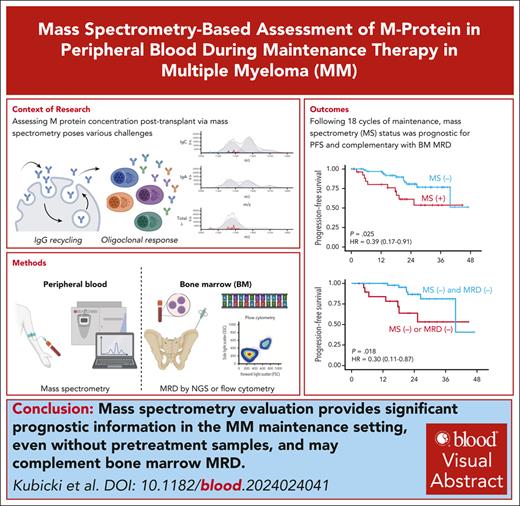
Key Points
MS provides significant prognostic information in the maintenance setting and complements BM MRD in multiple myeloma.
Without the aid of a baseline serum sample, MS’s prognostic performance is most significant 18 months after transplant.
Visual Abstract
Mass spectrometry (MS) can detect multiple myeloma–derived monoclonal proteins in the peripheral blood (PB) with high sensitivity, potentially serving as a PB assay for measurable residual disease (MRD). This study evaluated the significance of PB MS MRD negativity during posttransplant therapy in patients with newly diagnosed multiple myeloma. Serum samples from 138 patients treated in the phase 3 ATLAS trial of posttransplant maintenance with either carfilzomib, lenalidomide, and dexamethasone, or with lenalidomide alone were analyzed using EXENT MS methodology. We established feasibility of measuring MRD by MS in the PB in the posttransplant setting, despite unavailability of pretreatment calibration samples. There was high agreement between MRD by MS in the PB and paired bone marrow (BM) MRD results at the 10–5 threshold, assessed by either next-generation sequencing (NGS) or multiparameter flow cytometry (MFC) (70% and 67%, respectively). Agreement between PB MS and both BM MRD methods was lowest early after transplant and increased with time. MS negativity was associated with improved progression-free survival (PFS), which, in landmark analysis, reached statistical significance after 18 cycles after transplant. Combined PB/BM MRD negativity by MFC or NGS was associated with superior PFS compared with MRD negativity by only 1 modality. Sustained MS negativity carried similar prognostic performance to sustained BM MRD negativity at the 10–5 threshold. Overall, posttransplant MS assessment was feasible and provided additional prognostic information to BM MRD negativity. Further studies are needed to confirm the role and optimal timing of MS in disease evaluation algorithms. The ATLAS trial is registered at www.clinicaltrials.gov as #NCT02659293.
Introduction
The tremendous progress in therapies for patients living with multiple myeloma has resulted in a significant improvement in prognosis, attributed mostly to increases in the depth and durability of response.1,2 These improvements have increased the need for more sensitive tools for response assessment, particularly in patients who achieve the deepest levels of response. Standard definitions of response to therapy, which rely on measuring myeloma-associated monoclonal protein through serum protein electrophoresis (SPEP) with immunofixation (IFE), assessing serum free light chains ratio and cytomorphological evaluation of bone marrow (BM) biopsies through immunohistochemistry, lack the necessary sensitivity for measuring residual disease beyond the level of complete response.3,4 Evaluation of measurable (minimal) residual disease (MRD) in the BM using either multiparameter flow cytometry (MFC) or next-generation sequencing (NGS) enables the identification of residual myeloma cells in patients who achieve complete response by standard response assessment, with a limit of detection (LoD) as low as 1 myeloma cell per million nucleated cells analyzed.5,6
In addition to providing a more sensitive measure of response, MRD negativity has proven to be 1 of the most important prognostic markers in multiple myeloma, regardless of the disease setting.7 With increasing evidence that MRD negativity is associated with improved progression-free survival (PFS) and overall survival, MRD is now being used as a primary end point in randomized phase 3 trials and as a guide to change treatment as part of MRD-adapted study designs.8-13 Nevertheless, the assessment of MRD in the BM using current prevailing methods has several important limitations, including a requirement for inconvenient BM biopsies and the possibility of false negative results due to the presence of extramedullary lesions or the patchy nature of the disease.14 These limitations could be overcome by peripheral blood-based assays following the paradigm of liquid biopsy. Recently, sensitive measurement of serum monoclonal (M) protein concentration with mass spectrometry (MS) has emerged as a powerful tool capable of capturing lower concentrations of the M protein than those detectable by SPEP and IFE.15 Several studies have already shown that MS outperforms SPEP and IFE as a more sensitive method of detecting residual M protein, and emerging evidence shows that it can prognostically complement BM-based MRD assessments.16-20
MS limitations include the potential to falsely indicate the persistence of disease because of delayed clearance of M protein from the serum (particularly for recirculating immunoglobulin G [IgG]21), and the need for a baseline serum sample to enable the tracking of the unique mass-to-charge ratio (m/z) of the myeloma-associated M protein.22 The feasibility and potential utility of disease assessment by MS in patients lacking this baseline spectral signature, particularly after autologous hematopoietic stem cell transplantation (ASCT), has not been studied to date.
Here, we present the results of MS-based assessment of M protein in the peripheral blood without availability of pretreatment samples, as performed on patients with multiple myeloma undergoing maintenance therapy after ASCT in the randomized phase 3 ATLAS trial.
Methods
Patients and study design
ATLAS (clinicaltrials.gov identifier: NCT02659293) is an ongoing, open-label, randomized, phase 3 trial comparing post-ASCT treatment with lenalidomide alone (R) vs MRD-directed, risk-adapted combination of carfilzomib, R, and dexamethasone (KRd). The study, its protocol, and amendments were approved by the institutional review board or ethics committee at each participating institution and the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products in Poland. The study is conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice guidelines.
The detailed design of the ATLAS trial has been described previously.8 Briefly, patients with multiple myeloma that completed any induction therapy and within 100 days of ASCT were eligible to enroll. Only patients with available stored serum samples and who consented for exploratory testing were included in this analysis.
Treatment
Details of the assigned treatments have been published previously.8 In short, patients were randomized in a 1:1 ratio to either R or KRd. Randomization was stratified by cytogenetic risk (high vs standard), response after ASCT (at least very good partial response vs less than very good partial response), and country of enrollment (Poland vs United States). Patients in the KRd arm who had no detectable MRD at the 10–5 threshold as defined by the International Myeloma Working Group guidelines23 after cycle 6, and had no high-risk cytogenetic features as defined in the protocol (absence of del13, t(4;14), t(14;16), del17p, or hypodiploidy), de-escalated to single-agent R starting with cycle 9. Patients in the R maintenance group received R alone. After 36 cycles of treatment on both arms, all patients were assigned to receive R maintenance. Treatment continued until disease progression, unacceptable toxicity, or patient’s decision.
MRD assessment
MRD was assessed by centralized NGS using clonoSEQ (performed at Adaptive Biotechnologies, Seattle, WA; LoD of 6.8 × 10−7 with input of 20 μg DNA) and centralized and standardized MFC (minimum sensitivity of 10−5 based on the method of the EuroFlow Consortium24,25) performed either at the University of Chicago (Chicago, IL) or Poznań University of Medical Sciences (Poznań, Poland). MRD evaluations were performed at screening and after cycles 6, 12, 18, 24, and 36. Unless stated otherwise, MRD positivity by NGS refers to patients with MRD detected above the 10–5 threshold. For MRD by NGS, tracking was only enabled if a baseline sample was available and evaluable for clonotypic tracking. This restriction did not apply to MRD by MFC.
MS
Stored serum samples collected at screening (3 months after transplant) and after cycles 6, 12, 18, 24, and 36 were analyzed using the EXENT Immunoglobulin isotypes (GAM) assay (EXENT Solution, The Binding Site, part of Thermo Fisher Scientific), which uses matrix-assisted laser desorption-ionization time-of-flight (MALDI-TOF) MS. EXENT is an integrated clinical analyzer under development that consists of an automated liquid handler (EXENT-iP500), a MALDI-TOF mass spectrometer (EXENT-iX500), and instrument control/data analysis software (EXENT-iQ) for the detection and quantification of monoclonal immunoglobulins in serum. The device uses quantitative IgG, IgA, and IgM results (generated on the patient sample with the Optilite IgG, IgA, and IgM assays) combined with the EXENT assay peak areas in the mass spectra to calculate M-protein concentrations. The method, as described previously, allows for identification of M protein with a lower LoD of 0.015 g/L.26-28 For the current analysis, no baseline diagnostic samples were available, and samples were not available for every patient at every time point. A sample was called positive by MS when the EXENT solution modeled a peak with a concentration higher than that of the LoD, matching the known (diagnostic) isotype of the M protein (supplemental Figure 1, available on the Blood website). If multiple peaks were detected with the same isotype as the M protein at baseline, the peak with the highest concentration was assumed to represent the baseline M protein.
Statistical analysis
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated, with MRD by NGS or MFC serving as a reference. The numbers are presented with 95% confidence intervals (CIs). Time-to-event analyses such as PFS and overall survival were performed using the Kaplan-Meier method and groups were compared using the log-rank test. Hazard ratios (HRs) and 95% CIs for survival data were derived from Cox proportional hazards regression models. Statistical analysis was performed using GraphPad Prism version 10.1 and R software (version 4.3.0). All survival analyses were conducted using the same data set from the unplanned interim report.8
The study, its protocol, and amendments were approved by the institutional review board or ethics committee at each participating institution and the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products in Poland.
Results
Of 180 patients enrolled in the ATLAS trial, serum samples for MS were evaluable for 138 patients from at least 1 time point. This analysis was performed using clinical data from the previously reported cutoff (31 December 2021), the median follow-up from randomization for these 138 patients was 33.3 months (interquartile range, 9.2-40.7). Baseline characteristics are summarized in Table 1. Overall, M protein was assessed in 585 serum samples across all time points, including 349 paired results for MS and MRD by NGS, as well as 499 paired results for MS and MRD by MFC. A complete breakdown of samples evaluated at different time points can be found in supplemental Table 1. At screening, MS was positive in 66 (55%) patients, decreasing to 46 (39%) after cycle 6, 39 (34%) after cycle 12, 28 (29%) after cycle 18, 24 (30%) after cycle 24, and 12 (22%) after cycle 36. The respective numbers among patients with paired BM MRD and MS results available were 38 (54%), 30 (41%), 23 (64%), 16 (28%), 15 (29%), and 12 (35%) for MRD by NGS; whereas for MRD by MFC, the counts were 34 (32%), 16 (15%), 17 (17%), 10 (13%), 15 (21%), and 7 (18%). Notably, MS was positive in a significant proportion (21%) of IFE-negative samples (supplemental Table 3). This further translated into better outcomes of patients in complete response who achieved MS negativity as a best response, than those who remained MS positive (HR, 0.33 [0.11-0.98]; P = .003; supplemental Figure 7).
Baseline characteristics
| Variable . | Subcategory . | n (%) . |
|---|---|---|
| Age (y, median, IQR) | 57 (47-63) | |
| Sex | Male | 73 (53%) |
| Female | 65 (47%) | |
| Study arm | KRd | 73 (53%) |
| R | 65 (47%) | |
| M protein isotype | IgG κ | 75 (54%) |
| IgG λ | 27 (19%) | |
| IgA κ | 16 (12%) | |
| IgA λ | 4 (3%) | |
| IgM κ | 1 (1%) | |
| IgM λ | 3 (2%) | |
| FLC κ | 10 (7%) | |
| FLC λ | 2 (2%) | |
| IMWG response at study entry | <VGPR | 16 (12%) |
| VGPR | 73 (53%) | |
| CR | 31 (22%) | |
| sCR | 18 (13%) | |
| MRD by NGS at study entry | Negative | 35 (25%) |
| Positive | 40 (29%) | |
| Not evaluated | 63 (46%) | |
| MRD by MFC at study entry | Negative | 82 (59%) |
| Positive | 39 (29%) | |
| Not evaluated | 17 (12%) | |
| ISS | 1 | 49 (35%) |
| 2 | 67 (48%) | |
| 3 | 22 (17%) | |
| Cytogenetic risk | Standard | 104 (75%) |
| High | 34 (25%) | |
| Time from ASCT (d, median, IQR) | 92.5 (76-114.3) |
| Variable . | Subcategory . | n (%) . |
|---|---|---|
| Age (y, median, IQR) | 57 (47-63) | |
| Sex | Male | 73 (53%) |
| Female | 65 (47%) | |
| Study arm | KRd | 73 (53%) |
| R | 65 (47%) | |
| M protein isotype | IgG κ | 75 (54%) |
| IgG λ | 27 (19%) | |
| IgA κ | 16 (12%) | |
| IgA λ | 4 (3%) | |
| IgM κ | 1 (1%) | |
| IgM λ | 3 (2%) | |
| FLC κ | 10 (7%) | |
| FLC λ | 2 (2%) | |
| IMWG response at study entry | <VGPR | 16 (12%) |
| VGPR | 73 (53%) | |
| CR | 31 (22%) | |
| sCR | 18 (13%) | |
| MRD by NGS at study entry | Negative | 35 (25%) |
| Positive | 40 (29%) | |
| Not evaluated | 63 (46%) | |
| MRD by MFC at study entry | Negative | 82 (59%) |
| Positive | 39 (29%) | |
| Not evaluated | 17 (12%) | |
| ISS | 1 | 49 (35%) |
| 2 | 67 (48%) | |
| 3 | 22 (17%) | |
| Cytogenetic risk | Standard | 104 (75%) |
| High | 34 (25%) | |
| Time from ASCT (d, median, IQR) | 92.5 (76-114.3) |
CR, complete response; FLC, free light chain; IMWG, International Myeloma Working Group; IQR, interquartile range; ISS, International Staging System; sCR, stringent complete repsonse; VGPR, very good partial response.
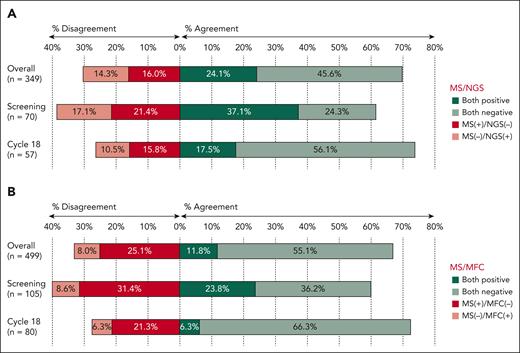
When patients with MRD of ≥10–5 were considered MRD positive by NGS, the overall agreement between MS and NGS was 70%. With NGS as the reference standard, sensitivity and specificity of MS was 63% (54%-71%) and 74% (68%-80%), respectively. Discordant cases were split between MS+/NGS– (56 of 349, 16%) and MS–/NGS+ (50 of 349, 14%) cases, with the PPV of 60% (54%-66%) and NPV of 76% (72%-80%). The agreement was lowest at screening (61% agreement) but improved in the subsequent evaluations, reaching the highest level at cycle 18 (74% agreement; Figure 1A). Additionally, the highest values for NPV and specificity were observed at cycle 18, whereas PPV was highest at cycle 6 and sensitivity at screening (supplemental Table 2). We also analyzed the agreement between MS evaluation and NGS results at different cutoffs for MRD positivity (supplemental Figure 2). As expected, when we considered MRD of ≥10–4 as positive, we observed higher proportion of MS+/NGS– samples (82 of 349, 23%) as opposed to MS–/NGS+ (20 of 349, 6%). Accordingly, when we used the more sensitive threshold of 10–6 for MRD negativity, there were less MS+/NGS– samples (28 of 294, 9%) than MS–/NGS+ samples (75 of 294, 26%). The smaller denominator in the latter analysis is because of the fact that not all samples reached the sensitivity of 10–6 for the NGS evaluation.
Agreement between peripheral blood MS and MRD in the BM. Rates of concordant and discordant cases assessed at different time points by NGS at the 10–5 threshold (A) and by MFC (B).
Agreement between peripheral blood MS and MRD in the BM. Rates of concordant and discordant cases assessed at different time points by NGS at the 10–5 threshold (A) and by MFC (B).
With MRD assessment by MFC as the reference standard, the overall agreement with MS was 67%, with a sensitivity of 60% (49%-69%) and specificity of 69% (64%-73%). The discrepancy between MRD by MFC and MS was mostly driven by patients with MS+/MFC– patients (125 of 499, 25%), whereas MS–/MFC+ was less frequent (40 of 499, 8%). The PPV was 32% (28%-37%) and the NPV was 87% (84%-90%). The agreement was lowest at screening (60% agreement) and increased at the subsequent timepoints, with the highest agreement observed after cycle 18 (73% agreement; Figure 1B). Sensitivity and NPV were highest after cycle 6, specificity peaked after cycle 18, and the PPV was at its maximum at screening (supplemental Table 2).
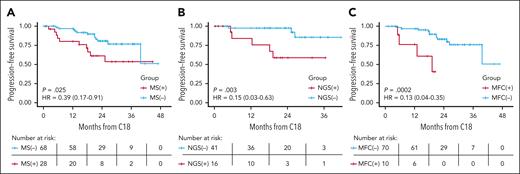
We then analyzed the prognostic implications of M protein detection by MS at different time points, focusing on the first 4 landmarks because of lower sample sizes at the later time points. Landmark analyses did not confirm a statistically significant association of MS status with PFS when assessed at screening and after cycles 6 or 12 (supplemental Figure 3), although the effect size at screening (HR, 0.52; 95% CI, 0.26-1.05; P = .06) and cycle 12 (HR, 0.61 [0.30-1.25]; P = .18) were suggestive of a possible association with PFS. This was, however, not true for cycle 6 (HR, 1.20 [0.6-2.39]; P = .6). Finally, using the landmark method, MS negativity after cycle 18 was associated with significantly superior PFS (HR, 0.39 [0.17-0.91]; P = .025); the direction and magnitude of benefit were similar regardless of the treatment arm. Similarly, C18 BM MRD-negative status was associated with superior PFS for both MFC (HR, 0.13 [0.04-0.35]; P = .0002) and NGS (HR, 0.15 [0.03-0.63]; P = .003; Figure 2).
Landmark analysis (after cycle 18) of PFS. PFS differences between positive and negative patients by (A) MS, (B) NGS at the 10–5 threshold, and (C) MFC. Not all patients included in this analysis had the BM MRD testing results available, hence the lower numbers of cases presented in panels B and C.
Landmark analysis (after cycle 18) of PFS. PFS differences between positive and negative patients by (A) MS, (B) NGS at the 10–5 threshold, and (C) MFC. Not all patients included in this analysis had the BM MRD testing results available, hence the lower numbers of cases presented in panels B and C.
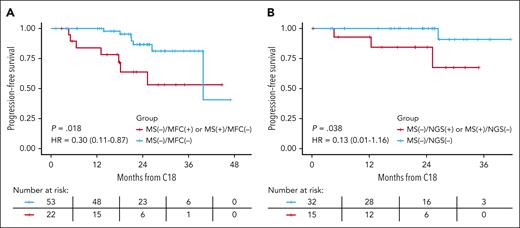
We then evaluated the effect of combined peripheral blood MS and BM MRD status on PFS (Figure 3; supplemental Figure 4). Using the same landmark analysis after cycle 18, combined peripheral blood and BM negativity (“double negative”) was associated with superior PFS compared with those negative by only 1 modality; this was true for both MS/MFC (PFS: HR, 0.30 [0.11-0.87]; P = .018) and for MS/NGS (PFS: HR, 0.13 [0.01-1.16]; P = .038).
Combined impact of peripheral blood MS and BM MRD on PFS. Comparison of PFS in landmark analysis after cycle 18 between those who were double negative (BM and peripheral blood) and those negative in 1 but positive in the other modality. (A) MS and MFC and (B) MS and NGS at the 10–5 threshold.
Combined impact of peripheral blood MS and BM MRD on PFS. Comparison of PFS in landmark analysis after cycle 18 between those who were double negative (BM and peripheral blood) and those negative in 1 but positive in the other modality. (A) MS and MFC and (B) MS and NGS at the 10–5 threshold.
When we examined the added value of the MS result (positive or negative) among only BM MRD-negative cases at the same specific landmark time point, we observed a potentially favorable PFS benefit for patients with concurrent BM MRD negativity and MS negativity but the difference did not reach statistical significance: NGS−/MS− vs NGS−/MS+ (HR, 0.14 [0.01-1.58]; P = .06) and MFC−/MS− vs MFC−/MS+ (HR, 0.40 [0.13-1.27]; P = .11; supplemental Figure 5). This benefit was further suggested when we analyzed best responses, throughout all analyzed time points. We observed improved PFS for patients who reached BM MRD negativity (at the 10–5 threshold, when both NGS and MFC results were available, at least 1 positive result qualified a sample as positive) and MS negativity as best response compared with MS positivity with BM MRD negativity at the same time points (HR, 0.36 [0.10-1.31]; P = .03; supplemental Figure 6).
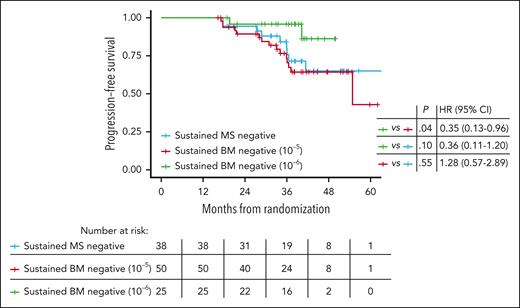
Finally, we examined the prognostic implications of sustained (≥12 months) negativity in the peripheral blood and BM at different sensitivity thresholds. This analysis included only patients that achieved 2 consecutive negative test results in landmark time points (screening, and cycles 6, 12, 18, 24, and 36) separated by at least 12 months. Among the 38 patients with sustained MS negativity, 34 also had sustained BM MRD negativity at the 10–5 threshold. PFS was similar for those with sustained BM MRD negativity at 10–5 vs sustained peripheral blood MS negativity (Figure 4). Sustained BM MRD negativity at 10–6 assessed by NGS appeared to be associated with potentially better PFS than sustained MS negativity by EXENT, although it did not yet reach statistical significance at the current data cutoff (Figure 4).
Sustained MS and MRD negativity. PFS of patients with sustained (>12 months) undetectable M protein in the peripheral blood by MS and MRD negative in the BM at the 10–5 and 10–6 thresholds.
Sustained MS and MRD negativity. PFS of patients with sustained (>12 months) undetectable M protein in the peripheral blood by MS and MRD negative in the BM at the 10–5 and 10–6 thresholds.
Discussion
To our knowledge, this is the first report on the feasibility of MS-based assessment of M protein presence in patients with multiple myeloma after ASCT, performed without access to pretreatment baseline serum calibration samples. Consistent with previous reports,17 MS was able to identify the presence of an M protein in a significant proportion of IFE-negative samples. Moreover, our results indicate that peripheral blood-based MRD status determined by EXENT MS tracks closely with BM-based MRD by NGS or MFC at the 10–5 level of sensitivity. In addition, it appears that when added to BM MRD status, MS status further discriminates outcomes in patients with multiple myeloma receiving posttransplant maintenance.
These results show the feasibility of measuring M protein by EXENT MS platform in the posttransplant setting, even without availability of pretreatment calibration samples. Pretreatment samples were also not available in the Mass-Fix analysis in the STAMINA trial.18 However, in that study, the first serum samples were obtained before ASCT, when residual M protein is expected to be higher than after ASCT, and the proportion of patients in very good partial response or better was lower than in our analysis (47% vs 88%), suggesting that there was higher likelihood of sufficient concentration of paraprotein at screening to allow for the assignment of the monoclonal light chain m/z value. Another key difference is that our analysis includes longer serial sampling for MS, extending as far as 36 cycles, compared with only 1 year of serial testing in the STAMINA trial.
MS assessment of residual disease in the blood in the post-ASCT setting investigated in our study by the EXENT platform and by Mass-Fix in the STAMINA trial, both illustrate the emerging value of MS evaluations as a prognostic tool. The results from both studies indicate that procuring samples for calibration in the peri-ASCT period can still allow for MS evaluation of M protein in the posttransplant setting to be feasible. This is important when considering that many patients with myeloma start treatment in nonacademic settings, in which serum samples may not be stored for initial MS evaluation. The clonotypic peptide approach may enable the use of archived SPEP gels for identifying clonotypic peptides and therefore detect M protein in tracking samples.22,29
Our study, to our knowledge, contains 1 of the largest sets of paired MS results along with standard BM MRD assessments published to date. Unsurprisingly, the discrepancies between M protein assessment in the peripheral blood and disease burden in the BM in this study were highest early after ASCT (within 100 days). We attribute these differences to a higher rate of MS positive results, which could be attributed to either prolonged clearance of IgG monoclonal proteins (which were present in 73% of patients) or the presence of posttransplant oligoclonal responses.21,30 False positives from oligoclonal bands can be circumvented if the m/z value for the M protein light chain is derived from a pretreatment serum sample. At later time points, the agreement between the BM and MS methods increased; however, ∼30% were still discrepant, especially when MS was compared with MFC MRD evaluation. This suggests superior sensitivity of MS compared with MFC at the 10–5 threshold, which is aligned with the recent findings by Claveau et al and a large analysis of the STAMINA trial.18,31 These studies, however, did not include MRD analysis by NGS or MRD testing with a similar lower LoD (10–6), which is another unique aspect of this analysis.
The observed significant difference in PFS by MS status using a landmark analysis after cycle 18 is consistent with observations regarding delayed clearance of paraprotein after tumor lysis in the BM. This finding corroborates the findings of a previous study, in which MS status after KRd induction, ASCT, and KRd consolidation was associated with PFS,16 along with results from several other studies in which the prognostic significance of MS evaluation increased with time.18,19 The seemingly paradoxical results observed after cycle 6 may be, at least in part, related to the study design which includes de-escalation of the therapy in the subset of standard risk patients after cycle 8, based on BM MRD status assessed after cycle 6. This landmark analysis may be particularly susceptible to confounding factors arising from different treatment. Importantly, in the landmark analysis of MS status after cycle 18, HRs for PFS were similar in both ATLAS study arms.
Our results further support the potential role of multimodal response assessment as evidenced by the superior prognosis for patients with “double MRD negativity” (negative in peripheral blood and in BM) compared with single modality MRD negativity. Although this complementarity has already been reported when calibration samples have been available,19,32 it is encouraging that the same principle applies in the post-ASCT setting without available calibration samples. Although our results suggest a complementarity of blood-based MS and BM-based NGS or MFC MRD evaluations, they do not justify forgoing a BM evaluation at this time. The impact of sustained BM and blood-based MRD negativity on PFS support future evaluations of the prognostic impact of other blood-based MRD methodologies, including more sensitive MS techniques along with alternative methods such as blood-based NGS. For the time being, our data show that sustained BM MRD negativity at the 10–6 threshold still carries the greatest prognostic significance and remains the most desirable goal of therapy. However, this may be challenged in future by applying more sensitive methods of MS-based disease assessment, using liquid chromatography MS and clonotypic peptide approach that are ∼10 and 100 times more sensitive than the EXENT platform.28,33,34 Nonetheless, these methods are time consuming, labor intensive, and not automated. Their widespread applicability requires further studies.
Perhaps the most significant limitation of this study is the retrospective nature of the MS assessment, in which all stored serum samples were analyzed in the same batch and were not available for every patient at every time point. However, based on the criteria used to classify a sample as negative via MS, the subsequent identification of the specific m/z value for the M protein light chain for later time points resulted in a shift from positive to negative in only a small subset of patients (6 of 138). This alteration was observed exclusively during the early time point screening and cycle 6. Another limitation may lie in the fact that, for the landmark analyses, time of follow-up is relatively short and the numbers of patients at later time points preclude definitive conclusions. Consistent with other similar studies,18,19 we refrained from adjusting P value for comparisons at multiple time points, guided by our primary focus on avoiding type 2 rather than type 1 errors in this exploratory analysis,35 the kinetics of MRD that lead to a different composition of patients at each time point, and the aforementioned biological plausibility that later time points are more reliable because of immunoglobulin recycling.21 It is also essential to acknowledge that MALDI-TOF intact immunoglobulin MS for detecting low concentrations of M proteins is most effective when a baseline sample is available. Future studies on multimodal disease assessment should strive to include the calibration step, as, for example, in the currently ongoing prospective EMN33 TAURUS study.
In summary, this analysis provides rationale for MS-based disease evaluation even in the posttransplant setting without a pretreatment calibration sample. In this study posttransplant cycle 18 appeared to be an optimal time point for prognostic assessment by MS. Further implementation of this technique requires subsequent prospective investigations to confirm the optimal timing for MS assessment to best inform prognosis; clinical practice; and, potentially, 1 day, to guide treatment intensity and duration.
Acknowledgments
The authors thank Gabriella Lakos (The Binding Site, part of Thermo Fisher, Rochester, NY) for her feedback on the manuscript text and study design.
The ATLAS study was funded by Amgen and Celgene.
The sponsors had no role in data collection, analysis, or interpretation.
Authorship
Contribution: D.D. and A.J.J. conceptualized, designed, and acquired funding, and were responsible for administration and supervision of the ATLAS trial; T.K., D.D., A.J.J., and B.A.D. designed the study; D.B. and D.S. performed laboratory evaluations; T.K., D.D., D.B., D.S., A.J.J., and B.A.D. analyzed the results; T.K. made the figures; T.K., D.D., D.B., A.J.J., and B.A.D. drafted the first version of the manuscript; and all authors were involved in data collection and edited and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.K. reports financial support for attending meetings from Janssen. D.D. reports speaker honoraria and participation in advisory boards for Amgen and Celgene (Bristol Myers Squibb); and had conference fees paid by Amgen. D.B. is a current employee of The Binding Site. D.S. is a current employee of The Binding Site. B.P. served as a consultant for AbbVie, Roche, and Sandoz; and received honoraria and research funding from AbbVie, Amgen, Gilead, Celgene, and Janssen. J.W. reports consultancy for AbbVie, Gilead, Novartis, Roche, and Takeda; reports research or clinical trial funding from GlaxoSmithKline, Novartis, and Roche; and received honoraria from AbbVie, Amgen, Gilead, GlaxoSmithKline, Novartis, Roche, and Takeda. A.J.J. reports consulting fees and honoraria for lectures and presentations from AbbVie, Amgen, Celgene (Bristol Myers Squibb), Gracell, GlaxoSmithKline, Janssen, and Sanofi-Aventis. B.A.D. reports consulting fees from COTA Healthcare, Janssen, and Sanofi; and reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from MJH Life Sciences and Plexus Communications. A.D.-S. reports honoraria for lectures and presentations, travel grants, and participation in advisory boards for Amgen and Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Benjamin A. Derman, University of Chicago, 5841 South Maryland Ave, M/C 2115, Chicago, IL 60637; email: bderman@bsd.uchicago.edu.
References
Author notes
A.J.J. and B.A.D. are joint senior authors and contributed equally to this study.
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 9 December 2023.
Deidentified participant data will be made available to investigators upon reasonable request from the corresponding author, Benjamin A. Derman (bderman@bsd.uchicago.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal