Key Points
αKG dehydrogenase is a critical dependency in AML, maintaining leukemia by fulfilling biosynthetic demands.
Targeting the TCA cycle at the αKG dehydrogenase step disrupts nucleotide synthesis, offering a broadly applicable antileukemic approach.
Visual Abstract
Perturbations in intermediary metabolism contribute to the pathogenesis of acute myeloid leukemia (AML) and can produce therapeutically actionable dependencies. Here, we probed whether α-ketoglutarate (αKG) metabolism represents a specific vulnerability in AML. Using functional genomics, metabolomics, and mouse models, we identified the αKG dehydrogenase complex, which catalyzes the conversion of αKG to succinyl coenzyme A, as a molecular dependency across multiple models of adverse-risk AML. Inhibition of 2-oxoglutarate dehydrogenase (OGDH), the E1 subunit of the αKG dehydrogenase complex, impaired AML progression and drove differentiation. Mechanistically, hindrance of αKG flux through the tricarboxylic acid (TCA) cycle resulted in rapid exhaustion of aspartate pools and blockade of de novo nucleotide biosynthesis, whereas cellular bioenergetics was largely preserved. Additionally, increased αKG levels after OGDH inhibition affected the biosynthesis of other critical amino acids. Thus, this work has identified a previously undescribed, functional link between certain TCA cycle components and nucleotide biosynthesis enzymes across AML. This metabolic node may serve as a cancer-specific vulnerability, amenable to therapeutic targeting in AML and perhaps in other cancers with similar metabolic wiring.
Introduction
Acute myeloid leukemia (AML) is a group of hematopoietic cancers characterized by a clonal outgrowth of myeloid precursor cells. A backbone of AML treatment has long been cytarabine, a nucleoside analog, which is frequently combined with anthracyclines. These regimens, which affect nucleotide metabolism and interfere with DNA replication, often achieve remissions and are curative in some patients. However, relapse remains common, emphasizing a need for more rational therapeutic approaches.1
One molecularly targeted approach involves the inhibition of isocitrate dehydrogenase (IDH) mutant proteins, which aberrantly convert α-ketoglutarate (αKG) into an oncogenic metabolite, R-2-HG (R-2-hydroxyglutarate).2-4 Mechanistically, R-2-HG competitively inhibits αKG-dependent dioxygenases, many of which play roles in DNA or histone demethylation, to drive leukemogenesis.4-9 Consequently, selective inhibitors of IDH-mutant proteins trigger leukemia cell differentiation and have proven effective in AMLs harboring IDH1 or IDH2 mutant enzymes.10-14
AMLs without IDH mutations also depend on altered metabolism for leukemia maintenance. These leukemias invariably contain perturbations in central carbon metabolism, which includes elements of energy production, macromolecule biosynthesis, and reduction-oxidation homeostasis.15,16 They are also hyperdependent on certain metabolic programs. For example, AMLs require enhanced glycolysis, prompting investigation of inhibitors that target this pathway.17-20 Additionally, maintenance of leukemia stem cells requires oxidative phosphorylation (OXPHOS), a process targeted by venetoclax, the US Food and Drug Administration–approved B-cell lymphoma 2 (BCL2) antagonist.21,22 This requirement for OXPHOS also provides a rationale for electron transport chain (ETC) inhibition. However, ETC inhibitors proved too toxic in clinical trials,23,24 highlighting the need for more specific, targetable metabolic dependencies.
Appealing targets exist within biosynthetic pathways that produce macromolecules required to sustain AML growth, including certain amino acids25-27 and nucleic acids.1,28,29 Notably, dihydroorotate dehydrogenase (DHODH), which participates in de novo pyrimidine synthesis, was identified as an AML dependency, and DHODH inhibition drives differentiation.29 Although DHODH inhibitors have not proven efficacious for AML in early clinical trials,30-32 the concept of suppressing nucleotide generation, rather than their incorporation into DNA, opens the door for alternative strategies to achieve similar ends.
Previous work from our laboratory and others indicates that αKG, a key intermediate in central carbon metabolism, can exert control of cell fate and facilitate tumor suppression.33-35 Because αKG, a substrate of IDH enzymes, is also implicated in the pathogenesis of IDH-mutant leukemia, we probed whether αKG metabolism represents a specific vulnerability in AML. Using functional genomics, metabolomics, and orthogonal approaches in mouse models, we identified the αKG dehydrogenase complex as a dependency in adverse-risk AML subsets. Interrogation of the mechanistic consequences of depleting its E1 subunit, 2-oxoglutarate dehydrogenase (OGDH), led us to identify a cancer-sustaining metabolic node that links αKG flux through the tricarboxylic acid (TCA) cycle to de novo nucleotide production. Inhibiting this node blocked cell cycle progression and induced differentiation. These results provide a rationale for therapeutically targeting the αKG dehydrogenase complex in AML.
Methods
Cell culture
Cell lines derived from C57BL/6 Mll-Af9; NrasG12D (“MLL-fusion”) and Trp53R172H;shMll3;shNf1 (“p53-mutant”) AMLs were previously generated.36-39 Human patient-derived xenograft (PDX) cells were previously established. In vitro assessments included viability measurement, morphological staining, RNA sequencing, colony-formation assays, and flow cytometry.
Transplantation experiments
AML cell lines derived from MLL-fusion or p53-mutant primary leukemias were transduced with doxycycline-inducible short hairpin RNAs (shRNAs) and injected into sublethally irradiated recipient mice. Engraftment or in vivo leukemic burden was tracked by bioluminescence imaging or fluorophore measurement in peripheral blood.
Bioenergetic assessment, metabolomics, and isotope tracing
For measurement of mitochondrial respiration, the Seahorse XF Cell Mito Stress Test assay was performed. Metabolomic profiling and isotope tracing were performed using liquid chromatography-mass spectrometry (LC-MS) platforms.
Public data set dependency analysis
To measure differential gene dependency and codependency, we analyzed gene effect values from the DepMap Public 22Q4 database, which includes 24 AML cell lines and 1054 cell lines of other cancer types.
Research involving vertebrate animals was performed in accordance with our approved Institutional Animal Care and Use Committee protocol (Memorial Sloan Kettering Cancer Center). PDX models were previously established and deidentified, not meeting the criteria for human subjects research per the National Cancer Institute Decision Tool.
For detailed descriptions of methods and reagents, please refer to the supplemental Materials, available on the Blood website.
Results
OGDH is a preferential AML dependency across genotypes
Given the importance of αKG biology in AML, we analyzed the dependency of AML on genes involved in the biosynthesis or utilization of αKG in genome-wide CRISPR-CRISPR associated protein 9 (Cas9) screening data in >1000 human cancer cell lines, including 24 AML lines.40 To identify specific AML dependencies, we compared normalized gene dependency scores across AML vs non-AML cell lines. Among αKG and TCA cycle–related genes demonstrating significant AML specificity (q < 0.05), the most preferential dependencies in AML included OGDH, DLST, and DLD, which encode the subunits of αKG dehydrogenase (Figure 1A-C; Table 1; supplemental Table 1), and members of the succinate dehydrogenase complex (Table 1; supplemental Table 1). OGDH similarly ranked higher as a dependency in AML and, in fact, more broadly in leukemias vs nonleukemia cell lines (Figure 1D; supplemental Figure 1A). Although dihydrolipoamide dehydrogenase (DLD) is shared among additional enzymes, including pyruvate dehydrogenase and branched-chain α-ketoacid dehydrogenase, other components of these complexes were not AML dependencies, suggesting that DLD dependency is driven through its participation in the αKG dehydrogenase complex (supplemental Figure 1B). Given its direct role in αKG generation and our prior work demonstrating its tumor-sustaining effects,35 we focused our studies on αKG dehydrogenase.
OGDH is a bona fide genetic dependency across AML systems. (A) Schematic of the TCA cycle highlighting the αKG dehydrogenase complex. (B) Differential gene dependency in AML vs all non-AML (other) cancer types, based on difference of mean depletion or enrichment of sgRNAs targeting individual genes across available CRISPR screens. An y-axis value <0 indicates preferential dependency in AML and a value >0 indicates preferential dependency in other. Each data point represents an individual gene. Selected genes are labeled. (C) OGDH, DLST, and DLD gene effect across CRISPR screens for AML vs other cell lines included in the DepMap database. A lower gene effect indicates an increased likelihood of gene dependency, with 0 indicating nondependency and –1 representing the median of all pan-essential genes. Each data point represents an individual cell line, and the mean ± standard error are shown. P values indicated (Wilcoxon rank-sum test). (D) OGDH gene dependency ranking across CRISPR screens for AML vs other cell lines included in the DepMap database. Each data point represents an individual cell line, and the mean ± standard error are shown. P value is indicated (Wilcoxon rank-sum test). (E) For each indicated gene, the violin plot shows change in sgRNA abundance (measured as log2 fold change) in publicly available whole-genome CRISPR screens across multiple AML vs other cancer cell lines. P values are indicated (Wilcoxon rank-sum test). (F-G) Western blot analysis showing the expression of OGDH in AML cell lines transduced with the indicated shRNA after 3 days of doxycycline treatment. (F) NrasG12D; MLL-AF9 cells. (G) p53R172H cells. (H-I) Depletion of Ogdh shRNAs in the competition assay. (H) NrasG12D; MLL-AF9 murine leukemia cells were infected with retroviruses encoding the indicated doxycycline-inducible shRNAs linked to a GFP reporter and admixed with 20% uninfected (GFP–) cells. The relative percentages of GFP+ cells (normalized to day 1 for each shRNA) were counted on the indicated days after doxycycline treatment. P values of shOgdh vs shControl groups indicated (2-way analysis of variance [ANOVA] with Sidak multiple comparisons test). (I) p53R172H murine leukemia cells were infected with lentiviruses encoding reverse tetracycline-controlled transactivator and the indicated doxycycline-inducible shRNAs linked to a blue fluorescent protein (BFP) reporter on single backbone (LT3 BEPIR). Infected cells were admixed with 20% uninfected cells. The relative percentages of BFP+ cells (normalized to day 2 for each shRNA) were counted on the indicated days after doxycycline treatment. (J) Western blot analysis showing expression of OGDH in PDX1 AML cells 4 days after transduction with the indicated shRNAs. (K) Cell proliferation of PDX1 AML cells transduced with the indicated shRNAs (normalized to day 6 after infection for each shRNA). (L) Depletion of OGDH shRNAs in a competition assay in PDX2 cells. PDX2 AML cells were infected at day –7 with a lentivirus encoding shControl or shOGDH linked to a GFP reporter and a second lentivirus encoding shControl linked to a red fluorescent protein (RFP) reporter. The GFP:RFP ratio (normalized to day 0 for each shRNA pair) was measured on the indicated days. P value of shOGDH vs shControl group is indicated (2-way ANOVA). αKGDC, α-ketoglutarate dehydrogenase complex; ns, not significant; OAA, oxaloacetate.
OGDH is a bona fide genetic dependency across AML systems. (A) Schematic of the TCA cycle highlighting the αKG dehydrogenase complex. (B) Differential gene dependency in AML vs all non-AML (other) cancer types, based on difference of mean depletion or enrichment of sgRNAs targeting individual genes across available CRISPR screens. An y-axis value <0 indicates preferential dependency in AML and a value >0 indicates preferential dependency in other. Each data point represents an individual gene. Selected genes are labeled. (C) OGDH, DLST, and DLD gene effect across CRISPR screens for AML vs other cell lines included in the DepMap database. A lower gene effect indicates an increased likelihood of gene dependency, with 0 indicating nondependency and –1 representing the median of all pan-essential genes. Each data point represents an individual cell line, and the mean ± standard error are shown. P values indicated (Wilcoxon rank-sum test). (D) OGDH gene dependency ranking across CRISPR screens for AML vs other cell lines included in the DepMap database. Each data point represents an individual cell line, and the mean ± standard error are shown. P value is indicated (Wilcoxon rank-sum test). (E) For each indicated gene, the violin plot shows change in sgRNA abundance (measured as log2 fold change) in publicly available whole-genome CRISPR screens across multiple AML vs other cancer cell lines. P values are indicated (Wilcoxon rank-sum test). (F-G) Western blot analysis showing the expression of OGDH in AML cell lines transduced with the indicated shRNA after 3 days of doxycycline treatment. (F) NrasG12D; MLL-AF9 cells. (G) p53R172H cells. (H-I) Depletion of Ogdh shRNAs in the competition assay. (H) NrasG12D; MLL-AF9 murine leukemia cells were infected with retroviruses encoding the indicated doxycycline-inducible shRNAs linked to a GFP reporter and admixed with 20% uninfected (GFP–) cells. The relative percentages of GFP+ cells (normalized to day 1 for each shRNA) were counted on the indicated days after doxycycline treatment. P values of shOgdh vs shControl groups indicated (2-way analysis of variance [ANOVA] with Sidak multiple comparisons test). (I) p53R172H murine leukemia cells were infected with lentiviruses encoding reverse tetracycline-controlled transactivator and the indicated doxycycline-inducible shRNAs linked to a blue fluorescent protein (BFP) reporter on single backbone (LT3 BEPIR). Infected cells were admixed with 20% uninfected cells. The relative percentages of BFP+ cells (normalized to day 2 for each shRNA) were counted on the indicated days after doxycycline treatment. (J) Western blot analysis showing expression of OGDH in PDX1 AML cells 4 days after transduction with the indicated shRNAs. (K) Cell proliferation of PDX1 AML cells transduced with the indicated shRNAs (normalized to day 6 after infection for each shRNA). (L) Depletion of OGDH shRNAs in a competition assay in PDX2 cells. PDX2 AML cells were infected at day –7 with a lentivirus encoding shControl or shOGDH linked to a GFP reporter and a second lentivirus encoding shControl linked to a red fluorescent protein (RFP) reporter. The GFP:RFP ratio (normalized to day 0 for each shRNA pair) was measured on the indicated days. P value of shOGDH vs shControl group is indicated (2-way ANOVA). αKGDC, α-ketoglutarate dehydrogenase complex; ns, not significant; OAA, oxaloacetate.
Selected differential gene dependencies in AML
| Gene symbol . | Differential gene dependency∗ . | P value . | q value . |
|---|---|---|---|
| MYB | –1.04 | 3.8E-76 | 6.4E-84 |
| SDHB | –0.39 | 1.1E-08 | 8.7E-07 |
| DHODH | –0.36 | 1.9E-11 | 7.2E-10 |
| OGDH | –0.33 | 3.3E-06 | 2.1E-04 |
| DLD | –0.29 | 8.8E-08 | 3.1E-06 |
| BCL2 | –0.27 | 1.3E-13 | 3.0E-12 |
| DLST | –0.27 | 1.7E-08 | 5.5E-07 |
| SDHC | –0.25 | 2.1E-05 | 6.9E-04 |
| SDHD | –0.24 | 1.2E-06 | 2.9E-05 |
| SDHA | –0.24 | 2.0E-07 | 6.3E-06 |
| PDXK | –0.12 | 3.9E-07 | 7.3E-06 |
| Gene symbol . | Differential gene dependency∗ . | P value . | q value . |
|---|---|---|---|
| MYB | –1.04 | 3.8E-76 | 6.4E-84 |
| SDHB | –0.39 | 1.1E-08 | 8.7E-07 |
| DHODH | –0.36 | 1.9E-11 | 7.2E-10 |
| OGDH | –0.33 | 3.3E-06 | 2.1E-04 |
| DLD | –0.29 | 8.8E-08 | 3.1E-06 |
| BCL2 | –0.27 | 1.3E-13 | 3.0E-12 |
| DLST | –0.27 | 1.7E-08 | 5.5E-07 |
| SDHC | –0.25 | 2.1E-05 | 6.9E-04 |
| SDHD | –0.24 | 1.2E-06 | 2.9E-05 |
| SDHA | –0.24 | 2.0E-07 | 6.3E-06 |
| PDXK | –0.12 | 3.9E-07 | 7.3E-06 |
Members of the αKG dehydrogenase complex are shown in bold.
Difference in AML vs other cancers of normalized depletion of sgRNAs targeting individual genes across available CRISPR screens.
Confirming the differential αKG dehydrogenase dependency gene by gene, we found that single guide RNAs (sgRNAs) targeting each component were selectively depleted in AML lines relative to those from other cancer types (Figure 1E). The effect sizes were comparable with our positive controls: BCL2 and PDXK, which are highly selective AML dependencies.41 In contrast, BRD4 and SF3B1, which have been investigated as AML targets, were depleted across cancer cell lines and therefore were nonselective. Importantly, the dependency of AML cells on OGDH was not associated with the presence or absence of specific driver mutations, suggesting applicability as a target across AML genotypes (supplemental Figure 1C). Of note, OGDH was nonessential in 2 independent CRISPR screens performed in human pluripotent stem cells42,43 (supplemental Figure 1D-E).
We next tested whether αKG dehydrogenase serves as a genetic dependency, primarily using cell lines derived from 2 murine models of AML. The first is an NrasG12D; Mll-Af9–driven model that allows for doxycycline-inducible expression of shRNAs via the reverse tetracycline-controlled transactivator and in vivo imaging via firefly luciferase (supplemental Figure 2A).36 The second is a model of p53-mutant, complex karyotype (CK) AML, which also incorporates shRNAs targeting Nf1 and Mll3, recapitulates hallmark copy number abnormalities of human disease, and displays an abnormal immunophenotype with erythroid features (supplemental Figure 2B-D).37-39 These models were chosen because they represent adverse-risk disease subsets and harbor genetic elements that facilitate examination of gene dependencies.
We first performed whole-genome CRISPR dependency screens using either Cas9-MLL-AF9 (a variation of the MLL-fusion model that expresses Cas9 in place of luciferase and NrasG12D) or Cas9-expressing p53-mutant AML cell lines. As expected, sgRNAs targeting known AML dependencies (MYB44 and DHODH29) were depleted in both cell lines (supplemental Table 2). Consistent with the human AML data, sgRNAs targeting all 3 αKG dehydrogenase subunits were rapidly depleted in both murine models (supplemental Figure 2E-F). Next, we used an shRNA-based approach, which might better approximate the partial inhibition typical of pharmacological targeting. A vector system was used that links a microRNA-based shRNA to a transcript for a fluorescent protein, enabling tracking and sorting of shRNA-expressing cells. Comparing 2 independent OGDH-targeted shRNAs with a neutral shRNA (targeting Renilla luciferase [shControl]), the OGDH shRNAs efficiently suppressed Ogdh (Figure 1F-G) and, confirming on-target activity, increased steady-state levels of αKG and the αKG-to-succinate ratio (supplemental Figure 2G). As expected, shRNA-mediated depletion of OGDH inhibited the proliferation of MLL-fusion and p53-mutant AMLs in vitro in a competition assay (Figure 1H-I; supplemental Figure 2H), effects that were also observed in shOgdh-transduced AML cells harboring Idh2, Flt3, or Npm1 mutations (supplemental Figure 2I).
Last, we examined OGDH dependency in human AML using cells derived from PDX models established from patients with CK AML harboring TP53 mutations (supplemental Figure 3A-B). Fortuitously, PDX1 cells propagated ex vivo and were efficiently transduced with green fluorescent protein (GFP)–linked human OGDH shRNAs (supplemental Figure 3C). OGDH depletion, confirmed by immunoblot, impeded cell growth in culture (Figure 1J-K). PDX2 AML cells, which had a lower transduction efficiency, showed OGDH dependency in a competition assay (Figure 1L). Hence, in both human and murine AML cells, OGDH behaves as bona fide genetic dependency.
OGDH is required to maintain AML
We next examined whether OGDH inhibition can have antileukemic effects in vivo. First, we transduced clonal isolates of MLL-fusion AML with a vector encoding a doxycycline-inducible shRNA targeting Ogdh (or shControl) and transplanted the cells into sublethally irradiated syngeneic mice (Figure 2A). Establishment of leukemia was confirmed by bioluminescence imaging (Figure 2B). Mice that underwent transplant with shOgdh-containing AML and treated with doxycycline demonstrated a dramatic reduction in leukemic burden (Figure 2B-C) accompanied by significant improvements in platelet counts (supplemental Figure 4A) and overall survival, with some mice achieving durable remissions (Figure 2D). Although most of these mice eventually succumbed to leukemia, their relapsed disease was GFP negative and OGDH positive, implying selection for loss or inactivation of the vector (supplemental Figure 4B-D). By contrast, shControl leukemias maintained GFP expression on doxycycline. These observations imply that only cells with intact OGDH expression can sustain leukemic progression.
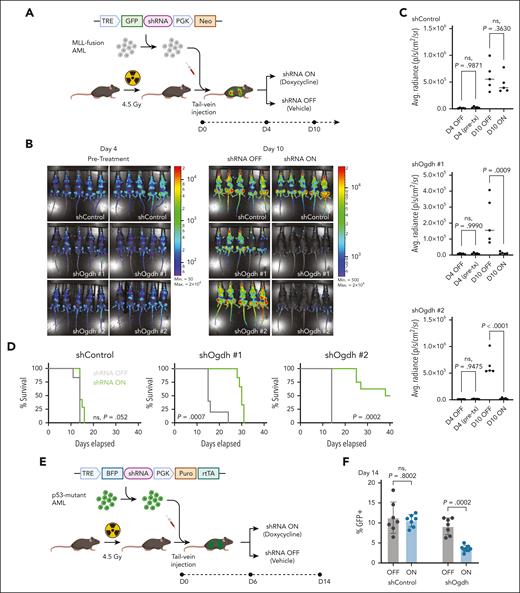
OGDH is required for AML progression in vivo. (A) Schematic of in vivo knockdown experiment using transplantation of shRNA-transduced NrasG12D; MLL-AF9 AML. (B) Leukemia burden after shRNA knockdown of Ogdh in MLL-fusion AML. NrasG12D; MLL-AF9 AML clones transduced with retroviruses encoding doxycycline-inducible shControl or shOgdh were transplanted into sublethally irradiated recipient mice. Luminescence imaging was performed before (day 4) and 6 days after treatment with doxycycline vs vehicle (day 10). (C) Quantification of average radiance for the bioluminescence imaging shown in panel B. P values indicated (1-way ANOVA with Sidak multiple comparisons test; n = 5 per condition). (D) Survival curves are shown, and P values are indicated (log-rank test; n = 5-7 per condition). (E) Schematic of in vivo knockdown experiment using transplantation of shRNA-transduced p53R172H AML. (F) Leukemia burden after shRNA knockdown of Ogdh in p53-mutant AML. p53R172H AML clones transduced with retroviruses encoding doxycycline-inducible shControl or shOgdh were transplanted into sublethally irradiated recipient mice. GFP+ percentage in peripheral blood was measured at day 14. P values indicated (1-way ANOVA with Sidak multiple comparisons test; n = 7-8 per condition). Avg., average; max, maximum; min, minimum; Neo, neomycin resistance cassette; ns, not significant; p, photons; pre-tx, pre-treatment; puro, puromycin resistance cassette; PGK, phosphoglycerate kinase 1 promoter; rtTA, reverse tetracycline-controlled transactivator; s, seconds; sr, steradian; TRE, tetracycline response element.
OGDH is required for AML progression in vivo. (A) Schematic of in vivo knockdown experiment using transplantation of shRNA-transduced NrasG12D; MLL-AF9 AML. (B) Leukemia burden after shRNA knockdown of Ogdh in MLL-fusion AML. NrasG12D; MLL-AF9 AML clones transduced with retroviruses encoding doxycycline-inducible shControl or shOgdh were transplanted into sublethally irradiated recipient mice. Luminescence imaging was performed before (day 4) and 6 days after treatment with doxycycline vs vehicle (day 10). (C) Quantification of average radiance for the bioluminescence imaging shown in panel B. P values indicated (1-way ANOVA with Sidak multiple comparisons test; n = 5 per condition). (D) Survival curves are shown, and P values are indicated (log-rank test; n = 5-7 per condition). (E) Schematic of in vivo knockdown experiment using transplantation of shRNA-transduced p53R172H AML. (F) Leukemia burden after shRNA knockdown of Ogdh in p53-mutant AML. p53R172H AML clones transduced with retroviruses encoding doxycycline-inducible shControl or shOgdh were transplanted into sublethally irradiated recipient mice. GFP+ percentage in peripheral blood was measured at day 14. P values indicated (1-way ANOVA with Sidak multiple comparisons test; n = 7-8 per condition). Avg., average; max, maximum; min, minimum; Neo, neomycin resistance cassette; ns, not significant; p, photons; pre-tx, pre-treatment; puro, puromycin resistance cassette; PGK, phosphoglycerate kinase 1 promoter; rtTA, reverse tetracycline-controlled transactivator; s, seconds; sr, steradian; TRE, tetracycline response element.
We also tested the antileukemic effects of OGDH depletion in p53-mutant AML (Figure 2E). These knockdowns relied on a lentiviral system that links reverse tetracycline-controlled transactivator to the tetracycline-inducible shRNA in the same vector and results in shRNA induction in only ∼60% of leukemia cells. Despite this inefficiency, we were able to monitor overall leukemic burden (indicated by GFP positivity) and shRNA activation (indicated by blue fluorescent protein positivity) in the peripheral blood. Mice that underwent transplant with shOgdh-expressing AML (vs shControl-expressing AML) and treated with doxycycline (vs vehicle) showed a significant reduction in leukemic burden (Figure 2F) and a significant improvement in platelet counts (supplemental Figure 4E). Similar to the MLL-fusion model, the Ogdh shRNA was selected against following doxycycline addition, with only ∼30% of shOgdh-transduced p53-mutant AML cells retaining blue fluorescent protein positivity by day 14 (supplemental Figure 4F-G). This again implies that OGDH expression is required for leukemic progression.
To assess leukemia-initiating capacity, we first examined the colony-forming potential of AML cells in methylcellulose culture as a surrogate for leukemia stem cell activity.45 The number of colonies formed by shOgdh-transduced (vs shControl) AMLs was drastically reduced in both murine models (supplemental Figure 4H-K). Accordingly, the engraftment potential of MLL-fusion AML cells, first depleted of OGDH and then transplanted into sublethally irradiated recipient mice, was completely ablated (supplemental Figure 4L-O). Furthermore, mice that received transplant with human PDX1 AML cells transduced with OGDH-targeting shRNAs, unlike controls, showed no CD45 or GFP positivity, markers of human AML engraftment and shRNA expression, respectively (supplemental Figure 4P). Together, these data suggest that OGDH suppression not only debulks AMLs but also inhibits leukemia-initiating capacity.
αKG flux through the TCA cycle is blocked upon OGDH inhibition
Because OGDH is a component of the TCA cycle, we next studied the impact of OGDH inhibition on metabolic outputs. The reaction catalyzed by OGDH and αKG dehydrogenase not only generates NADH to fuel OXPHOS and support the bioenergetic needs of the cell but also produces TCA cycle intermediates essential for the biosynthesis of amino acids, lipids, nucleic acids, and various metabolic cofactors.
To assess bioenergetics, we measured the mitochondrial oxygen consumption rate of cells after pharmacologically inhibiting individual ETC components, thereby assessing basal respiration, spare respiratory capacity, and related parameters (supplemental Figure 5A). In both MLL-fusion and p53-mutant AMLs, OGDH depletion caused minimal impairment in basal respiration (measured before addition of any ETC inhibitors) but greatly attenuated spare respiratory capacity (Figure 3A-B; supplemental Figure 5B). Thus, OGDH suppression compromises the metabolic flexibility of AML cells under stress but does not trigger bioenergetic collapse.
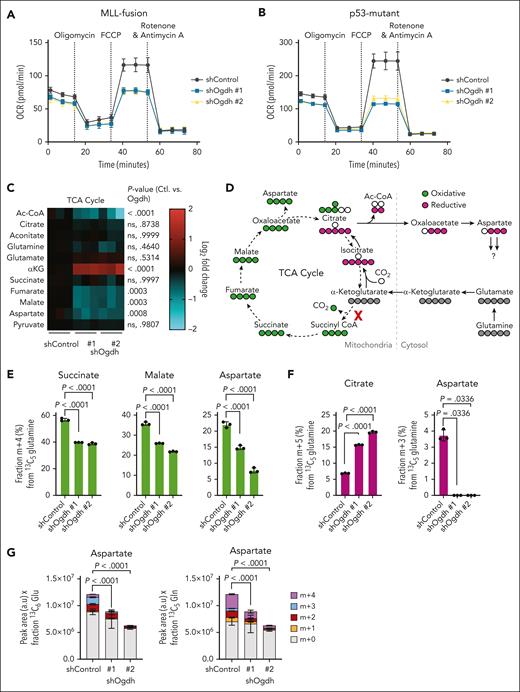
OGDH inhibition reduces TCA cycle flux and aspartate biosynthesis. (A-B) Analysis of mitochondrial bioenergetics. Agilent Seahorse XF Cell Mito Stress Test assay was performed on NrasG12D; MLL-AF9 AML cells (A) and p53R172H AML cells (B) at 48 hours after OGDH depletion for the measurement of cellular oxygen consumption rate (OCR). Mitochondrial inhibitors are individually labeled and were added at the indicated time points. (C) TCA cycle and related metabolite levels in p53R172H AML cells. LC-MS was performed at 48 hours after knockdown with the indicated shRNAs. Data are shown as a heat map of log2 fold change relative to shControl-treated cells. Red color represents upregulated; blue color, downregulated. P values are indicated (2-way ANOVA with Sidak multiple comparisons test; n = 3-6 per condition). (D) Schematic of oxidative flux (carbons labeled in green) or reductive flux (carbons labeled in purple) through the TCA cycle by 13C5-glutamine tracing. Red “X” indicates block observed in p53R172H AML after OGDH knockdown. Labeled carbons are indicated in gray, green, or purple. (E) Fraction (%) of m+4 labeling from 13C5-glutamine for the indicated metabolites in p53R172H AML after induction of shRNAs targeting OGDH (or control) for 48 hours and labeling for 6 hours. P values indicated (1-way ANOVA with Dunnett multiple comparisons test; n = 3 per condition). (F) Fraction (%) of m+5 labeled citrate or m+3 labeled aspartate from 13C5-glutamine in p53R172H AML after induction of shRNAs targeting OGDH (or control) for 48 hours and labeling for 6 hours. P values indicated (1-way ANOVA with Dunnett multiple comparisons test or Kruskal-Wallis with Dunn multiple comparisons test as appropriate; n = 3 per condition). (G) After induction of shRNAs targeting OGDH (or control) in p53R172H AML for 48 hours and labeling for 6 hours, LC-MS was used to determine the steady-state level and fraction of aspartate labeling from 13C6-glucose (left) or 13C5-glutamine (right). With 13C6-glucose tracing, the m+2 and m+4 fractions represent 1 and 2 forward rotations through the TCA cycle, respectively, whereas with 13C5-glutamine tracing m+2 represents 2 forward rotations and m+4 represents 1. Aspartate levels are shown as peak area in arbitrary units (a.u.) × fractional percentage of labeling with each tracer. P values are indicated for total labeled aspartate in shControl vs shOgdh groups (1-way ANOVA with Dunnett multiple comparisons test; n = 3 per condition). Ac-CoA, acetyl-coenzyme A; ctl, shControl; FCCP, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; ns, not significant.
OGDH inhibition reduces TCA cycle flux and aspartate biosynthesis. (A-B) Analysis of mitochondrial bioenergetics. Agilent Seahorse XF Cell Mito Stress Test assay was performed on NrasG12D; MLL-AF9 AML cells (A) and p53R172H AML cells (B) at 48 hours after OGDH depletion for the measurement of cellular oxygen consumption rate (OCR). Mitochondrial inhibitors are individually labeled and were added at the indicated time points. (C) TCA cycle and related metabolite levels in p53R172H AML cells. LC-MS was performed at 48 hours after knockdown with the indicated shRNAs. Data are shown as a heat map of log2 fold change relative to shControl-treated cells. Red color represents upregulated; blue color, downregulated. P values are indicated (2-way ANOVA with Sidak multiple comparisons test; n = 3-6 per condition). (D) Schematic of oxidative flux (carbons labeled in green) or reductive flux (carbons labeled in purple) through the TCA cycle by 13C5-glutamine tracing. Red “X” indicates block observed in p53R172H AML after OGDH knockdown. Labeled carbons are indicated in gray, green, or purple. (E) Fraction (%) of m+4 labeling from 13C5-glutamine for the indicated metabolites in p53R172H AML after induction of shRNAs targeting OGDH (or control) for 48 hours and labeling for 6 hours. P values indicated (1-way ANOVA with Dunnett multiple comparisons test; n = 3 per condition). (F) Fraction (%) of m+5 labeled citrate or m+3 labeled aspartate from 13C5-glutamine in p53R172H AML after induction of shRNAs targeting OGDH (or control) for 48 hours and labeling for 6 hours. P values indicated (1-way ANOVA with Dunnett multiple comparisons test or Kruskal-Wallis with Dunn multiple comparisons test as appropriate; n = 3 per condition). (G) After induction of shRNAs targeting OGDH (or control) in p53R172H AML for 48 hours and labeling for 6 hours, LC-MS was used to determine the steady-state level and fraction of aspartate labeling from 13C6-glucose (left) or 13C5-glutamine (right). With 13C6-glucose tracing, the m+2 and m+4 fractions represent 1 and 2 forward rotations through the TCA cycle, respectively, whereas with 13C5-glutamine tracing m+2 represents 2 forward rotations and m+4 represents 1. Aspartate levels are shown as peak area in arbitrary units (a.u.) × fractional percentage of labeling with each tracer. P values are indicated for total labeled aspartate in shControl vs shOgdh groups (1-way ANOVA with Dunnett multiple comparisons test; n = 3 per condition). Ac-CoA, acetyl-coenzyme A; ctl, shControl; FCCP, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; ns, not significant.
To assess the impact of OGDH depletion on biosynthetic reactions, we used LC-MS metabolomic profiling. Steady-state profiling of the p53-mutant CK AML model revealed that OGDH inhibition (supplemental Figure 5C) markedly impaired the generation of biosynthetic precursors from the TCA cycle. Specifically, although steady-state levels of citrate and aconitate (measured as a proxy for isocitrate) were relatively unchanged, levels of fumarate, malate, and aspartate (derived from oxaloacetate) were reduced (Figure 3C). Stable isotope tracing was then used to evaluate whether these changes resulted from impairment in the forward, oxidative reactions of the TCA cycle that generate NADH and, indirectly, adenosine triphosphate (ATP). Cells were fed with 13C5-glutamine, and then the fractions of TCA metabolites with 4 13C atoms (m+4 fraction) were measured (Figure 3D). As expected for αKG dehydrogenase blockade, OGDH inhibition reduced the synthesis of intermediates downstream of αKG, including succinate, malate, and aspartate in the oxidative TCA cycle (Figure 3E). OGDH inhibition also caused an increase in reductive carboxylation (ie, glutamine contribution to citrate by reversal of the IDH reaction; Figure 3D,F). Despite the redirection of carbons from reduced glutamine toward aspartate synthesis, the resultant m+3 fraction of aspartate itself was nearly undetectable (Figure 3F). These results are in line with prior reports that reductive carboxylation depends on reducing equivalents produced by oxidative TCA cycle flux.46
Confirming that OGDH inhibition impairs aspartate generation, both total levels and labeling of aspartate were reduced, regardless of whether 13C6-glucose or 13C5-glutamine was the carbon source (Figure 3G). Collectively, these results indicate that disrupted forward αKG flux through the TCA cycle impairs aspartate production in AML and verify that aspartate levels can be used as a proxy for TCA cycle activity.47,48 Reductive carboxylation, although increased, is apparently insufficient to preserve aspartate pools that are likely quickly used in AML cells. These observations reinforce the view that aspartate is a key biosynthetic metabolite in AML.23,49
TCA cycle disruption in AML compromises nucleotide biosynthesis
The above results raise 2 questions: what aspartate-dependent biosynthetic processes are impaired upon OGDH suppression; and why are AML cells so exquisitely sensitive? Nucleotide functions are targeted by the most successful AML chemotherapies,1,28 and interestingly, their biogenesis requires aspartate50 (Figure 4A). Moreover, disrupted nucleotide biogenesis can impair AML cell proliferation and trigger terminal differentiation.29,51 Consistent with OGDH inhibition acting to impair de novo purine biosynthesis precisely at the aspartate-dependent step, OGDH inhibition produced increased levels of upstream purine intermediates, AICAR (5-aminoimidazole-4-carboxamide ribonucleotide) and inosine monophosphate (IMP), and a concomitant decrease in downstream adenosine monophosphate (AMP; Figure 4B). The accumulated AICAR and IMP, similar to αKG, were released from cells at elevated levels (supplemental Figure 5D-F). Additionally, OGDH inhibition broadly reduced the levels of pyrimidine intermediates downstream of aspartate utilization (Figure 4B). Isotope tracing reinforced these findings, showing reduced AMP generation from 13C6-glucose or amide-15N1-glutamine (Figure 4C-D). Although IMP levels increased, its fractional labeling decreased, indicating end product inhibition (Figure 4C-D). In the pyrimidine pathway, tracing with 13C6-glucose, 13C5-glutamine, or 15N1-glutamine showed reduced uridine monophosphate (UMP) synthesis regardless of carbon source (Figure 4E-G). Notably, we observed neither reduction-oxidation imbalance, as indicated by largely unchanged ratios of NAD+/NADH and glutathione (GSH/GSSG) forms in both AML models (supplemental Figure 5G-H), nor evidence of oxidative stress (supplemental Figure 5I). Reductions in ATP were largely proportional to reductions in AMP, consistent with a biosynthetic deficit (supplemental Figure 5J-K). Collectively, these results imply that AML cells are highly dependent on aspartate-dependent reactions needed for purine and pyrimidine biosynthesis, and this underlies their dependence on OGDH.
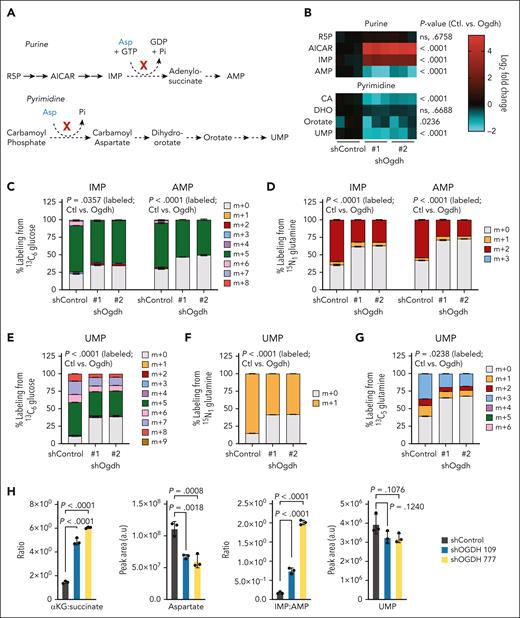
TCA cycle disruption compromises nucleotide biosynthesis in AML. (A) Schematics of the de novo purine (upper) and pyrimidine (lower) biosynthesis pathways highlighting aspartate (Asp)-dependent steps. (B) LC-MS for measurement of the levels of selected nucleotide intermediates (shown in heat map) was performed on p53R172H AML cells at 48 hours after knockdown with the indicated shRNAs. Data are shown as log2 fold change relative to shControl-treated cells. P values are indicated (2-way ANOVA with Sidak multiple comparisons test; n = 3-6 per condition). (C-D) Labeling of IMP and AMP. After induction of shRNAs targeting Ogdh (or Control) in p53R172H AML for 48 hours and labeling for 6 hours, LC-MS was used to determine the fraction (%) of IMP or AMP labeling from 13C6-glucose (C) or 15N1 glutamine (D). (E-G) The fraction (%) of UMP labeling from 13C6-glucose (E), 15N1-glutamine (F), or 13C5-glutamine (G) was measured. For panels C-G, P values are indicated for total percentage of labeled metabolites in shControl (Ctl) vs shOgdh groups (Wilcoxon rank-sum test or unpaired t test as appropriate; n = 3-6 per condition). (H) Levels or ratios of selected TCA cycle and nucleotide metabolites in PDX1 cells. LC-MS for measurement of the indicated metabolites was performed at 7 days after knockdown with the indicated shRNAs. Data are shown as either peak area (a.u) or a ratio. P values are indicated (1-way ANOVA with Sidak multiple comparisons test; n = 3 per condition). CA, carbamoyl aspartate; GDP; guanosine diphosphate; GTP, guanosine-5'-triphosphate; Pi; inorganic phosphate; R5P, ribose 5-phosphate.
TCA cycle disruption compromises nucleotide biosynthesis in AML. (A) Schematics of the de novo purine (upper) and pyrimidine (lower) biosynthesis pathways highlighting aspartate (Asp)-dependent steps. (B) LC-MS for measurement of the levels of selected nucleotide intermediates (shown in heat map) was performed on p53R172H AML cells at 48 hours after knockdown with the indicated shRNAs. Data are shown as log2 fold change relative to shControl-treated cells. P values are indicated (2-way ANOVA with Sidak multiple comparisons test; n = 3-6 per condition). (C-D) Labeling of IMP and AMP. After induction of shRNAs targeting Ogdh (or Control) in p53R172H AML for 48 hours and labeling for 6 hours, LC-MS was used to determine the fraction (%) of IMP or AMP labeling from 13C6-glucose (C) or 15N1 glutamine (D). (E-G) The fraction (%) of UMP labeling from 13C6-glucose (E), 15N1-glutamine (F), or 13C5-glutamine (G) was measured. For panels C-G, P values are indicated for total percentage of labeled metabolites in shControl (Ctl) vs shOgdh groups (Wilcoxon rank-sum test or unpaired t test as appropriate; n = 3-6 per condition). (H) Levels or ratios of selected TCA cycle and nucleotide metabolites in PDX1 cells. LC-MS for measurement of the indicated metabolites was performed at 7 days after knockdown with the indicated shRNAs. Data are shown as either peak area (a.u) or a ratio. P values are indicated (1-way ANOVA with Sidak multiple comparisons test; n = 3 per condition). CA, carbamoyl aspartate; GDP; guanosine diphosphate; GTP, guanosine-5'-triphosphate; Pi; inorganic phosphate; R5P, ribose 5-phosphate.
Finally, we were able to confirm these findings in human AML. PDX1 cells were subject to LC-MS analysis after 7 days of knockdown, accounting for a slower kinetic of the antiproliferative phenotype in primary cells. In accordance with the data from the murine AML lines, after OGDH suppression, we observed the expected increase in αKG-to-succinate ratio, a reduction in total aspartate levels, and an increased IMP:AMP ratio, indicative of purine biosynthesis disruption (Figure 4H). Even at this early time point and with media repletion, we began to observe reductions (albeit not meeting statistical significance) in UMP levels (Figure 4H). Thus, αKG-dehydrogenase inhibition in AML impairs aspartate generation, causing distinct blocks in nucleotide biosynthesis.
OGDH inhibition also perturbed steady-state levels of αKG-associated metabolites associated with pathways beyond nucleotide biosynthesis. For example, de novo biosynthesis of serine or alanine generates αKG (supplemental Figure 6A-B), and accordingly, OGDH inhibition reduced steady-state levels of both amino acids by shifting transaminase activity from production to consumption (supplemental Figure 6C-D). Isotope tracing confirmed decreased m+3 labeling of alanine from 13C6-glucose (supplemental Figure 6E), despite unchanged pyruvate labeling (supplemental Figure 6F) and glutamate levels (Figure 3C). Given that AML cells are highly dependent on serine and alanine,27,52 reductions in these amino acids likely contribute to the antileukemic effects of OGDH inhibition.
Another αKG-dependent biosynthetic pathway involves the synthesis of branched-chain amino acids through branched-chain aminotransferase (BCAT; supplemental Figure 6G). Highly expressed in AML, BCAT has oncogenic potential, although its role remains debated. One model suggests BCAT’s catabolic activity limits αKG in AML cells, whereas another proposes it drives αKG production in the anabolic direction.53,54 After OGDH inhibition, branched-chain ketoacids increased substantially (supplemental Figure 6H-I), but elevated αKG levels persisted (Figure 3C). Depending on the dominant mechanism, excess αKG may either overwhelm BCAT’s oncogenic activity or alter the BCAT reaction balance. Thus, OGDH inhibition primarily disrupts nucleotide synthesis while also targeting amino acid dependencies and undermining oncogenic pathways.
Biosynthetic failure in AML impairs cell cycle progression and drives differentiation
Given the role of αKG as a cofactor for dioxygenases involved in cell fate specification,33-35 we also assessed whether OGDH inhibition induced differentiation in our models. Morphologically, the MLL-fusion model displayed features of nuclear maturation after OGDH depletion (Figure 5A; supplemental Figure 7A-B), upregulated the myeloid differentiation marker Cd11b, and downregulated the stemness marker Cd117 (supplemental Figure 8A). This myeloid differentiation pattern was confirmed by gene set enrichment analysis (Figure 5B), with many of the genes upregulated after OGDH knockdown being targets of transcription factors (TFs) that drive myeloid cell fate (supplemental Figure 8B). p53-mutant AML also showed signs of differentiation, upregulating Cd11b (supplemental Figure 8C) and, consistent with an erythroid bias, the erythroid marker Gypa and targets of the erythroid lineage–specific TFs TAL1 and GATA1. They also downregulated the expression of the stemness TF GATA2 (Figure 5C-D; supplemental Figure 8D). Both models showed marked decreases in transcriptional signatures linked to MYC, which have been linked to self-renewal of leukemia stem cells (supplemental Figure 8B,D).55-57
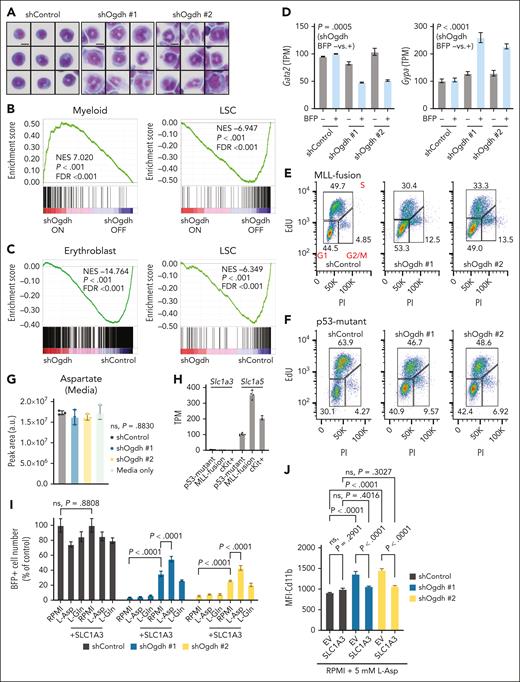
Biosynthetic failure in AML impairs cell cycle progression and drives differentiation. (A) Representative Cytospin and Hema3 stains of NrasG12D; MLL-AF9 clones transduced with the indicated shRNAs after 4 days of doxycycline treatment (n = 9 per condition). Scale bar, 10 μm. (B-C) Gene set enrichment analysis (GSEA) after OGDH depletion. NrasG12D; MLL-AF9 AML clones (B) or p53R172H AML cells (C) transduced with retroviruses encoding doxycycline-inducible shControl or shOgdh (2 independent shRNAs sequenced separately and pooled during analysis) were subjected to RNA sequencing after 4 days of treatment with doxycycline. GSEA identified pathways of interest that were upregulated or downregulated. (D) Transcript levels of Gata2 (left) and Gypa (right) in p53R172H AML cells treated with doxycycline for 4 days and sorted for BFP. BFP+ indicates shRNA on; BFP– indicates shRNA off. P values for shOgdh off (BFP–) vs on (BFP+) indicated (unpaired t test; n = 4 per condition) (E-F) Cell cycle analysis by 5-ethynyl-2′-deoxyuridine (EdU) tracing. NrasG12D; MLL-AF9 (E) or p53R172H (F) AML cells treated with doxycycline for 4 days and sorted for GFP or BFP, respectively, were labeled with EdU for 1 hour. They were then fixed, permeabilized, stained with propidium iodide (PI), and subjected to cell cycle analysis by flow cytometry. Left lower gate represents G1 phase; upper gate, S phase; right lower gate, G2 phase/mitosis (M). Percentages of cells falling within each gate are indicated. (G) Extracellular aspartate levels in media were measured at 48 hours after knockdown of p53R172H AML cells with the indicated shRNAs. (H) Baseline transcript levels of Slc1a3 and Slc1a5 in p53R172H AML cells, MLL-fusion AML cells, and normal murine cKit-positive progenitor cells. (I) Relative number (% of control) of BFP+ cells after 4 days of induction of the indicated shRNA in p53R172H AML cells transduced with an empty vector or an exogenous Asp transporter (+SLC1A3). Media containing fetal bovine serum (FBS) was either unmodified (RPMI) or supplemented with 5 mM L-aspartate (L-Asp) or 10 mM L-glutamine (L-Gln) as indicated. P values are indicated (1-way ANOVA with either Dunn or Sidak multiple comparisons test as appropriate). (J) Median fluorescent intensity (MFI) of Cd11b after 4 days of induction of the indicated shRNAs in p53R172H AML cells transduced with an empty vector or vector for expression of an exogenous aspartate transporter (+SLC1A3). Media containing FBS was supplemented with 5 mM L-Asp. P values are indicated (1-way ANOVA with Sidak multiple comparisons test). FDR, false discovery rate; LSC, leukemia stem cell; NES, normalized enrichment score; ns, not significant; TPM, transcripts per million.
Biosynthetic failure in AML impairs cell cycle progression and drives differentiation. (A) Representative Cytospin and Hema3 stains of NrasG12D; MLL-AF9 clones transduced with the indicated shRNAs after 4 days of doxycycline treatment (n = 9 per condition). Scale bar, 10 μm. (B-C) Gene set enrichment analysis (GSEA) after OGDH depletion. NrasG12D; MLL-AF9 AML clones (B) or p53R172H AML cells (C) transduced with retroviruses encoding doxycycline-inducible shControl or shOgdh (2 independent shRNAs sequenced separately and pooled during analysis) were subjected to RNA sequencing after 4 days of treatment with doxycycline. GSEA identified pathways of interest that were upregulated or downregulated. (D) Transcript levels of Gata2 (left) and Gypa (right) in p53R172H AML cells treated with doxycycline for 4 days and sorted for BFP. BFP+ indicates shRNA on; BFP– indicates shRNA off. P values for shOgdh off (BFP–) vs on (BFP+) indicated (unpaired t test; n = 4 per condition) (E-F) Cell cycle analysis by 5-ethynyl-2′-deoxyuridine (EdU) tracing. NrasG12D; MLL-AF9 (E) or p53R172H (F) AML cells treated with doxycycline for 4 days and sorted for GFP or BFP, respectively, were labeled with EdU for 1 hour. They were then fixed, permeabilized, stained with propidium iodide (PI), and subjected to cell cycle analysis by flow cytometry. Left lower gate represents G1 phase; upper gate, S phase; right lower gate, G2 phase/mitosis (M). Percentages of cells falling within each gate are indicated. (G) Extracellular aspartate levels in media were measured at 48 hours after knockdown of p53R172H AML cells with the indicated shRNAs. (H) Baseline transcript levels of Slc1a3 and Slc1a5 in p53R172H AML cells, MLL-fusion AML cells, and normal murine cKit-positive progenitor cells. (I) Relative number (% of control) of BFP+ cells after 4 days of induction of the indicated shRNA in p53R172H AML cells transduced with an empty vector or an exogenous Asp transporter (+SLC1A3). Media containing fetal bovine serum (FBS) was either unmodified (RPMI) or supplemented with 5 mM L-aspartate (L-Asp) or 10 mM L-glutamine (L-Gln) as indicated. P values are indicated (1-way ANOVA with either Dunn or Sidak multiple comparisons test as appropriate). (J) Median fluorescent intensity (MFI) of Cd11b after 4 days of induction of the indicated shRNAs in p53R172H AML cells transduced with an empty vector or vector for expression of an exogenous aspartate transporter (+SLC1A3). Media containing FBS was supplemented with 5 mM L-Asp. P values are indicated (1-way ANOVA with Sidak multiple comparisons test). FDR, false discovery rate; LSC, leukemia stem cell; NES, normalized enrichment score; ns, not significant; TPM, transcripts per million.
Key αKG-dependent enzymes that act as effectors of αKG-mediated differentiation in other systems33-35 include the Ten eleven translocation (TET) methylcytosine dioxygenases that convert 5-methylcytosine to 5-hydroxymethylcytosine. However, upon OGDH knockdown, our MLL-fusion model showed no significant change in global 5-hydroxymethylcytosine levels (supplemental Figure 8E). Moreover, CRISPR-mediated disruption of TET2 and TET3 did not prevent differentiation after OGDH depletion (supplemental Figure 8F-I). Alternatively, a recent study suggests that nucleotide-targeting drugs may induce AML differentiation by slowing the cell cycle.51,58 In agreement, 5-ethynyl-2′-deoxyuridine profiling indicated reduced S-phase progression in both MLL-fusion and p53-mutant AML cells after OGDH depletion (Figure 5E-F). These findings imply that OGDH inhibition induces differentiation in non–IDH-mutant AML cells via proliferative defects due to impaired aspartate and nucleotide biosynthesis.
Despite reduced aspartate biosynthesis, OGDH-depleted cells did not show a compensatory increase in the uptake of the abundant aspartate in the media (Figure 5G). However, this requires the SLC1A3 transporter,59 which was expressed at lower levels in AML and normal hematopoietic progenitor cells than, for example, the primary glutamine transporter, SLC1A5 (Figure 5H). To determine whether aspartate depletion is critical for AML dependency on OGDH, we tested whether SLC1A3 overexpression could raise intracellular aspartate levels and, as reported in other contexts,60-62 rescue the proliferative defects produced by OGDH inhibition. p53-mutant AML cells were transduced with a construct expressing an SLC1A3 complementary DNA and doxycycline-inducible control or Ogdh-targeting shRNAs, and cells were analyzed for their proliferative capacity after doxycycline addition. Enforced SLC1A3 expression restored proliferation after OGDH suppression, an effect enhanced by aspartate (but not glutamine) supplementation (Figure 5I). Concurrent SLC1A3 expression and aspartate addition also prevented differentiation after OGDH knockdown, as shown by the reduced Cd11b expression to baseline (Figure 5J).
We used stable isotope tracing to further characterize how decreased aspartate levels after OGDH depletion affected metabolism. Cells expressing the SLC1A3 transporter showed robust 13C4-aspartate uptake, enhanced further upon OGDH suppression (supplemental Figure 9A). A proportion of aspartate-derived carbons re-entered the oxidative TCA cycle, producing m+4 labeled citrate and subsequently m+3 labeled αKG and glutamate (but not glutamine; supplemental Figure 9A-B). Notably, OGDH suppression increased m+4 labeled malate, an effect again enhanced by OGDH suppression (supplemental Figure 9A), suggesting that exogenous aspartate carbons are directed to malate via reductive TCA reactions, especially when malate pools are decreased. We also observed increased UMP labeling upon OGDH suppression (supplemental Figure 9C), indicating that AML cells use exogenous aspartate for de novo pyrimidine biosynthesis when available.
Our findings indicate that AML cells rely on αKG-dehydrogenase–driven aspartate production for proliferation and differentiation suppression. To evaluate whether normal hematopoietic cells exhibit similar dependency, we developed a genetically engineered mouse model with systemic, doxycycline-inducible OGDH suppression (Chaves-Perez et al, unpublished data, October 2024), including in the bone marrow (supplemental Figure 10A-C). Morphological assessment of bone marrow aspirates at times of OGDH depletion required for antileukemic effects (Figure 2B) indicated that multilineage hematopoiesis remained intact (supplemental Figure 10D). In cKit-positive progenitors from shOgdh mice treated ex vivo with doxycycline, basal bioenergetics and ATP levels showed minimal changes (supplemental Figure 11A-E), although LC-MS confirmed on-target OGDH knockdown with increased αKG-to-succinate and IMP:AMP ratios and reduced aspartate (supplemental Figure 11E). Unlike murine AML cells, cKit-positive progenitors showed only partial colony suppression after OGDH depletion (P1; supplemental Figure 11F-G) and retained GFP positivity up to 2 weeks. Colony formation declined further with prolonged OGDH suppression (P2) but was reversible upon doxycycline withdrawal and OGDH restoration (supplemental Figure 11H-I). These data suggest a therapeutic window between AML and normal hematopoietic cells.
A functional genetic link between αKG-dehydrogenase activity and de novo nucleotide metabolism exists across cancers
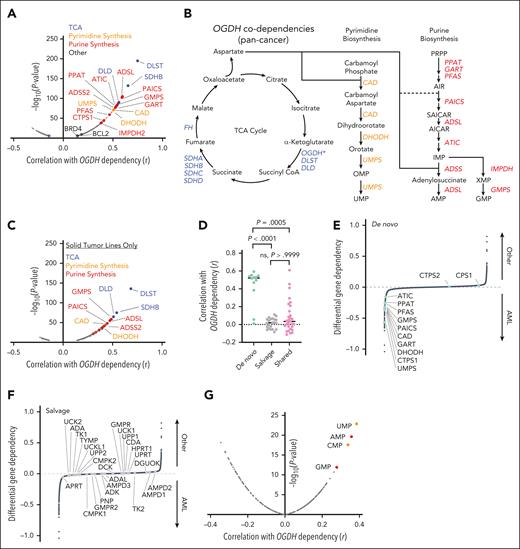
Our results imply that AML cells are particularly dependent on the TCA cycle to produce nucleotides and other key metabolites to support their rapid expansion. To extend our observations to additional models and human systems, we integrated dependency data from whole-genome CRISPR screening40 with corresponding metabolite profiles.40,63 Strikingly, nearly all the genes encoding enzymes catalyzing de novo nucleotide biosynthesis were highly codependent with OGDH (Figure 6A-B). This codependency was also observed when we excluded AML and other hematologic cancers (Figure 6C).
A functional genetic link between αKG dehydrogenase activity and de novo nucleotide biosynthesis exists across cancers. (A) Gene codependency with OGDH across all cancers. The graph shows correlation (r; x-axis) and –log10(P value) (y-axis; genes with P > 10E-5 not shown) of gene dependency with OGDH dependency across all cancer types, based on composite depletion or enrichment of sgRNAs targeting individual genes across available CRISPR screens. A positive Pearson coefficient indicates gene codependency with OGDH. Selected genes of interest are labeled with blue indicating TCA enzymes; yellow, pyrimidine biosynthesis enzymes; and red, purine biosynthesis enzymes. (B) Schematic of the TCA cycle and its connectivity to purine and pyrimidine biosynthesis, highlighting significantly correlated OGDH codependencies as determined in the analysis depicted in panel A. (C) Gene codependency with OGDH in nonhematologic cancers. The analysis was conducted as in panel A, except that hematologic cancers were excluded. (D) Quantification of the correlation between OGDH and all genes encoding components of the de novo nucleotide biosynthesis pathway, nucleotide salvage pathway, or shared between these 2 pathways. P values are indicated (Kruskal-Wallis with Dunn multiple comparisons test). (E-F) Differential gene dependency in AML vs all non-AML (other) cancer types, based on difference of mean depletion or enrichment of sgRNAs targeting individual genes across available CRISPR screens. An y-axis value <0 indicates preferential dependency in AML, and an y-axis value >0 indicates preferential dependency in other cancer types. The highlighted genes are those encoding components of the de novo nucleotide synthesis pathway (E; green) and those encoding components of the nucleotide salvage pathway (F; gray). Each data point represents an individual gene. (G) Correlation of relative metabolite levels with OGDH dependency across all cancer cell types. A positive Pearson coefficient indicates relative enrichment of an individual metabolite in cells demonstrating OGDH dependency. Selected nucleotide precursors are labeled. CMP, cytidine 5′-monophosphate; GMP, guanosine monophosphate; ns, not significant.
A functional genetic link between αKG dehydrogenase activity and de novo nucleotide biosynthesis exists across cancers. (A) Gene codependency with OGDH across all cancers. The graph shows correlation (r; x-axis) and –log10(P value) (y-axis; genes with P > 10E-5 not shown) of gene dependency with OGDH dependency across all cancer types, based on composite depletion or enrichment of sgRNAs targeting individual genes across available CRISPR screens. A positive Pearson coefficient indicates gene codependency with OGDH. Selected genes of interest are labeled with blue indicating TCA enzymes; yellow, pyrimidine biosynthesis enzymes; and red, purine biosynthesis enzymes. (B) Schematic of the TCA cycle and its connectivity to purine and pyrimidine biosynthesis, highlighting significantly correlated OGDH codependencies as determined in the analysis depicted in panel A. (C) Gene codependency with OGDH in nonhematologic cancers. The analysis was conducted as in panel A, except that hematologic cancers were excluded. (D) Quantification of the correlation between OGDH and all genes encoding components of the de novo nucleotide biosynthesis pathway, nucleotide salvage pathway, or shared between these 2 pathways. P values are indicated (Kruskal-Wallis with Dunn multiple comparisons test). (E-F) Differential gene dependency in AML vs all non-AML (other) cancer types, based on difference of mean depletion or enrichment of sgRNAs targeting individual genes across available CRISPR screens. An y-axis value <0 indicates preferential dependency in AML, and an y-axis value >0 indicates preferential dependency in other cancer types. The highlighted genes are those encoding components of the de novo nucleotide synthesis pathway (E; green) and those encoding components of the nucleotide salvage pathway (F; gray). Each data point represents an individual gene. (G) Correlation of relative metabolite levels with OGDH dependency across all cancer cell types. A positive Pearson coefficient indicates relative enrichment of an individual metabolite in cells demonstrating OGDH dependency. Selected nucleotide precursors are labeled. CMP, cytidine 5′-monophosphate; GMP, guanosine monophosphate; ns, not significant.
The codependencies of OGDH with de novo nucleotide biosynthesis genes were of similar magnitude as the codependencies of OGDH with its interacting partners DLST and DLD and with other TCA components such as succinate dehydrogenase. By contrast, OGDH was not codependent with genes encoding enzymes involved in salvage pathways of nucleotide biosynthesis (Figure 6D; supplemental Figure 12A); accordingly, although de novo nucleotide biosynthesis enzymes were strong AML dependencies, those involved in salvage pathways were not (Figure 6E-F; supplemental Figure 12B). Importantly, the codependencies between OGDH and de novo nucleotide biosynthesis genes were independent of population doubling rates or OGDH transcript levels (supplemental Figure 12C-D), which also do not correlate with patient outcomes, but instead correlated tightly with nucleoside monophosphate levels (Figure 6G; supplemental Figure 12E). SLC1A3 expression, which was low in most AML cell lines, anticorrelated with OGDH dependency (supplemental Figure 12F-G). Together, these findings indicate that AML’s preferential requirement for αKG dehydrogenase is driven by a heightened dependence on de novo nucleic acid biosynthesis across murine and human models.
Discussion
Exploiting functional genomics data and genetic perturbations in murine models, we established the αKG dehydrogenase complex as a molecular dependency with preferential specificity for leukemias, including AML, and showed that inhibition of OGDH, the E1 subunit of αKG dehydrogenase, impairs leukemic growth and drives AML differentiation. Our results point to unique metabolic demands for AML cells, provide insights into the serendipitous success of nucleotide-targeting chemotherapies in AML, and suggest new strategies to harness this metabolic dependency therapeutically.
We show that OGDH depletion selectively impairs AML proliferation by disrupting biosynthetic, rather than ETC and OXPHOS, reactions, particularly by restricting αKG utilization for aspartate synthesis essential to various biosynthetic processes. These observations are in line with prior studies implicating aspartate utilization as a determinant of OGDH sensitivity48,61 but go further, showing that aspartate pools are critical for the de novo generation of both pyrimidines and purines required for AML proliferation. Accordingly, OGDH shows a strong codependency with enzymes involved in nucleotide biosynthesis across a series of AML lines. By contrast, TCA disruption via OGDH suppression did not induce bioenergetic collapse, suggesting that the minimum reducing equivalents required for ETC function may be generated before the αKG dehydrogenase step or through anaplerotic reactions or noncanonical TCA cycle configurations.64 Alternatively, biosynthetic failure may simply outpace bioenergetic deficiencies.
Although αKG-dependent dioxygenases are crucial for cell fate determination, their role in promoting differentiation via chromatin modification does not explain OGDH dependency in AML. This was unexpected, considering the dominant role that these αKG-dependent dioxygenases play in stem cells, IDH-mutant AMLs, and certain models of pancreatic cancer.4,33,35 We speculate that the rapid nucleotide-associated phenotype of OGDH depletion likely obscures the slower chromatin-based mechanisms of differentiation. In agreement, mutant IDH2 inhibition requires nearly 3 weeks to induce AML differentiation in mice and a median of 2 months to elicit responses in patients with AML.6,11,13 Similarly, forthcoming work from our group (Chaves-Perez et al, unpublished data, October 2024) suggests 2 modes of OGDH dependency during intestinal regeneration, with 1 cell lineage relying on biosynthetic mechanisms akin to AML and another demonstrating epigenetic-mediated differentiation. These observations highlight how cells differentially use the TCA cycle to support cell type–specific needs that maintain proliferation and/or cell state.
Our finding that OGDH inhibition triggers differentiation in AML models via biosynthetic deficits aligns with recent reports suggesting that replication stress, leading to reduced cell cycle progression, may enable the accumulation of fate-determining TFs to levels capable of triggering differentiation.29,51,58,65,66 Thus, the mechanisms of OGDH inhibition may be related to those underlying certain chemotherapies, such as cytarabine, whose differentiation effects were first described >4 decades ago.67,68 The effects of OGDH inhibition are also reminiscent of those observed with DHODH inhibition, which affects pyrimidine biosynthesis and similarly induces differentiation.29 Other approaches targeting nucleotide biosynthesis, including inhibition of the purine biosynthesis enzyme inosine 5′-monophosphate dehydrogenase (IMPDH)69,70 or the vitamin B6 pathway enzyme pyridoxal kinase (PDXK),41 likely converge on similar biology. Unfortunately, early-phase clinical trials of DHODH inhibitors in AML have shown limited efficacy, possibly owing to pyrimidine salvage pathways or increased uridine transport.71,72
Targeting OGDH instead may provide several advantages, including simultaneous disruption of de novo biosynthesis of both pyrimidines and purines to minimize resistance, depletion of other key macromolecules such as serine, impairment of stress-responsive mitochondrial activity, and engagement of αKG-dependent tumor suppression. Toxicity may be an issue; however, our results indicate that AML cells are much more sensitive to OGDH inhibition than normal hematopoietic progenitors and are in line with a recent report establishing markedly variable requirements for TCA cycle components across cell states.64 This suggests that agents that inhibit but do not completely inactivate OGDH may show a therapeutic window. Beyond AML, certain other cancers also display strong genetic dependency on OGDH.48,61 We propose that such cancers, such as AMLs, may be hypersensitive to nucleoside analog chemotherapy and should be among the best candidates for the development of novel, αKG dehydrogenase–directed therapies.
Acknowledgments
The authors thank J. Simon and the Memorial Sloan Kettering Cancer Center animal facility for technical support with animal colonies and F. M. Barriga, K. M. Tsanov, and other members of the Lowe laboratory for experimental advice and discussion. Janet Novak edited the manuscript.
S.E.M. received support from an American Society of Hematology (ASH) Research Training Award for Fellows, an ASH Scholar Award, National Institutes of Health (NIH), National Cancer Institute (NCI) Clinical Scholars Biomedical Research Training Program (T32 CA009512), and a grant from the NIH, NCI (K08CA259453). L.W.S.F. is a New York Stem Cell Foundation (NYSCF)–Robertson Investigator and supported by an NIH, NCI grant (R37CA252305) and NYSCF. This work is also supported by an NIH/NCI Cancer Center Support grant to Memorial Sloan Kettering (MSK) (P30 CA008748), an NIH, NCI Specialized Programs of Research Excellence (SPORE) grant (5P50CA254838), an MSK Experimental Therapeutics Center Award (S.W.L), and grant support from the Emerson Collective (S.W.L.). S.W.L. is an investigator in the Howard Hughes Medical Institute and the Geoffrey Beene Chair for Cancer Biology.
Authorship
Contribution: S.E.M. contributed to conceptualization, methodology, writing the original draft, reviewing and editing, and visualization; A.C.-P. contributed to conceptualization, methodology, investigation, and writing including reviewing and editing; S.J.-R. contributed to methodology, investigation, formal analysis, visualization, and writing including review and editing; Y.-J.H. contributed to formal analysis and visualization; J.P.M. contributed to methodology, investigation, and writing including review and editing; V.N. contributed to methodology, investigation, and formal analysis; C.-C.C. contributed to methodology and investigation; B.T.J. contributed to formal analysis and visualization; J.J.Y. contributed to investigation and formal analysis; R.M. contributed to methodology and investigation; T.I.D. contributed to investigation; V.J.A.B. contributed to formal analysis; M.S. contributed to methodology, investigation, and formal analysis; T.B. contributed to formal analysis; S.T. contributed to investigation; Z.S. contributed to methodology and resources; L.W.S.F. contributed to resources, conceptualization, and writing including review and editing; J.R.C. contributed to resources, conceptualization, and writing including review and editing; and S.W.L. contributed to conceptualization, supervision, and writing the original draft, reviewing, and editing.
Conflict-of-interest disclosure: S.W.L. declares outside consultancy and equity for Oric Pharmaceuticals, Blueprint Medicines, Mirimus, Senecea Therapeutics, Faeth Therapeutics, Selectin Therapeutics, and PMV Pharmaceuticals and outside consultancy (no equity) for Fate Therapeutics (unrelated to this manuscript). Z.S. has received research funds and reagents from Stemline Therapeutics and research reagents from Jazz Pharmaceuticals (neither of which are relevant to this study). The remaining authors declare no competing financial interests.
Correspondence: Scott W. Lowe, Memorial Sloan Kettering Cancer Center, 417 East 68th St, ZRC-1104, New York, NY 10065; email: lowes@mskcc.org.
References
Author notes
S.E.M. and A.C.-P. contributed equally to this study.
Sequencing data generated in this study are available in the Gene Expression Omnibus database under series records GSE282977 and GSE282980.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![OGDH is a bona fide genetic dependency across AML systems. (A) Schematic of the TCA cycle highlighting the αKG dehydrogenase complex. (B) Differential gene dependency in AML vs all non-AML (other) cancer types, based on difference of mean depletion or enrichment of sgRNAs targeting individual genes across available CRISPR screens. An y-axis value <0 indicates preferential dependency in AML and a value >0 indicates preferential dependency in other. Each data point represents an individual gene. Selected genes are labeled. (C) OGDH, DLST, and DLD gene effect across CRISPR screens for AML vs other cell lines included in the DepMap database. A lower gene effect indicates an increased likelihood of gene dependency, with 0 indicating nondependency and –1 representing the median of all pan-essential genes. Each data point represents an individual cell line, and the mean ± standard error are shown. P values indicated (Wilcoxon rank-sum test). (D) OGDH gene dependency ranking across CRISPR screens for AML vs other cell lines included in the DepMap database. Each data point represents an individual cell line, and the mean ± standard error are shown. P value is indicated (Wilcoxon rank-sum test). (E) For each indicated gene, the violin plot shows change in sgRNA abundance (measured as log2 fold change) in publicly available whole-genome CRISPR screens across multiple AML vs other cancer cell lines. P values are indicated (Wilcoxon rank-sum test). (F-G) Western blot analysis showing the expression of OGDH in AML cell lines transduced with the indicated shRNA after 3 days of doxycycline treatment. (F) NrasG12D; MLL-AF9 cells. (G) p53R172H cells. (H-I) Depletion of Ogdh shRNAs in the competition assay. (H) NrasG12D; MLL-AF9 murine leukemia cells were infected with retroviruses encoding the indicated doxycycline-inducible shRNAs linked to a GFP reporter and admixed with 20% uninfected (GFP–) cells. The relative percentages of GFP+ cells (normalized to day 1 for each shRNA) were counted on the indicated days after doxycycline treatment. P values of shOgdh vs shControl groups indicated (2-way analysis of variance [ANOVA] with Sidak multiple comparisons test). (I) p53R172H murine leukemia cells were infected with lentiviruses encoding reverse tetracycline-controlled transactivator and the indicated doxycycline-inducible shRNAs linked to a blue fluorescent protein (BFP) reporter on single backbone (LT3 BEPIR). Infected cells were admixed with 20% uninfected cells. The relative percentages of BFP+ cells (normalized to day 2 for each shRNA) were counted on the indicated days after doxycycline treatment. (J) Western blot analysis showing expression of OGDH in PDX1 AML cells 4 days after transduction with the indicated shRNAs. (K) Cell proliferation of PDX1 AML cells transduced with the indicated shRNAs (normalized to day 6 after infection for each shRNA). (L) Depletion of OGDH shRNAs in a competition assay in PDX2 cells. PDX2 AML cells were infected at day –7 with a lentivirus encoding shControl or shOGDH linked to a GFP reporter and a second lentivirus encoding shControl linked to a red fluorescent protein (RFP) reporter. The GFP:RFP ratio (normalized to day 0 for each shRNA pair) was measured on the indicated days. P value of shOGDH vs shControl group is indicated (2-way ANOVA). αKGDC, α-ketoglutarate dehydrogenase complex; ns, not significant; OAA, oxaloacetate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/13/10.1182_blood.2024025245/3/m_blood_bld-2024-025245-gr1.jpeg?Expires=1769093158&Signature=z3BIxbtR~C9spb3o2DjUUdsW0hbmWQYg6nR68pLTaWE2eGGh2BDpNP~NPnXokqq5t34mTm7KAHXwg9VCxqKZ5p8xg57tENG~QtYuWHAern56TM6RG4i-Z3O5Z-XS0V6I~eubHNOAsXbhciAl419WubEGcZ-PXVhX7MbpVFZwTYmrmjslumhICM4K8RapdaO~9TUmqWFGeMsFYyh-cvoR2F0IjejFLx7HH9guMvo~wa6KZJC-XaPeKJip7arEgn-PDL4JU2kJq4aPDExoZO3oDuOIBFc-qu-p6aa9I6ZK2N1m3pI-Q~qB9Fx8rhb8rb-RSqyQRhocoJO27~d9XKxrzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal