib mitigates IFN-γ–mediated ISC and organoid loss.17,19 We also previously reported that ruxolitinib protected skin and intestinal stem cells against GVHD.8,19 Unlike intestinal organoids, we found that liver organoids are resistant to IFN-γ and highly susceptible to TGF-β. Consistent with these findings from the organoid system, SB-431542, an SMAD2/3 inhibitor, preserved the organoid-forming capacity of BECs and mitigated jaundice in GVHD. It has been shown that macrophage-derived TGF-β plays a critical role in injury-induced hepatocyte senescence. Inhibition of TGF-β leads to the emergence of a stem/progenitor phenotype in hepatocytes and promotes liver regeneration after liver injury.38,39 In contrast, hepatocytes transdifferentiate into BECs in a TGF-β–dependent manner in a bile duct–deficient mouse model produced by knocking down NOTCH in the liver.40 This discrepancy suggests that the role of TGF-β signaling in the BEC differentiation is context dependent: development in the fetus vs repairment in the adult, presence vs absence of NOTCH signaling, or hepatocyte vs BEC derived.
It has been shown that metabolomic analyses of patients' plasma demonstrate a significant increase in primary and secondary bile acids in acute GVHD, possibly due to impaired bile duct functions.41 In line with this finding, it has been shown that bile acids were significantly reduced in both gut tissue and content in a mouse model. Importantly, the prophylactic administration of tauroursodeoxycholic acid suppressed gut epithelial injury in GVHD, according to this report.42 Therefore, it has been speculated that protecting the bile ducts with SB-431542 could ameliorate gut GVHD by restoring protective bile acid levels in the gut. However, we found that the SMAD2/3 inhibitor SB-431542 did not ameliorate intestinal acute GVHD. We speculate this is because we initiated the SB-431542 treatment on day 14 after allogeneic HCT, when gut epithelial injury had already culminated in this model. It is still possible that protection of the bile ducts ameliorated gut GVHD, which subsequently developed after bile injury. Indeed, it has been reported that TGF-β–neutralizing antibodies ameliorated gut GVHD in a mouse chronic GVHD model.43
MMP7 is primarily expressed on extrahepatic bile duct and gallbladder epithelial cells, not on the intrahepatic BECs in a steady state. Emerging evidence indicates that Mmp7 is induced on damaged intrahepatic BECs and released into the circulation.15,16,44 It is of note that BEC injury has already been detected, as indicated by increased apoptosis and Mmp7 expression, at a time point when the bile duct was still increased and compensating for biliary function. This finding suggests that serum MMP7 may serve as a predictive biomarker for hepatic GVHD.15 Furthermore, ectopic expression MMP7 on BECs enhances tissue injury by inducing inflammatory cytokines and chemokines, and an MMP7 inhibitor suppresses the biliary atresia phenotype in mice, suggesting that MMP7 could be a therapeutic target of hepatic GVHD.15
In summary, our study demonstrated the emergence and depletion of organoid-forming BDSCs in hepatic GVHD, and a novel TGF-β–dependent mechanism by which donor immune cells damage organoid-forming capacities in the liver. Our findings pave the way for future studies to develop protection of BDSCs as a novel strategy for the prophylaxis and treatment of hepatic GVHD.
Acknowledgments
This study was supported by Japan Society for the Promotion of Science KAKENHI (21K16259 to T.A., 20K17366 to H.O., 21H02944 and 19K17849 to S.T., 23K18296 and 23K21439 to T. Teshima, and 21K08409 and 24K02475 to D.H.).
Authorship
Contribution: D.H. and T. Teshima developed the conceptual framework of the study, designed the experiments, analyzed data, and wrote the manuscript; Y.H. designed the experiments, conducted experiments, analyzed data, and wrote the manuscript; Z.Z. and R.K. designed the experiments and conducted experiments; T.M., Y.S., W.L., H.S., T.S., T. Tateno, X.C., E.Y., S.T., H.O., T.A., and E.H. conducted experiments; and I.Y. conducted statistical analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daigo Hashimoto, Department of Hematology, Hokkaido University Faculty of Medicine, N15W7, Kita-ku, Sapporo, 060-8638, Japan; email: d5hash@pop.med.hokudai.ac.jp.
References
Author notes
This article does not report original code. For original data, please contact the corresponding author, Daigo Hashimoto (d5hash@pop.med.hokudai.ac.jp).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal