Visual Abstract
For patients with myelodysplastic neoplasm/syndrome (MDS), allogeneic hematopoietic cell transplantation (allo-HCT) represents the only potentially curative treatment, capable of eradicating disease-related mutant hematopoietic cells and establishing normal donor hematopoiesis. Biologic-assignment clinical trials have indicated that in eligible patients, allo-HCT is associated with superior clinical outcomes compared with nontransplant therapy. However, this therapeutic option is only available to a subset of patients, and the outcome is influenced by multiple factors inherent to the patient, the MDS subtype, and the allo-HCT procedure itself. In 2017, the European Society for Blood and Marrow Transplantation (EBMT) published recommendations for allo-HCT in MDS to guide practical decision making. In the contemporary era, genomic profiling has become routine clinical practice in many centers, and the most recent classification systems include MDS entities that are defined by genetic abnormalities. In particular, the molecular International Prognostic Scoring System offers more precise prognostication across all clinical end points and currently represents the standard tool for estimating patient survival in the absence of disease-modifying treatment. Evidence from multiple sources increasingly indicates that allo-HCT should be considered at the time of diagnosis in all eligible patients with MDS. Therefore, genomic profiling for somatic mutations and testing for germ line predisposition variants are integral to determining a patient’s eligibility for transplantation. Although all patients with higher-risk MDS are potential candidates for immediate transplantation, a subset of those with lower-risk MDS may also derive benefit from this procedure at an earlier disease stage. Comprehensive recommendations on behalf of an expert international panel for clinical practice and future clinical studies of relevance are presented.
Introduction
Myelodysplastic syndromes or neoplasms (MDS) are cytopenic disorders resulting from a clonal proliferation of hematopoietic cells driven by somatic genetic alterations.1-3 Because different driver genes may be responsible for clonal proliferation, the resultant disorders are heterogeneous with respect to clinical features and outcomes, particularly the risk of progression to acute myeloid leukemia (AML). Patients at low risk of leukemic transformation may be considered for medical treatment aimed at improving cytopenias, primarily anemia, and in turn quality-of-life (QoL). Conversely, individuals at high risk of progression to AML require more intensive treatments with the potential for disease modification.
To date, allogeneic hematopoietic cell transplantation (allo-HCT) remains the only potentially curative treatment option for MDS.4,5 In 2017, an international expert panel, comprising members of the European Society for Blood and Marrow Transplantation (EBMT), developed recommendations for allo-HCT in MDS.6 Since then, significant advances in the understanding of the genetics of MDS and associated clinical implications have occurred. Currently, genomic profiling is used to aid both diagnosis and risk stratification in routine clinical practice and several studies illustrate the pivotal role of integrating genomic features into clinical decision-making processes.7-14 In particular, the presence of TP53 mutations and its allelic state have been demonstrated to be critical for genome stability, clinical presentation, and outcomes, underscoring the necessity of its evaluation.15 The recently developed molecular International Prognostic Scoring System (IPSS-M) for MDS has been validated as an important tool for clinical decision making, including in the context of transplantation.16-20 Finally, a molecular taxonomy of MDS has been defined, including novel molecular entities, such as those associated with DDX41 mutations and VEXAS (vacuoles, E1 enzyme, X linked, autoinflammatory, somatic) syndrome.21-24 A review on the role of genome sequencing in the management of MDS and related disorders has recently been published.25
In the setting of these advances, the EBMT Chronic Malignancies Working Party (CMWP) determined that an update to the 2017 recommendations for allo-HCT in MDS was warranted.
Design and methods
In April 2024, the MDS subcommittee of the Chronic Malignancies Working Party of the EBMT agreed to revise its previous recommendations for allo-HCT in MDS. To this end, the subcommittee appointed a project leader (C.G.) and 2 senior authors (D.P.M. and M.C.), who, in turn, identified a team of MDS and allo-HCT experts chosen from within the EBMT or because of internationally recognized expertise to undertake this revision. The experts were initially contacted via email and all consented to participate in the project. They agreed with its goal to develop recommendations to support practical decision making for allo-HCT in patients with MDS, specifically taking into consideration the recent genomic and clinical advances within the field.
Once the expert team was established, a systematic review of the literature was conducted. This encompassed both indexed papers (PubMed) and abstracts of meeting proceedings. In particular, the meeting proceedings of the American Society of Hematology, the American Society of Clinical Oncology, and the European Hematology Association were considered. EBMT registry data were used when required.
Based on the systematic review of the literature, ad hoc working groups developed a list of questions potentially relevant to decision making in the transplant setting for MDS. These questions were then gathered and made available to all experts using electronic shared documents; responses were collected and analyzed. Iterative rounds of voting were needed to reach the level of consensus (≥66%, namely two-thirds of the expert panel) as per Delphi-modified methodology via controlled feedback.26 Details are provided in the supplemental Methods (available on the Blood website).
Advances in allogeneic transplantation for MDS
A systematic review of the literature was conducted with a focus on studies published since 2017, that is, after the publication of the previous recommendations.6
Biologic-assignment clinical trials comparing allogeneic transplantation with medical treatment
Despite the absence of randomized trials directly comparing allo-HCT and conventional therapy in MDS, valuable studies have been conducted to investigate the comparative efficacy of these 2 approaches.27-29 In these so-called biologic-assignment clinical trials, patients with MDS were stratified according to the availability of a biologically matched HLA-compatible donor (donor vs no donor studies).30 These studies have demonstrated improvement in long-term outcomes with transplantation, favoring the superiority of allo-HCT over non-HCT therapy, at least for patients with higher-risk MDS up to the age of 75 years.20
The biologic-assignment trial conducted by the Blood and Marrow Transplant Clinical Trials Network compared reduced-intensity conditioning (RIC) allo-HCT with nontransplant therapy (hypomethylating agent [HMA] or best supportive care) in patients aged 50 to 75 years with higher-risk de novo MDS.29 The trial enrolled 384 patients at 34 centers: 260 patients were enrolled in the donor arm and 124 in the no-donor arm. In an intention-to-treat analysis, the adjusted overall survival (OS) rate at 3-years was 48% in the transplant cohort, compared with 27% in the nontransplant arm. Additionally, the survival advantage afforded by allo-HCT was cost-effective and there was no decrease in QoL for patients who had received HCT compared with controls.31,32 A secondary analysis demonstrates that RIC allo-HCT improved OS also in high-risk genetic subgroups, including both TP53-mutated MDS and patients with very high IPSS-M risk.18
Clinical studies comparing MAC with RIC
Treatment-related mortality represents a significant challenge associated with allo-HCT, particularly in older patients and those with comorbidities. To optimize transplantation outcomes in these individuals, prospective randomized studies have been designed that compared different conditioning strategies and intensities. The working hypothesis was that RIC platforms would result in improved OS compared with myeloablative conditioning (MAC).33-39 These studies suggest that although MAC may offer improved disease control resulting in survival benefit for fitter and higher-risk patients with MDS, RIC expands transplantation suitability, particularly for older individuals and those with more comorbidities, without a significantly higher risk of relapse.40
Contemporary practice in Europe: what can we learn from EBMT registry data of MDS allo-HCT between 2010 and 2020?
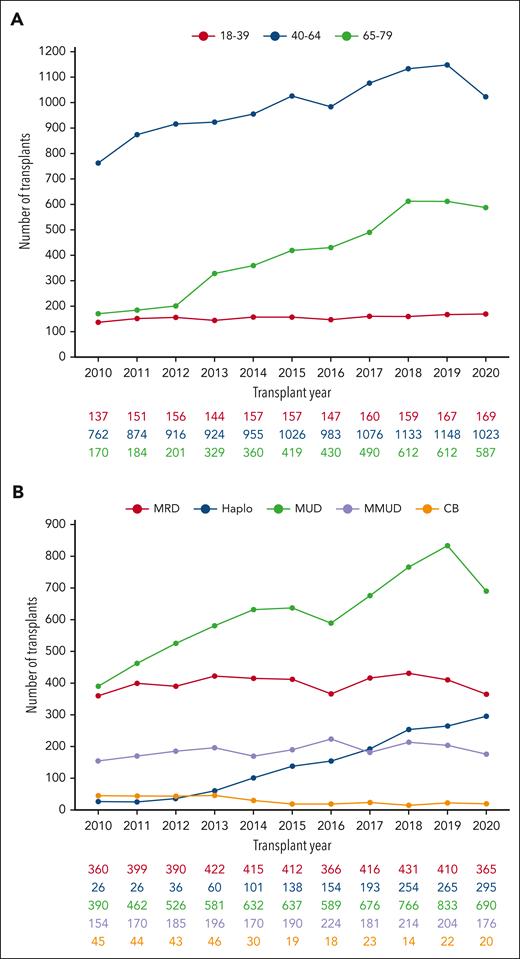
A total of 16 918 allo-HCTs in patients with MDS were reported to the EBMT registry between 2010 and 2020. This represents a 66.4% increase over a 10-year period, with the number of procedures rising from 1069 in 2010 to 1779 in 2020 (supplemental Figure 1). A descriptive analysis of these data illustrates the dynamic advances in MDS allo-HCT activity. The median age of patients undergoing transplantation has increased significantly, and, in 2020, approximately one-third of patients were aged between 65 and 79 years (Figure 1A). Concerning donor type, there has been a significant increase in the use of haploidentical donors (2.4% in 2010 vs 16.5% in 2020; Figure 1B), whereas peripheral blood has become the predominant stem cell source in lieu of bone marrow (supplemental Figure 2). Regarding conditioning intensity, RIC regimens remain the most frequently used, taking into consideration the higher median age of this group of patients (59% in both 2010 and 2020; supplemental Figure 3).
Contemporary practice in Europe: a dynamic review of MDS allo-HCT activity from the EBMT registry between 2010 and 2020. (A) Illustrating the number of allo-HCTs for MDS by age ranges. (B) Evolution by type of donors: HLA-id donors, MUD, and MMUD. CB, cord blood; Haplo, haploidentical; HLA-id, haploidentical; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
Contemporary practice in Europe: a dynamic review of MDS allo-HCT activity from the EBMT registry between 2010 and 2020. (A) Illustrating the number of allo-HCTs for MDS by age ranges. (B) Evolution by type of donors: HLA-id donors, MUD, and MMUD. CB, cord blood; Haplo, haploidentical; HLA-id, haploidentical; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
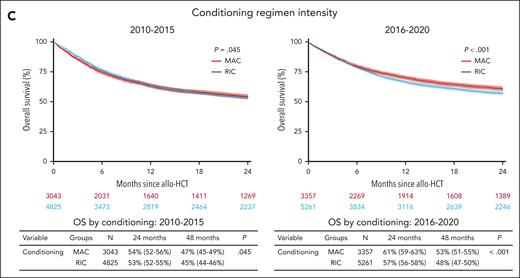
The EBMT registry facilitates longer-term outcome analysis with regard to OS, progression-free survival, treatment-related mortality, and relapse rates in a large number of patients who had received transplantation, with the continuing improvement in outcome being attained by better treatment of infections, refinement of conditioning regimens (major use of RIC regimens), as well as improvements in monitoring and supportive care. Particularly, in this period, we have seen the consolidation of the posttransplant cyclophosphamide (PTCy) platform as prophylaxis for graft-versus-host disease (GVHD; of note, used in <1% of transplants in 2010 vs 59% in 2020). Pivotal studies from the EBMT on evaluating outcomes and adverse effects of allo-HCT for MDS are discussed hereafter, when relevant, whereas a cartography of survival analyses of EBMT registry based on donor type, age, and conditioning intensity over time is provided in Figure 2. No differences were noticed in terms of outcomes when comparing stem cell source (supplemental Figure 4).
Contemporary practice in Europe: outcomes of patients with MDS enrolled in the EBMT registry and allografted between 2010 and 2020. Kaplan-Meier curves show OS outcomes of patients with MDS in the time periods 2010 to 2015 and 2016 to 2020 according to the age at allo-HCT (A), type of donor (B), and conditioning regimen intensity (C). Numbers at risk are indicated below the curves and color-coded. Table below each curve provides OS estimates at 24 and 48 months; median follow-up for the entire cohort is 49 months (95% confidence interval, 48-50). CB, cord blood; Haplo, haploidentical; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
Contemporary practice in Europe: outcomes of patients with MDS enrolled in the EBMT registry and allografted between 2010 and 2020. Kaplan-Meier curves show OS outcomes of patients with MDS in the time periods 2010 to 2015 and 2016 to 2020 according to the age at allo-HCT (A), type of donor (B), and conditioning regimen intensity (C). Numbers at risk are indicated below the curves and color-coded. Table below each curve provides OS estimates at 24 and 48 months; median follow-up for the entire cohort is 49 months (95% confidence interval, 48-50). CB, cord blood; Haplo, haploidentical; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
Genomic profiling in the management of patients with MDS undergoing allogeneic transplantation
The advent of genomic profiling has fundamentally transformed the diagnostic approach to MDS in the current era. Conventional methods were essentially focused on the detection and characterization of myelodysplastic features, namely, morphological abnormalities. In contrast, molecular profiling is primarily used for the identification of the clonal (malignant) nature of aberrant hematopoiesis, as well as the identification of specific genetic lesions that are associated with distinct clinical phenotypes and prognosis in MDS.1,25 The results of the expert panel survey on the diagnosis and classification of MDS are summarized in the supplemental Results.
The backbone of MDS diagnosis and classification has historically been the assessment of the degree of cytopenia, blast count, and presence of dysplasia. The most recent classifications of MDS, namely the International Consensus Classification and the fifth edition of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemias (WHO-HAEM5), have now included genetic features in the diagnostic criteria, defining 3 MDS entities by genetic abnormalities (Tables 1 and 2).2,3 Both classifications emphasize that MDS is a myeloid neoplasm that lies on a continuum between clonal hematopoiesis (clonal hematopoiesis of indeterminate potential and clonal cytopenia of undetermined significance) and AML. The conventional threshold for blast counts in the bone marrow that separates MDS from AML has historically been 20%; however, recent genetic data have shown that some genetic aberrations are associated with “AML-like” biology even in cases with <20% blasts.41,42 Accordingly, both the WHO-HAEM5 and International Consensus Classification allow a diagnosis of AML in the presence of certain specific gene fusions or mutations, regardless of the blast count.
MDS entities according to WHO-HAEM5 2022 and ICC 2022 classifications
| WHO-HAEM5 2022 . | ICC 2022 . |
|---|---|
| MDS with defining genetic abnormalities | |
| MDS with low blasts and isolated 5q deletion | MDS with del(5q) |
| MDS with low blasts and SF3B1 mutation∗ | MDS with mutated SF3B1 |
| MDS with biallelic TP53 inactivation | MDS with mutated TP53 |
| MDS, morphologically defined | |
| MDS with low blasts (<5% blasts; dysplasia is a prerequisite) | |
| MDS with low blasts and single-lineage dysplasia (MDS-LB-SLD) MDS with low blasts and multilineage dysplasia (MDS-LB-MLD) MDS with ring sideroblasts (MDS-RS) | MDS, NOS without dysplasia MDS, NOS with single lineage MDS, NOS with multilineage dysplasia |
| MDS, hypoplastic (<5% blasts) | |
| MDS with increased blasts (5% to <20% blasts) | |
| MDS-IB1 (5% to <10% blasts) MDS-IB2 (10% to <20% blasts) MDS with fibrosis (5% to <20% blasts) | MDS with excess blasts (5% to <10% blasts) MDS/AML (10% to <20% blasts) |
| WHO-HAEM5 2022 . | ICC 2022 . |
|---|---|
| MDS with defining genetic abnormalities | |
| MDS with low blasts and isolated 5q deletion | MDS with del(5q) |
| MDS with low blasts and SF3B1 mutation∗ | MDS with mutated SF3B1 |
| MDS with biallelic TP53 inactivation | MDS with mutated TP53 |
| MDS, morphologically defined | |
| MDS with low blasts (<5% blasts; dysplasia is a prerequisite) | |
| MDS with low blasts and single-lineage dysplasia (MDS-LB-SLD) MDS with low blasts and multilineage dysplasia (MDS-LB-MLD) MDS with ring sideroblasts (MDS-RS) | MDS, NOS without dysplasia MDS, NOS with single lineage MDS, NOS with multilineage dysplasia |
| MDS, hypoplastic (<5% blasts) | |
| MDS with increased blasts (5% to <20% blasts) | |
| MDS-IB1 (5% to <10% blasts) MDS-IB2 (10% to <20% blasts) MDS with fibrosis (5% to <20% blasts) | MDS with excess blasts (5% to <10% blasts) MDS/AML (10% to <20% blasts) |
IB1/2, increased blasts type 1/2; ICC, International Consensus Classification; LB, low blasts; MLD, multilineage dysplasia; NOS, not otherwise specified; RS, ring sideroblasts; SLD, single-lineage dysplasia.
When SF3B1 mutation analysis is not available and ring sideroblasts constitute ≥15% of erythroid precursors.
MDS entities defined by genetic abnormalities in the ICC and WHO-HAEM5
| WHO-HAEM5 . | ICC . | Implications of genomic profiling . |
|---|---|---|
| MDS with low blasts and isolated 5q deletion | MDS with del(5q) | |
| BM blasts of <5% del(5q) alone or with 1 additional abnormality, except −7/del(7q) Any somatic mutation, except biallelic TP53 inactivation | BM blasts of <5% del(5q) alone or with 1 additional abnormality, except −7/del(7q) Any somatic mutation, except biallelic TP53 | Genomic profiling is fundamental to diagnosis. Patients with biallelic inactivation of TP53 have poor outcomes and are therefore classified as TP53-mutant MDS. |
| MDS with low blasts and SF3B1 mutation | MDS with mutated SF3B1 | |
| BM blasts of <5% Absence of del(5q), −7, or complex karyotype SF3B1 mutation, no biallelic TP53 | BM blasts of <5% Absence of del(5q), −7/del(7q), abn3q26.2, or complex karyotype SF3B1 mutation (VAF ≥10%), no biallelic TP53 inactivation, no RUNX1 mutation | Genomic profiling is fundamental to diagnosis (exclusion of biallelic inactivation of TP53) and important for prognosis. Patients with comutation patterns in other genes such as BCOR, BCORL1, NRAS, RUNX1, or STAG2 have worse clinical outcomes. |
| MDS with biallelic TP53 inactivation | MDS with mutated TP53 | |
| BM blasts of <20% Two or more TP53 mutations, or one mutation with evidence of TP53 copy number loss or cnLOH Usually complex karyotype | BM blasts of 0%-9% Biallelic TP53 inactivation∗ or TP53 mutation (VAF >10%) and complex karyotype often with loss of 17p† | This condition is associated with exceptionally poor clinical outcomes. The identification of a biallelic TP53 inactivation requires the implementation of ad hoc copy number and LOH analysis. |
| MDS/AML with mutated TP53 | ||
| BM blasts of 10%-19% Any somatic TP53 mutation (VAF >10%) |
| WHO-HAEM5 . | ICC . | Implications of genomic profiling . |
|---|---|---|
| MDS with low blasts and isolated 5q deletion | MDS with del(5q) | |
| BM blasts of <5% del(5q) alone or with 1 additional abnormality, except −7/del(7q) Any somatic mutation, except biallelic TP53 inactivation | BM blasts of <5% del(5q) alone or with 1 additional abnormality, except −7/del(7q) Any somatic mutation, except biallelic TP53 | Genomic profiling is fundamental to diagnosis. Patients with biallelic inactivation of TP53 have poor outcomes and are therefore classified as TP53-mutant MDS. |
| MDS with low blasts and SF3B1 mutation | MDS with mutated SF3B1 | |
| BM blasts of <5% Absence of del(5q), −7, or complex karyotype SF3B1 mutation, no biallelic TP53 | BM blasts of <5% Absence of del(5q), −7/del(7q), abn3q26.2, or complex karyotype SF3B1 mutation (VAF ≥10%), no biallelic TP53 inactivation, no RUNX1 mutation | Genomic profiling is fundamental to diagnosis (exclusion of biallelic inactivation of TP53) and important for prognosis. Patients with comutation patterns in other genes such as BCOR, BCORL1, NRAS, RUNX1, or STAG2 have worse clinical outcomes. |
| MDS with biallelic TP53 inactivation | MDS with mutated TP53 | |
| BM blasts of <20% Two or more TP53 mutations, or one mutation with evidence of TP53 copy number loss or cnLOH Usually complex karyotype | BM blasts of 0%-9% Biallelic TP53 inactivation∗ or TP53 mutation (VAF >10%) and complex karyotype often with loss of 17p† | This condition is associated with exceptionally poor clinical outcomes. The identification of a biallelic TP53 inactivation requires the implementation of ad hoc copy number and LOH analysis. |
| MDS/AML with mutated TP53 | ||
| BM blasts of 10%-19% Any somatic TP53 mutation (VAF >10%) |
BM, bone marrow; cnLOH, copy-neutral loss of heterozygosity; VAF, variant allele frequency.
Defined as 2 distinct TP53 mutations (each VAF of >10%) OR a single TP53 mutation with (1) 17p deletion on cytogenetics; (2) VAF of >50%; or (3) cnLOH at the 17p TP53 locus.
If TP53 locus LOH information is not available.
A comprehensive bone marrow evaluation including cytomorphology, chromosome banding analysis, and gene sequencing is essential to establishing the diagnosis and classification of patients with MDS.43,44 For classification purposes, only SF3B1 and TP53 mutation assessment is required, but for prognostic classification according to IPSS-M, a substantially larger gene panel is mandatory.13 The blast count, karyotype, and gene mutation profile should be provided in any bone marrow sample taken immediately before transplantation, because this may differ from the original presentation (if time has elapsed) and provide a baseline to compare with bone marrow samples taken after the therapeutic procedure.45 Next-generation sequencing (NGS) and cytogenetics are generally available in a marrow sample, given its necessity for MDS diagnosis to distinguish from other clonal cytopenic states, but peripheral blood can also be a good source of DNA for genomic profiling and if NGS has already been performed on a recent blood specimen, it may not need to be repeated on the bone marrow.46,47 Regardless of the source of DNA, genomic studies should be interpreted in the context of marrow morphology and blast percentage to establish an MDS diagnosis.
The IPSS-M
For >10 years, the revised IPSS (IPSS-R) has been used for risk stratification in MDS.48 The advent of genomic profiling has revealed a significant limitation of the IPSS-R, namely its inability to account for somatic gene mutations and their prognostic implications.25 In pursuit of molecular precision, the International Working Group for Prognosis in MDS developed the IPSS-M, which is applicable to both primary and secondary/therapy-related MDS (Table 3).16
IPSS-M risk score, risk categories, and clinical outcomes
| Risk category . | IPSS-M score . | Median leukemia-free survival (y) . | Median OS (y) . | AML transformation by 1 y (%) . |
|---|---|---|---|---|
| Six-category risk schema | ||||
| Very low (14% of all patients) | Less than or equal to −1.5 | 9.7 | 10.6 | 0 |
| Low (33%) | More than −1.5 to −0.5 | 5.9 | 6.0 | 1.7 |
| Moderate low (11%) | More than −0.5 to 0 | 4.5 | 4.6 | 4.9 |
| Moderate high (11%) | >0 to 0.5 | 2.3 | 2.8 | 9.5 |
| High (14%) | >0.5 to 1.5 | 1.5 | 1.7 | 14.3 |
| Very high (17%) | >1.5 | 0.7 | 1.0 | 28.2 |
| Lower-risk vs higher-risk MDS | ||||
| Lower-risk MDS (58%) | ≤0 (negative value) | 6.0 (95% CI, 5.7-6.7) | 6.3 (95% CI, 5.8-7.2) | 2.0 |
| Higher-risk MDS (42%) | >0 (positive value) | 1.2 (95% CI, 1.1-1.3) | 1.5 (95% CI, 1.4-1.6) | 18.9 |
| Risk category . | IPSS-M score . | Median leukemia-free survival (y) . | Median OS (y) . | AML transformation by 1 y (%) . |
|---|---|---|---|---|
| Six-category risk schema | ||||
| Very low (14% of all patients) | Less than or equal to −1.5 | 9.7 | 10.6 | 0 |
| Low (33%) | More than −1.5 to −0.5 | 5.9 | 6.0 | 1.7 |
| Moderate low (11%) | More than −0.5 to 0 | 4.5 | 4.6 | 4.9 |
| Moderate high (11%) | >0 to 0.5 | 2.3 | 2.8 | 9.5 |
| High (14%) | >0.5 to 1.5 | 1.5 | 1.7 | 14.3 |
| Very high (17%) | >1.5 | 0.7 | 1.0 | 28.2 |
| Lower-risk vs higher-risk MDS | ||||
| Lower-risk MDS (58%) | ≤0 (negative value) | 6.0 (95% CI, 5.7-6.7) | 6.3 (95% CI, 5.8-7.2) | 2.0 |
| Higher-risk MDS (42%) | >0 (positive value) | 1.2 (95% CI, 1.1-1.3) | 1.5 (95% CI, 1.4-1.6) | 18.9 |
Original information is from Bernard et al,16 as displayed by Cazzola and Malcovati (reproduced under a CC BY-NC license).25
The IPSS-M calculation uses information about the mutation status of the following genes in addition to del(5q): ASXL1, BCOR, BCORL1, CBL, CEBPA, DNMT3A, ETNK1, ETV6, EZH2, FLT3, GATA2, GNB1, IDH1, IDH2, KRAS, MLL (KMT2A), NF1, NPM1, NRAS, PHF6, PPM1D, PRPF8, PTPN11, RUNX1, SETBP1, SF3B1, SRSF2, STAG2, TP53, U2AF1, and WT1.
The International Working Group for Prognosis in MDS has developed the IPSS-M web-based calculator, which is freely available online.16 In addition to providing the individual IPSS-R and IPSS-M scores, the calculator also returns the expected leukemia-free survival (median value, y), (OS median value, y), and risk of AML transformation (% by 1 y). Notably, the calculator is capable of accounting for missing values, whereby the IPSS-M is calculated under three distinct scenarios: best, average, and worst.
CI, confidence interval.
Although the IPSS-M improves personalized prognostic discrimination across all clinical end points when compared with the IPSS-R,17,19 it must be emphasized that it was developed using a cohort of patients with MDS who had not yet received treatment to alter the natural history of the disease.49 As such, IPSS-M primarily represents a valuable tool for estimating patient survival in the absence of disease-modifying treatment. Nonetheless, the application of the IPSS-M has been found to enhance the estimation of both survival and risk of posttransplant relapse in comparison with the IPSS-R.17 Prognostic models that additionally include transplantation-related parameters likely provide improved estimation of outcomes after allo-HCT.49-51
Molecular taxonomy of MDS
The International Working Group for Prognosis in MDS has recently examined 3233 representative patients with MDS to determine whether and how genetic lesions delineate specific disease subtypes.24 Unsupervised clustering analysis through Bayesian Dirichlet processes enabled the formulation of a molecular taxonomy comprising 18 distinct groups (Table 4). The results of the expert panel survey on the molecular taxonomy of MDS are summarized in the supplemental Results.
Genetically derived subgroups of MDS that have been defined in the ad hoc study of the International Working Group for Prognosis in MDS
| Molecular subgroup . | Clinical features, outcomes, and disease-related eligibility for allogeneic transplantation . |
|---|---|
| Morphological MDS with the absence of recurrent genetic events in myeloid genes | Good OS with low risk of leukemic transformation. Most patients are not transplant candidates. Patients with VEXAS and severe rheumatic disease may be considered. |
| SF3B1-mutant MDS | Indolent clinical course with low risk of leukemic transformation. Most of these patients are not transplant candidates. |
| ZRSR2-mutant MDS | Male patients with refractory macrocytic anemia, no excess blasts, and indolent clinical course. These patients are not transplant candidates. |
| MDS NOS | Mild phenotype and favorable outcomes in most patients. Most of these patients are not transplant candidates. |
| CCUS-like MDS | Mild phenotype and favorable outcomes in most patients. Most of these patients are not transplant candidates. |
| MDS del(5q) | Well-established MDS subtype whose outcomes are determined by comutation patterns. Patients with comutation in SF3B1, RUNX1, or TP53 should be considered for allogeneic transplantation. |
| MDS with biallelic TET2 mutation | Older patients, monocytosis overlapping with CMML. Indolent clinical course in most patients. Most of these patients are not transplant candidates. |
| DDX41-mutant MDS | Cytopenia with hypoplastic bone marrow with excess blasts. Disorder with high risk of leukemic evolution, but favorable prognosis compared with other MDS with excess blasts. Genomic diagnosis is crucial in the transplantation setting for donor selection and prevention of acute GVHD with PTCy. All patients are potential transplant candidates. |
| U2AF1-mutant MDS SRSF2-mutant MDS BCOR/L1-mutant MDS IDH-STAG2-mutant MDS MDS with der(1;7) −7/SETBP1-mutant MDS EZH2-ASXL1-mutant MDS | Aggressive diseases with poor survival and high risk of leukemic transformation. Although specific agents targeting the driver mutation are being developed (such as spliceosome inhibitors, ivosidenib, or enasidenib). These patients are potential transplant candidates. |
| AML-like MDS | Biologically this condition resembles AML, except for <20% bone marrow blasts. All patients are potential transplant candidates. |
| TP53-complex MDS | Extremely aggressive disease with a median survival <1 year. Poorly responsive to any currently available treatment. High relapse rate after transplantation. All patients are potential transplant candidates, preferably within a clinical trial. |
| Molecular subgroup . | Clinical features, outcomes, and disease-related eligibility for allogeneic transplantation . |
|---|---|
| Morphological MDS with the absence of recurrent genetic events in myeloid genes | Good OS with low risk of leukemic transformation. Most patients are not transplant candidates. Patients with VEXAS and severe rheumatic disease may be considered. |
| SF3B1-mutant MDS | Indolent clinical course with low risk of leukemic transformation. Most of these patients are not transplant candidates. |
| ZRSR2-mutant MDS | Male patients with refractory macrocytic anemia, no excess blasts, and indolent clinical course. These patients are not transplant candidates. |
| MDS NOS | Mild phenotype and favorable outcomes in most patients. Most of these patients are not transplant candidates. |
| CCUS-like MDS | Mild phenotype and favorable outcomes in most patients. Most of these patients are not transplant candidates. |
| MDS del(5q) | Well-established MDS subtype whose outcomes are determined by comutation patterns. Patients with comutation in SF3B1, RUNX1, or TP53 should be considered for allogeneic transplantation. |
| MDS with biallelic TET2 mutation | Older patients, monocytosis overlapping with CMML. Indolent clinical course in most patients. Most of these patients are not transplant candidates. |
| DDX41-mutant MDS | Cytopenia with hypoplastic bone marrow with excess blasts. Disorder with high risk of leukemic evolution, but favorable prognosis compared with other MDS with excess blasts. Genomic diagnosis is crucial in the transplantation setting for donor selection and prevention of acute GVHD with PTCy. All patients are potential transplant candidates. |
| U2AF1-mutant MDS SRSF2-mutant MDS BCOR/L1-mutant MDS IDH-STAG2-mutant MDS MDS with der(1;7) −7/SETBP1-mutant MDS EZH2-ASXL1-mutant MDS | Aggressive diseases with poor survival and high risk of leukemic transformation. Although specific agents targeting the driver mutation are being developed (such as spliceosome inhibitors, ivosidenib, or enasidenib). These patients are potential transplant candidates. |
| AML-like MDS | Biologically this condition resembles AML, except for <20% bone marrow blasts. All patients are potential transplant candidates. |
| TP53-complex MDS | Extremely aggressive disease with a median survival <1 year. Poorly responsive to any currently available treatment. High relapse rate after transplantation. All patients are potential transplant candidates, preferably within a clinical trial. |
CCUS, clonal cytopenia of undetermined significance; CMML, chronic myelomonocytic leukemia; NOS, not otherwise specified.
Modified from Bernard et al.24
The molecular entities of Table 4 may be relevant to decision making in the transplantation setting. Although 2 patients with MDS belonging to different molecular subgroups may have the same IPSS-M risk of progression to AML, they may benefit from different management. For instance, for a patient with DDX41-mutant MDS, the identification of the molecular group is crucial for implementing donor selection strategy and prevention of acute GVHD with PTCy.52 By contrast, a patient with TP53-complex MDS could be considered for enrollment into a clinical trial given the poor outcome of conventional allo-HCT.
Germ line genetic predisposition to MDS
Up to 5% to 10% of adult patients with MDS may carry germ line variants that predispose to myeloid neoplasms.53 Clinical implications in the setting of transplant include the selection of familial donors, genetic counseling for relatives, and the implementation of dedicated conditioning strategies in specific conditions (eg, TERT/Fanconi anemia/DDX41 variants).54 The EBMT has recently provided guidance on this matter, with particular emphasis on recommendations for screening for germ line predisposition, testing modalities, DNA sources, and clinical actionability with regard to allo-HCT.55 The results of the expert panel survey on the germ line genetic predisposition to MDS are summarized in the supplemental Results.
Measurable residual disease assessment after allogeneic transplantation
To establish whether genomic profiling may enable the assessment of measurable residual disease for the monitoring of responses to allo-HCT in patients with MDS, Duncavage et al used enhanced exome sequencing of paired samples of bone marrow and normal tissue.45 Subsequently, error-corrected sequencing was used to genotype mutations in bone marrow samples obtained 30 days after transplantation. Patients who exhibited a mutation with a variant allele frequency of ≥0.5% at day 30 demonstrated an elevated risk of disease progression.45
The relationship between measurable residual disease using patient-specific somatic mutations and relapse after allo-HCT has recently been investigated in a prospective study of 266 patients with MDS.56 Before transplantation, patient-specific mutations were identified using a targeted sequencing panel of 54 genes. After transplantation, patient-specific mutations were evaluated using droplet digital polymerase chain reaction, a technique with a sensitivity of 0.1%. In 42 of 44 cases, disease relapse was preceded by a positive measurable residual disease in the bone marrow. Overall, this study demonstrates that genome profiling can be used to evaluate measurable residual disease in patients with MDS after allo-HCT.
The results of the expert panel survey on measurable residual disease assessment are summarized in the supplemental Results. The experts concluded that there is enough evidence to use measurable residual disease monitoring after allo-HCT in patients with MDS.
2024 EBMT recommendations on allogeneic transplantation for patients with MDS
In clinical practice, after a confirmed diagnosis of MDS, the next step is to determine whether the patient is eligible for allo-HCT.1
Assessing the patient’s eligibility for allogeneic transplantation at the time of diagnosis
The results of the expert panel survey on this topic are summarized in the supplemental Results. The patient’s eligibility for allo-HCT is contingent upon both disease-related and patient-related risk factors, as well as the availability of a suitable donor. Once this information is available, the decision regarding transplantation should be made in a fully shared process that explicitly considers the patient’s values and goals.1
Transplantation eligibility based on disease-related risk factors
The primary objective of disease-related risk factor assessment is to determine whether transplantation should be performed at the time of diagnosis or considered as a potential treatment option at a later stage of the disease.
Allo-HCT is primarily, although not exclusively, used in patients with MDS to prevent leukemic transformation and prolong survival. Nowadays, the IPSS-M should be used in determining whether a patient requires transplantation, and its timing (Table 3). All patients with higher-risk MDS are potential candidates for upfront allo-HCT, which should ideally be performed shortly after diagnosis.18,29
The question of whether any patient with lower-risk MDS (Table 3) may benefit from immediate allo-HCT (tentatively, within 6 months from diagnosis) represents a significant challenge in the absence of pertinent clinical data. Overall, patients with very-low IPSS-M risk are not routinely approached for transplantation. In those with a low or moderate-low IPSS-M risk, the possibility of transplantation in the first 6 months after diagnosis should be considered on an individual basis. Potential candidates include individuals with a germ line predisposition to myeloid neoplasms and those with transfusion-dependent cytopenias who are unresponsive or have lost a prior response to medical treatments.4 Likewise, allo-HCT should be an early consideration for therapy-related MDS. In hypoplastic MDS, the current risk prognosis tools are less rigorously established, and transplant indication may come from the evaluation of the severity of cytopenias, transfusion burden, and failure of immunosuppressive treatment.6,57 A special mention deserves the clinical dyad of VEXAS/MDS, generally belonging to the lower-risk IPSS-R/M categories.23,58 In this peculiar setting, the morbidity associated with the VEXAS diagnosis may prioritize the transplant option also in patients with lower-risk disease given the reported recalcitrant hyperinflammatory picture of these patients. Data from the most recent meta-analysis on VEXAS and transplant are presented in supplemental Table 1.59
Patients with MDS with known germ line predisposition should be preferentially managed within clinical programs or research protocols in partnership with experts in the field as indicated by recent guidelines (supplemental Table 2).1,55
The panel also agreed that a special consideration deserves the scenario of low-to-middle income countries in which NGS is not available, and when traditional risk factors such as cytogenetics, marrow blasts, and blood counts (namely IPSS-R) should be factored as disease-related variables in transplant decisions.
Transplantation suitability based on patient-related risk factors
The advent of RIC regimens, more effective prevention of GVHD, and more accurate assessment of the biological age have rendered allo-HCT a feasible option for patients with MDS up to the eighth decade of life.60-62 However, although chronological age is not an absolute contraindication, it remains one of the most impactful variables in predicting posttransplant outcomes. Thus, allo-HCT should be generally avoided in patients aged >80 years.63
Beyond age, several factors influence survival in patients with MDS undergoing allo-HCT. The results of the expert panel survey on this topic are summarized in the supplemental Results. A number of studies have demonstrated that the Karnofsky performance status and the HCT-specific comorbidity index have a significant prognostic impact on outcomes of allo-HCT, irrespective of the characteristics of the underlying disease.64,65 From a practical point of view, the panel agreed that suitability based on patient-related risk factors can be determined as shown in Table 5, using a cutoff of 80 (≥80 vs <80) for Karnofsky performance status, and of 2 (0-2 vs ≥3) for HCT-specific comorbidity index.67
Transplantation suitability based on patient-related risk factors
| Suitability . | Age, Karnofsky performance status, HCT-CI,∗ and MDS-specific FI† . |
|---|---|
| Fit patients | Age <80 y Karnofsky performance status score of ≥80% HCT-CI score of 0 to 2 (low or intermediate risk) MDS-specific FI of <0.3 |
| Patients who are unfit | Age ≥80 y Karnofsky performance status score of <80% HCT-CI score of ≥3 (high risk) MDS-specific FI of ≥0.3 |
| Suitability . | Age, Karnofsky performance status, HCT-CI,∗ and MDS-specific FI† . |
|---|---|
| Fit patients | Age <80 y Karnofsky performance status score of ≥80% HCT-CI score of 0 to 2 (low or intermediate risk) MDS-specific FI of <0.3 |
| Patients who are unfit | Age ≥80 y Karnofsky performance status score of <80% HCT-CI score of ≥3 (high risk) MDS-specific FI of ≥0.3 |
FI, frailty score; HCT-CI, HCT-specific comorbidity index.
HCT-CI scores identify 3 risk groups: 0 (low risk), 1 to 2 (intermediate risk), and ≥3 (high risk).64
MDS-specific FI takes into account 42 deficits measurements reflective of physical performance, comorbidities, laboratory values, instrumental activities of daily living, QoL, and performance status.66
Geriatric assessment is a critical component of evaluating a patient’s suitability for transplantation.61 Patients exhibiting clinically relevant frailty (estimated to be prevalent in 19.6% of individuals aged 50-64 years) are considered unfit for allo-HCT, because this syndrome is independently associated with poor survival.66,68,69 Evaluation of body iron status may be needed to assess transfusional iron overload and establish the need for chelation therapy before transplantation, but this should not delay the procedure in high-risk MDS.70
Patient informed decision-making: counseling on allogeneic transplantation to guide the patient’s choice based on personal goals
Counseling patients with MDS regarding allo-HCT requires a comprehensive approach to ensure these individuals are empowered to make informed decisions that align with their personal goals, particularly emphasizing the need to consider present or future QoL.71 The transplant physician has a crucial role in the informed decision-making process but must be involved in the patient management from the very beginning (at MDS onset) to plan donor search and have meaningful discussions regarding the transplant procedure. The results of the expert panel survey on this topic are summarized in the supplemental Results; Table 6 reports the main items to be discussed with the patient.
Counseling on allogeneic transplantation to inform the patient’s choice: key items to be considered and carefully discussed
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Timing of transplantation
The question of the optimal timing for allo-HCT within the disease course remains a topic of ongoing investigation.20 Studies, some of which also using Markov models, have described the natural history of the disease and assessed the effect of different timing policies on patient survival.72-75 The findings of these studies collectively indicate that patients with higher-risk MDS may derive greater benefit from immediate allo-HCT, whereas those with lower-risk MDS may have a longer survival with delayed transplantation; however, the expert panel did not reach agreement on this topic, emphasizing the role of characteristics beyond the mere IPSS-M to decide upon timing of allo-HCT.20
In a recent decision analysis based on both clinical features and the IPSS-M risk, immediate transplantation was estimated to be associated with a prolonged life expectancy in patients with higher-risk MDS (as defined in Table 3), regardless of age.19 In those with lower-risk MDS, age was found to have an independent effect on the potential survival benefit. A delayed transplantation policy was estimated to be beneficial for patients with lower-risk MDS up to the age of 60 years. Conversely, those aged >60 years were more likely to have a prolonged life expectancy with immediate transplantation.
Donor selection
In the last decade, an increasing number of allografts from haploidentical donors, the use of PTCy and its impact on HLA-matching, and data on the role of donor age in posttransplant outcomes have contributed to influence donor selection algorithms for MDS.76 Recent data from the Acute Leukemia Working Party of the EBMT showed that younger unrelated donors may be preferable over HLA-matched relatives in the PTCy setting.77 In a study from the Center for International Blood and Marrow Transplant Research, patients with MDS undergoing allo-HCT from haploidentical relatives and matched unrelated donors (MUD) had similar survival despite higher relapse rate in patients with haploidentical donors. This was because of an increase in the rate of chronic GVHD in patients receiving MUD allografts.78 Another study comparing outcomes of high-risk MDS undergoing allo-HCT with haploidentical donors, MUD or HLA-matched relatives showed comparable mortality rates, suggesting that donor age might affect recipient outcome beyond the degree of HLA matching.79 In the setting of haploidentical transplant and PTCy, parental donors were associated with inferior outcomes compared with sibling donors.80
The results of the expert panel survey on donor selection are summarized in the supplemental Results. A list of factors to be included in the donor selection algorithm is provided in supplemental Table 3.
Cytoreductive treatment before transplant conditioning
In 2017, based on limited evidence, the expert panel concluded that a cytoreductive treatment should be used before transplant conditioning in patients with MDS with ≥10% bone marrow blasts.6 To date, no prospective studies have been reported that compare pretransplant therapy with no therapy, and the studies published in recent years do not allow us to draw firm conclusions about the effect of cytoreductive therapy before transplant conditioning on transplant outcome in patients with MDS.81-85
The results of the expert panel survey on cytoreductive treatment are summarized in the supplemental Results. The panel maintained the 2017 recommendation that patients with MDS with blast excess (≥10%) may benefit from cytoreductive therapy before allogeneic transplantation, but there was no consensus on the type of cytoreductive treatment to use. The need for multidisciplinary input into transplant decisions and patient management before the procedure, was emphasized. Ongoing clinical trials of cytoreductive treatment before transplant conditioning, summarized in the ad hoc supplemental Information, will hopefully provide more definitive conclusions.
Choice of conditioning regimen and GVHD prophylaxis
Studies to date have shown that RIC and MAC result in similar OS.33,34,36,39,86 In an EBMT study comparing RIC (mainly fludarabine-busulfan regimens) with standard MAC (total body irradiation with cyclophosphamide or busulfan-cyclophosphamide),87 RIC was associated with a higher risk of relapse but a reduction in nonrelapse mortality compared with MAC, resulting in similar progression-free survival and OS.35 Other studies, including a large analysis by the Center for International Blood and Marrow Transplant Research, have supported these findings.88
Studies of treosulfan showed its noninferiority to busulfan, confirming the results of the original prospective trial, while highlighting that the main benefit of treosulfan-based HCT may be provided when used as RIC.36,86 However, there is no consensus on an established definition of treosulfan intensity compared with other regimens.89 Generally, a total dose of 14 g/m2 per day for 3 days is equivalent to fludarabine/busulfan (a MAC regimen) with similar nonrelapse mortality and relapse rate.90 A recent EBMT study in high-risk MDS with excess blasts found no significant differences in survival between sequential, MAC, or RIC platforms.91
The results of the expert panel survey on the choice of conditioning regimen and GVHD prophylaxis are summarized in the supplemental Results. The panel did not reach consensus on the use of sequential conditioning or the preferential use of antithymocyte globulin over PTCy as GVHD prophylaxis in patients with MDS undergoing allo-HCT.
Disease monitoring and therapy after transplantation
The results of the expert panel survey on this subject are summarized in the supplemental Results.
Chimerism is an established tool for monitoring graft function and disease recurrence after allo-HCT. Common methods available for monitoring mixed chimerism after transplant include the use of sex chromosome–specific probes for fluorescent in situ hybridization studies and polymerase chain reaction amplification of highly polymorphic short tandem repeat sequences.92-97 The sensitivity of these assays to detect admixtures ranges from 1% to 5% of cells.98 Depending on the indication, bulk, CD34+-selected, or fractionated cell populations can be used (eg, myeloid vs lymphoid cells). NGS assays are now being used to monitor chimerism after transplant and can detect admixtures as low as 0.3%.99-102
The aforementioned study of the relationship between measurable residual disease using patient-specific somatic mutations and relapse after allo-HCT suggests that measurable residual disease assessment is feasible.56 However, studies are now needed to standardize this procedure and define its clinical relevance with respect to relapse.
Relapse remains a significant problem after transplantation. Approaches to reduce relapse include preemptive treatment of patients identified as high risk before transplantation or who have persistent measurable residual disease–positive status after transplantation. Several treatment approaches are available, including palliative care, adoptive immunotherapy with donor lymphocyte infusion (DLI), and/or tapered immunosuppression, HMA, and cellular immunotherapy.6 HMA-based approaches have recently emerged to treat high-risk patients and those with measurable residual disease positivity.103-106
Frank MDS relapse that occurs after transplant can be treated with HMA, intensive chemotherapy, DLI, and/or tapering immunosuppressive therapy, cellular immunotherapy, and, potentially, second transplant. Prospective trials are needed to identify the most appropriate treatment approach for MDS relapse after transplant and the potential role of maintenance in this setting.
Conclusions
MDS is a complex and heterogeneous disease. Allo-HCT stands as the only potential cure for the disease and must be judiciously pondered in the disease treatment algorithm. The goal of the current consensus is to provide evidence-based expert recommendations (summarized in “BOX 1”) to improve patient selection and decision making in MDS, with respect to allo-HCT.
Acknowledgments
The authors thank the patients and their families. They are also grateful to all members of the European Society for Blood and Marrow Transplantation, particularly the Chronic Malignancies Working Party, Leiden office.
C.G. was supported by a grant from the Edward P. Evans Foundation.
Authorship
Contribution: C.G., D.P.M., and M.C. designed and conceptualized the overall project, and wrote the manuscript; M.R., N.G., J.D.-S., K.R., and N.K. helped in leading subgroup sections; and all authors participated in the discussion and writing of the manuscript sections, read and approved the final version, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: C.G. participated in advisory boards of Novartis and Sobi, and offered consultancy for Genesis Therapeutics. M.R. offered consultancy for and received honoraria from Bristol Myers Squibb, Novartis, AbbVie, Amgen, and Kura. A.A. received speakers bureau fees from Bristol Myers Squibb, Novartis, AbbVie, and Gilead. A.D. participated in advisory boards of, and/or had a consultancy with, and received honoraria from Bristol Myers Squibb, Agios, and Novartis and served on clinical trial committees or data and safety monitoring boards for Novartis, AbbVie, Kura, Geron, Servier, Keros, Shattuck Labs, and Bristol Myers Squibb. J.D.-S. received travel grants from Swixx, AbbVie, and AstraZeneca; received lecture honoraria from AbbVie, Roche, Janssen, AstraZeneca, Sobi, Takeda, Bristol Myers Squibb, Novartis, and Swixx; and participated in advisory board meetings for Bristol Myers Squibb, Janssen-Cilag, Sanofi, AstraZeneca, Roche, and BeiGene. C.H. and T.H. declare part ownership of Munich Leukemia Laboratory. A.K. received consultancy and honoraria from Celgene/Bristol Myers Squibb, Novartis, Agios, and Janssen. M.M. received consultancy fees from Rafa and Reddy and research funds from AbbVie and Bristol Myers Squibb. A.E.M. received consultancy/advisory board honoraria from Takeda, Bristol Myers Squibb, AstraZeneca, Immedica, and Medac; speaker fees from Novartis, Takeda, and Unimedic; and a research grant from Medac. V.S. participated in advisory boards of Ascentage, AbbVie, Curis, Bristol Myers Squibb, Geron, Novartis, Syros, and Servier. The remaining authors declare no competing financial interests.
Correspondence: Carmelo Gurnari, Department of Biomedicine and Prevention, Tor Vergata University, Viale Oxford 81, Rome, Italy; email: carmelogurnari31@gmail.com; and Mario Cazzola, Fondazione IRCCS Policlinico San Matteo, Department of Molecular Medicine, University of Pavia, Viale Golgi 19, 27100 Pavia, Italy; email: mario.cazzola@unipv.it.
References
Author notes
D.P.M. and M.C. contributed equally to this study.
The online version of this article contains a data supplement.


Comments
Authors response to “No proven benefit for cytoreduction prior to allo-HCT in MDS patients”
1) In the absence of prospective studies, the majority of experts decided to maintain the 2017 recommendation (2), namely that patients with MDS with blast excess (≥10%) may benefit from cytoreductive therapy before allogeneic transplantation, but results of ongoing clinical trials (a list is provided in the supplemental of our published article) are needed for more definitive conclusions;
2) Although interesting, the 2024 Scheid et al (3) paper is a retrospective registry-based study that does not allow any firm conclusions to be drawn on the efficacy of pretransplant chemotherapy or azacitidine on transplantation outcome.
Once more evidence on this matter will be gathered and clinical trials results will be published, EBMT recommendations might be updated. We once again emphasize that there is no evidence on mandatory cytoreductive treatment pre allo-HCT in high-risk MDS patients with ≥10% blasts.
References
1. Gurnari C, et al. Clinical-genomic profiling of MDS to inform allo-HCT: recommendations from an international panel on behalf of the EBMT Blood 2025
2. de Witte T, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017
3. Scheid C, et al. Does IPSS-R down staging before transplantation improve the prognosis of patients with Myelodysplastic neoplasms? Blood. 2024.
No proven benefit for cytoreduction prior to allo-HCT in MDS patients.
We are surprised by the strong recommendation in favor of cytoreductive treatment before allo-HCT in high-risk MDS patients with ≥10% blasts, despite limited evidence. One cited study is our recent retrospective analysis published in Blood, which showed that only the IPSS-R at diagnosis predicted survival and relapse post-allo-HCT in non-pretreated patients.1 Disease progression before transplant, mostly due to blast increase or cytopenia, did not affect outcomes. Patients with progression or non-response under cytoreduction had worse outcomes than non-pretreated patients, while only chemo-responders (not HMA) showed better survival. Recent data on cytoreduction efficacy in high-risk MDS are lacking; older studies using the same chemo as nowadays showed just 57 percent response.2 It is unpredictable who will respond. Multiple retrospective studies with several hundreds of patients support our finding that non-response to prior cytoreductive treatment before alloSCT results in inferior outcome.3-5 Why did the international panelists not consider these studies? While randomized trials are missing, current evidence does not support mandatory cytoreductive treatment before allo-HCT, which involves burdensome hospital stays and complications.
References
1. Scheid C, et al. Does IPSS-R down staging before transplantation improve the prognosis of patients with Myelodysplastic neoplasms? Blood. 2024.
2. de Witte T, et al. Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European Intergroup Trial. Haematologica. 2010;95(10):1754-1761.
3. Nakai K, et al. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia. 2005;19(3):396-401.
4. Alessandrino EP, et al. Should cytoreductive treatment be performed before transplantation in patients with high-risk myelodysplastic syndrome? J Clin Oncol. 2013;31(21):2761-2762.
5. Damaj G, et al. Upfront allogeneic stem cell transplantation after reduced-intensity/nonmyeloablative conditioning for patients with myelodysplastic syndrome: a study