In this issue of Blood, Alikarami et al1 studied the contribution of the hematopoietic stem cell (HSC) factor GATA2 to chemotherapy resistance in acute myeloid leukemia (AML) and reported an original molecular link between GATA2 and TP53 through the inhibition of the MDM2 modulator RASSF4.
AML remains an unmet medical need with up to 40% of pediatric patients experiencing relapse and a <10% overall survival rate for older patients. The relationship between leukemia aggressiveness and prognosis has been shown over the past 15 years to be the result of an interplay between the genetic alterations (frequently targeting the transcription regulators) and the epigenetic state (in part controlled by the normal hematopoietic cell of origin) of the leukemic cells. Indeed, high stemness gene expression signatures have been linked to poorer clinical outcomes. The balance between stemness and differentiation is partly controlled by the stoichiometry and activity of several components within higher-order transcription factories. As shown by Orkin and colleagues in 1994,2 GATA2 is a DNA-binding transcription factor involved in these transcription complexes and is essential for HSC. GATA2-inactivating mutations predispose to myeloid malignancies, and a tumor-suppressor function for GATA2 has been proposed in AML.3,4 However, the basis for the present study is that high GATA2 expression correlates with poor outcomes in AML.5 These findings suggest that a precise GATA2 dosage is crucial for hematopoietic homeostasis but a better knowledge of its molecular bases is required.
Here, single-cell analyses of pediatric AML patient samples indicated that GATA2 expression correlates with ERG expression and that GATA2 motif accessibility correlates with MECOM accessibility. Also, high GATA2 expression was associated with immatures cells but low GATA2 expression with more mature myeloid cells. Using an MLL-AF9 mouse model in which established leukemia showed various Gata2 levels, they observed that highGata2 leukemic cells are more resistant to in vitro treatment with doxorubicin than lowGata2 cells. Moreover, the most immature HSC and multipotent progenitor cells, expressing highGata2, were the most resistant to doxorubicin, thus supporting the link between stemness status, GATA2 expression, and treatment resistance. Importantly, Gata2 inactivation resensitized highGata2, but not lowGata2 leukemic cells, to doxorubicin and to a lesser extent to cytarabine, demonstrating a cell-context-dependent role for GATA2 in resistance to chemotherapy. Taken together, the authors propose that GATA2 expression level in the leukemia reflects its expression in the cell of origin, supporting the previous observation that MLL-AF9 induction in HSC drives an aggressive leukemia with a poor outcome signature.6 Through detailed molecular characterizations, they revealed a novel mediator of the proposed link between GATA2 and TP53 activities. The use of nutlin-3a to modulate TP53 activity showed that GATA2 negatively regulates TP53 target expression, but GATA2 did not regulate transcription of TP53 nor of its negative regulator MDM2. Rather, they identified Rassf4, a member of a family promoting MDM2 self-ubiquitination and stabilization, as directly bound and repressed by GATA2 in murine leukemia. Although GATA2 transcriptional activation is associated with general ETS and RUNX motifs, GATA2 repression was more correlated with SPI1 motifs, supporting a proposed GATA2/SPI1 antagonism. Importantly, there was a reverse correlation between RASSF4 and GATA2 expression levels in several human AML databases. Moreover, overexpression of RASSF4 in highGATA2 leukemic cells resensitized them to doxorubicin. Using the MLL-AF9 mouse model and highGATA2 leukemic cells, they show that the combination of doxorubicin and nutlin-3a resulted in increased mouse survival in vivo (see figure). Finally, this combination led to an increased cell death in highGATA2 primary pediatric leukemic cells compared with lowGATA2 samples, providing a proof of concept that this strategy may also resensitize resistant human leukemic cells presenting high GATA2/stemness signatures.
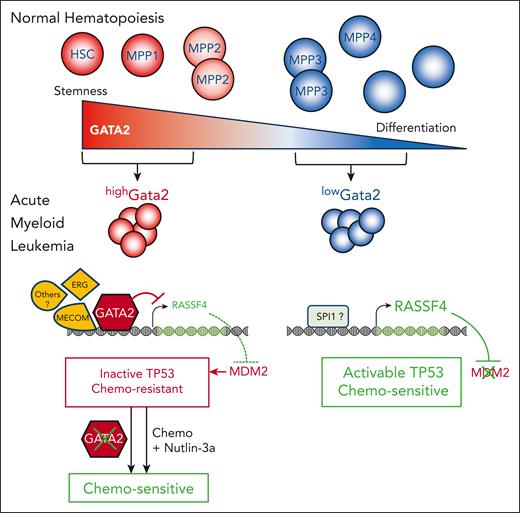
In normal hematopoiesis, GATA2 is highly expressed in the most immature HSCs and multipotent progenitors (MPP1 and MMP2) but less in other progenitors. Transformation by the MLLAF9 fusion oncogene generates highGata2-expressing cells (red) that are more resistant to chemotherapy than lowGata2-expressing cells (blue). Molecularly, highGata2 AML cells exhibited high ERG expression and high MECOM motif accessibility on chromatin, strongly repressed RASSF4 gene expression, TP53 inactivation through high MDM2 level, and chemoresistance. In contrast,lowGata2 leukemic cells showed higher RASSF4 expression, possibly through a positive regulation by SPI1. It is proposed that RASSF4 inhibits MDM2 favoring TP53 activation and a chemosensitive state. Inactivation of Gata2 in highGata2 as well as combined treatment with the TP53-MDM2 inhibitor nutlin-3a resensitized them to chemotherapy (Chemo).
In normal hematopoiesis, GATA2 is highly expressed in the most immature HSCs and multipotent progenitors (MPP1 and MMP2) but less in other progenitors. Transformation by the MLLAF9 fusion oncogene generates highGata2-expressing cells (red) that are more resistant to chemotherapy than lowGata2-expressing cells (blue). Molecularly, highGata2 AML cells exhibited high ERG expression and high MECOM motif accessibility on chromatin, strongly repressed RASSF4 gene expression, TP53 inactivation through high MDM2 level, and chemoresistance. In contrast,lowGata2 leukemic cells showed higher RASSF4 expression, possibly through a positive regulation by SPI1. It is proposed that RASSF4 inhibits MDM2 favoring TP53 activation and a chemosensitive state. Inactivation of Gata2 in highGata2 as well as combined treatment with the TP53-MDM2 inhibitor nutlin-3a resensitized them to chemotherapy (Chemo).
Intriguingly, this study suggests a wider role for GATA2 in therapy resistance. Here, Gata2 inactivation in highGata2 MLL-AF9 cells led to decreased Bcl2 expression and increased sensitivity to nutlin-3-dependent apoptosis. With another Hoxa9/Meis1 AML model mentioned in the study, these data support a role for GATA2 in resistance to BCL2 inhibition. Also, GATA2 mutations were identified in patient-derived clones of IDH2-mutated AML that became resistant to IDH2 inhibitor treatment.7 In other tumors, RASSF4 linked RAS to several prodeath pathways in multiple myeloma and increased response to MEK1/2 inhibitors,8 and GATA2 was involved in prostate cancer aggressiveness. Together, a GATA2/RASSF4-based mechanism may provide resistance to several therapies, including BCL2 and MEK1/2 inhibitors. Furthermore, the apoptosis-resistant property of highGata2 cells may explain the low percentage of TP53 mutations in de novo AML and raises the question of a potential selective pressure for TP53 mutation emergence upon highGATA2-targeting strategies.
Regarding the more global link between cell of origin, aggressiveness, and chemoresistance, this study raises interesting questions. Here, the authors have explored GATA2 partly based on the correlation that the most immature progenitors (HSC, MPP1, and MPP2) expressing high levels of Gata2 are also the most resistant to doxorubicin in vitro. Whether leukemias derived from highGata2 HSC and multipotent progenitors vs lowGata2 progenitors present different GATA2 levels and response to treatment in vivo remains to be investigated. If true, this would reinforce the idea that GATA2 expression in the cell of origin is a strong predictor of a resistance outcome. Also, would the inactivation of Gata2 in highGata2 HSC and progenitors resensitize them to in vivo treatment, and how could this be achieved in patients? Such studies may contribute to better characterize how leukemic cells adapt under treatment and whether a selective pressure toward another differentiation state may emerge from the combination between chemotherapy and nutlin-3a.
Finally, other stemness-controlling transcription regulators work in concert with GATA2 within large transcriptional complexes9 and are also altered in AML (eg, RUNX1, MYB, or CBFA2T3/ETO2).1,10 Therefore, it is tempting to speculate that the hijacking of these transcriptional complexes by various alterations may involve common mechanisms of aggressiveness and chemoresistance in leukemic cells from different molecular subgroups. In this regard, in AML with the RUNX1::RUNX1T1 fusion, GATA2 activity is associated with an immature state11 and RASSF2 is negatively controlled by the fusion. Therefore, it appears interesting to further investigate the relevance of relative GATA2/RASSF activities in the resistance to treatment for other AML molecular subgroups and a potential causal role for other transcriptional/epigenetic regulators in the control of the highGATA2 state.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal