Key Points
HbSC disease is the second most common form of SCD, which has largely been excluded from research and therapies.
We developed humanized HbSC mice that help identify the mechanism of hydroxyurea benefit in HbSC disease.
Visual Abstract
Sickle cell hemoglobin C (HbSC) disease results from compound heterozygosity of hemoglobin S (HbS) and hemoglobin C (HbC), comprising 30% of sickle cell disease (SCD). HbC induces red blood cell (RBC) dehydration/xerocytosis, which promotes sickling. HbSC-SCD causes significant morbidity despite being milder than homozygous HbSS-SCD. Current research/treatment strategies have focused on HbSS-SCD, whereas patients with HbSC are deprived of disease-modifying/transformative therapies because of lack of preclinical models. We generated HbSC mice, which resemble human HbSC-SCD: HbSC erythrocytes showed marked xerocytosis. Anemia, hemolysis, inflammation, and organ damage were milder than HbSS mice but hypoxia/reperfusion injury was similar. Retinopathy developed at higher frequency than HbSS mice (66.7% vs 16.7%; P < .05), as in patients with HbSC-SCD. Although HbSC RBCs sickled at lower oxygen tension than HbSS RBCs, they did not completely recover deformability after hypoxia/reoxygenation. Using the HbSC mice, we studied the mechanism by which hydroxyurea causes significant clinical benefit in patients with HbSC-SCD, despite minimal/modest increases in fetal Hb (HbF). We found hydroxyurea had distinct non-HbF and HbF effects. Hydroxyurea did not increase HbF in adult HbSC/HbSS mice but reduced RBC reactive oxygen species, ferryl Hb, and Heinz-body formation, thereby reducing membrane damage; however, RBC hydration was unaffected. When given to unborn pups before γ-globin expression was switched off, and continued postnatally, we could induce HbF in both HbSC and HbSS mice (higher HbF in HbSS vs HbSC mice). Minimal increases in HbF (∼1%) improved HbSC RBC hydration. Peak HbF levels of 7% in HbSC mice abrogated sickling. Overall, this HbSC model will help bridge the knowledge gap in mechanistic/therapeutic studies in this neglected disease.
Introduction
Sickle cell disease (SCD), a significant global health problem, which affects millions worldwide,1 results from a mutant HBB gene where valine is encoded instead of glutamic acid at the 6th codon (HBBE6V), and produces “sickle” hemoglobin (HbS) instead of normal adult Hb (HbA).2 HbS polymerizes upon deoxygenation, resulting in sickle-shaped red blood cells (RBCs). The homozygous form (HbSS) is the most severe and common form, comprising 60% of SCD. Sickle cell HbC (HbSC) disease, compound heterozygosity for HbS, and HbC (where lysine is encoded in place of glutamic acid at the 6th codon of the HBB gene, HBBE6K), is the second most common SCD genotype, comprising 30% of SCD (40%-50% of SCD in the Caribbean and Ghana).1,3-7 HbC crystallizes,8 and promotes KCl-cotransporter activity, causing RBC dehydration/xerocytosis.9,10 Xerocytosis increases the intracellular HbS concentration, promoting HbS polymerization and RBC sickling, despite HbS being in “trait-state” (50% of Hb).8 Hence, HbSC-SCD has the same symptoms as HbSS-SCD.
Recent studies have challenged the notion that HbSC-SCD is milder than HbSS-SCD,5,11 because it leads to significant morbidity and mortality.12 Besides, HbSC-SCD has some distinctive features, attributed to increased blood viscosity11: 33% to 43% of patients with HbSC develop proliferative retinopathy earlier (aged 15-24 years) compared with 3% to 14% of patients with HbSS (aged 25 to 39 years).8,13-15 Patients with HbSC-SCD also have a similar degree of avascular necrosis as HbSS-SCD patients and a higher frequency of splenic sequestration than those with HbSS-SCD.11,16 Understanding the unique properties of HbC and mechanism(s) of xerocytosis is critical to developing effective therapies for HbSC-SCD.17
Although extensive research/treatment strategies have focused on HbSS-SCD, patients with HbSC-SCD have mostly been excluded,16,18 and therefore deprived of specific therapies. For instance, they are excluded from transformative genetic therapies, because of the lack of preclinical models to assess their efficacy in the presence of HbC. A prospective randomized trial of hydroxyurea (HU) for HbSC-SCD was reported in 2024,19 37 years after being approved for HbSS-SCD.19-21 Perplexingly, both in this trial,19 and several previous retrospective/nonrandomized studies,22-27 HU resulted in negligible/small increases in fetal Hb (HbF) in patients with HbSC-SCD, but showed highly significant clinical benefit in SCD symptoms19,22-27 through an unknown mechanism. In contrast, in HbSS-SCD, HU not only upregulates HbF at significantly higher levels, but its levels determine the degree of correction of SCD symptoms.28 Generation of humanized SCD mice (Townes29 and Berkeley30 mice), which exclusively express human Hb, phenocopying human HbSS-SCD, revolutionized research and translation in HbSS-SCD. No animal model exists for HbSC-SCD, creating a large knowledge gap in identifying mechanisms or developing targeted/effective therapies for HbSC-SCD.
Herein, we generated HbSC mice on the same genetic background as HbSS mice, except changing the HbS to HbC mutation on 1 allele. This novel HbSC model phenocopies human HbSC-SCD, allowing direct comparisons of therapies currently effective in HbSS-SCD to HbSC-SCD. We found unique insights into the HbF-dependent and HbF-independent mechanisms by which HU improves HbSC-SCD.
Material and methods
Mice
The Townes HbSS (hα/hα::hβS/hβS) mice were obtained from Tim Townes29,31 and HbAA (hα/hα::hβA/hβA) mice, derived by Tim Townes, were kindly provided by Cheryl Hillary (Madison, WI). HbAS females were mated with HbSS/HbAA males to derive HbSS/HbAA mice. Mice were housed in the Cincinnati Children’s Hospital Medical Center vivarium and studied using institutional animal care and use committee–approved protocols.32
HU treatment
Adult HbSS/HbSC mice were given HU 50 mg/kg body weight (BW) intraperitoneally, 3 days per week for 4 weeks. Pregnant HbSC females (mated with HbSS males) were treated with HU (25 mg/kg BW per day) on days 15 through 21 of gestation by gavage. Pups (HbSC and HbSS) born from HU-treated females were given HU 50 mg/kg BW intraperitoneally, 3 days per week. Controls (pregnant dams, adult mice, and pups) received phosphate-buffered saline (PBS).
Human samples
Blood samples from patients with HbSS/HbSC and controls were obtained through an institutional review board-approved protocol.
Hb subtypes
RBC lysates were fractionated through ion-exchange high-performance liquid chromatography (HPLC; Alliance-2690), using a PolyCAT-A column (3.54CT0510; Poly-LC Inc).33
Hematological analysis
Complete blood counts were analyzed using Element HT5 (Heska) or ADVIA-2120 hematology analyzers. Reticulocytes were assessed either by staining with 0.01 mg/mL thiazole orange (BD Biosciences) on the fluorescence-activated cell sorter (FACS) BD FACSCanto flow cytometer (BD Biosciences), or via the Advia-2120 (Siemens Healthineers).
RBC half-life
RBC deformability
RBC deformability was measured on the Lorrca Maxsis, oxygen-gradient or osmoscan ektacytometer (RR Mechatronics, Zwaag, The Netherlands).33,34
Whole blood viscosity was measured at different shear rates on the DV3T rheometer (Ametek Brookfield Engineering).
Metabolic and inflammatory parameters
Plasma vascular cell adhesion molecule 1 (VCAM-1) was measured using an enzyme-linked immunosorbent assay (Abcam), and tumor necrosis factor α using the mouse Luminex assay (R&D Systems).
Retinal vasculature
Anesthetized mice received an intracardiac fluorescein isothiocyanate–dextran (10 kDa; Sigma) and were euthanized after 2 minutes; the eyes were removed, fixed in 4% paraformaldehyde (11 minutes, 4°C), and washed with PBS. Retinas were dissected, fixed in 4% paraformaldehyde (1.5 hours, room temperature), washed, and cut into leaflets for flat-mounting. Fluorescent images were captured using a Nikon C2 confocal microscope.
Scanning electron microscopy (SEM)
RBCs were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, followed by 1% osmium tetroxide,35 then dehydrated through a graded ethanol series, mounted on aluminum specimen stubs, and sputter-coated with 10 nm of carbon (Safematic CCU-010; Rave Scientific). The specimens were imaged using a Zeiss FE-SEM (Supra 35 VP; Zeiss).
Histopathology
Hypoxia/reoxygenation assay
Mice were challenged with 8% hypoxia for 10 hours followed by 2 hours of reoxygenation in ambient air to mimic acute vaso-occlusive crisis, as previously described.38
RBC reactive oxygen species detection by flow cytometry
Whole blood samples were stained with anti-Ter119, anti-CD41, and anti-CD45 antibodies (BD Biosciences) followed by washes and resuspension in 0.05 mM CM-H2-DCFDA (5′,6′-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; Invitrogen) at 37°C, 30 minutes; resuspended in 100 to 150 μL FACS buffer; and acquired on the flow cytometer.
Immunofluorescent staining
Whole blood (3 μL) was washed with Hanks balanced salt solution +0.1% bovine serum albumin, resuspended to 5% hematocrit, and deposited onto polylysine-coated coverslips. After 20 minutes, adherent cells were fixed (1% paraformaldehyde, 0.0075% glutaraldehyde), washed, permeabilized, and blocked (permeabilization/wash buffer, 1% bovine serum albumin). Cells were stained with anti–ferryl Hb antibody (1:100) for 1 hour,39 followed by secondary antibodies (anti-mouse immunoglobulin G–AlexaFluor488, 1:300; Ter119-BV421, 2:100) for 30 minutes. Coverslips were mounted with Fluoromount-G, dried, and imaged on an EVOS 7000 microscope (60×). Images were analyzed with EVOS software.
Heinz-body detection
Fresh EDTA-anticoagulated blood was mixed with 0.5% methyl violet (2:3 ratio), incubated for 60 minutes at room temperature, and smeared on glass slides for examination under a 100× oil-immersion objective (Nikon microscope). Positive controls used human blood treated with 1 mg/mL acetyl-2-phenylhydrazine (1:20 blood-to-reagent ratio) at 37°C for 2 hours.
Protein digestion, mass spectrometry, and quantification of C93-containing peptides from RBC ghosts
RBC ghosts were processed for mass spectrometry for β-globin93Cys–containing peptides, quantified using Proteome Discoverer 3.040 (details provided in the supplemental Methods; supplemental Figure 6, available on the Blood website).
Statistical analysis
Groups were compared using analysis of variance, and pairs using Mann-Whitney U test using the GraphPad Prism 10.0 (San Diego, CA). P < .05 was considered significant.
Results
Generation of humanized HbSC mice
Fertilized eggs from the Townes HbSS×HbAS mouse timed-crosses were retrieved, electroporated with single guide RNA–CRISPR–associated protein 9 ribonucleoprotein and single-stranded HBB donor DNA carrying the HBBE6K mutation and implanted into pseudopregnant females. Pups were screened by polymerase chain reaction amplification of the targeted locus and confirmed by Sanger sequencing. Founder HbSC mice exhibited both the HBBE6K (aAG) and the HBBE6V (GtG) alleles (supplemental Figure 1). HPLC on blood from founder mice confirmed HbC and HbS protein expression (Figure 1A-C). Germ line transmission was ascertained before the expansion of the HbSC colony. HbSC (expressing HBBE6V/E6K) mice were compared with HbSS (expressing HBBE6V/E6V) and HbAA (expressing HBBE6/E6) controls.
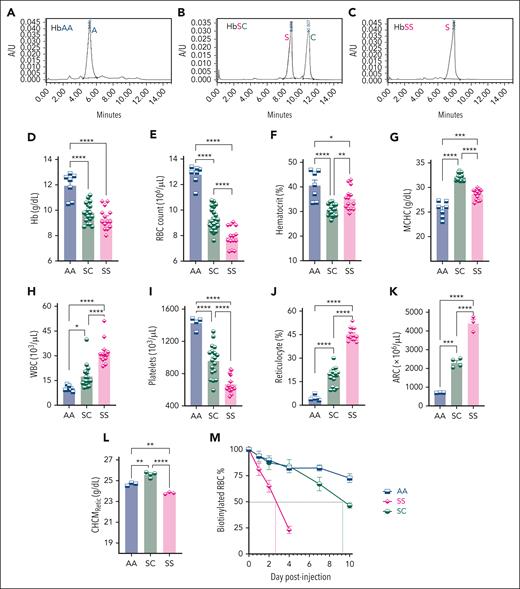
Derivation of HbSC mice and their hematological characteristics. (A-C) HPLC analysis on the peripheral blood of HbSC founder mouse along with HbAA (AA) and HbSS (SS) controls, showing expression of HbA (A), both HbS and HbC in the HbSC (SC) (B), and HbS (C). (D-I) Complete blood counts of blood from AA, SC, and SS mice, performed on the HT-5 hematology analyzer. Hb concentration (D), RBC counts (E), hematocrit (%) (F), MCHC (G), WBC counts (H), and platelets (I) are shown. (J) The percentage of reticulocytes was determined by flow cytometry on peripheral blood stained with thiazole orange. ARC (K) and CHCMRetic (L) were analyzed using the reticulocyte channel using the ADVIA-2010 hematology analyzer. For panels D through L: each symbol represents an individual mouse, and bars represent mean ± standard error of the mean. Statistics were performed using analysis of variance (ANOVA). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .00001. (M) RBC half-life was determined by serial blood analysis by flow cytometry for the percentage of biotinylated RBCs remaining after an initial intravenous injection of N-hydroxysulfosuccinimidobiotin. Symbols represent mean ± standard error of the mean of 3 to 5 mice per group. ARC, absolute reticulocyte count; WBC, white blood cell.
Derivation of HbSC mice and their hematological characteristics. (A-C) HPLC analysis on the peripheral blood of HbSC founder mouse along with HbAA (AA) and HbSS (SS) controls, showing expression of HbA (A), both HbS and HbC in the HbSC (SC) (B), and HbS (C). (D-I) Complete blood counts of blood from AA, SC, and SS mice, performed on the HT-5 hematology analyzer. Hb concentration (D), RBC counts (E), hematocrit (%) (F), MCHC (G), WBC counts (H), and platelets (I) are shown. (J) The percentage of reticulocytes was determined by flow cytometry on peripheral blood stained with thiazole orange. ARC (K) and CHCMRetic (L) were analyzed using the reticulocyte channel using the ADVIA-2010 hematology analyzer. For panels D through L: each symbol represents an individual mouse, and bars represent mean ± standard error of the mean. Statistics were performed using analysis of variance (ANOVA). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .00001. (M) RBC half-life was determined by serial blood analysis by flow cytometry for the percentage of biotinylated RBCs remaining after an initial intravenous injection of N-hydroxysulfosuccinimidobiotin. Symbols represent mean ± standard error of the mean of 3 to 5 mice per group. ARC, absolute reticulocyte count; WBC, white blood cell.
Hematological parameters of HbSC mice resemble those in patients with HbSC-SCD
HbSC mice were anemic compared with HbAA mice. Although their Hb and RBC counts were higher than HbSS mice, their hematocrits were lower (Figure 1D-F), suggesting that HbSC RBCs were more dehydrated than HbSS. Indeed, their mean corpuscular Hb concentration (MCHC) was higher than HbSS/HbAA RBCs (Figure 1G). HbSC mice showed less pronounced reticulocytosis than HbSS (Figure 1J), suggesting lesser hemolysis. Because mean corpuscular volume (MCV) is higher in reticulocytes than RBCs, MCV was highest in HbSS mice (supplemental Figure 2A-B). Despite much higher reticulocytosis, HbSC erythrocytes had a similar MCV as HbAA RBCs because of xerocytosis. HbSC mice also had leukocytosis, albeit milder than HbSS mice, consistent with chronic inflammation in SCD (Figure 1H).
Similar RBC and white blood cell features were present in patients with HbSC-SCD when compared with normal (HbAA) controls and patients with HbSS-SCD, except for the MCV, which was higher in HbSC and HbSS mice from the high reticulocytosis compared with patients with SCD (supplemental Figure 3A-J); and platelets, which were normal in human patients with HbSS/SC but lower in HbSS and HbSC mice (Figure 1I).
HbSC reticulocyte parameters, analyzed using the ADVIA-2120 analyzer showed similar reticulocyte counts to those obtained by flowcytometry (Figure 1J-K). The corpuscular Hb concentration mean of reticulocytes (CHCMRetic) was highest in HbSC reticulocytes, significantly exceeding HbAA and HbSS, indicating xerocytosis is also present in HbSC reticulocytes (Figure 1L). Furthermore, dense RBC and reticulocytes were higher in HbSC vs HbSS mice (supplemental Figure 2C-D).
HbSC RBCs had a longer half-life (9.2 days) than HbSS RBCs (2.6 days; Figure 1M). Overall, the RBC parameters of the HbSC mice were similar to those in patients with HbSC (supplemental Figure 3A-I). However, it is important to note that many patients with HbSS-SCD were taking HU and, therefore, may have a less severe hematological profile.
HbSC RBCs have remarkable xerocytosis but milder sickling kinetics than HbSS RBCs
SEM showed severely dehydrated HbSC erythrocytes folded into “pita shapes,” “pretzel shapes,” or with a double dimple from membrane folding around scant cytoplasm (Figure 2B,D-F). HbSS controls showed irreversibly sickled RBCs, larger-sized reticulocytes, and some folded dehydrated RBCs; HbAA controls had classic doughnut-shaped RBC (Figure 2A,C).
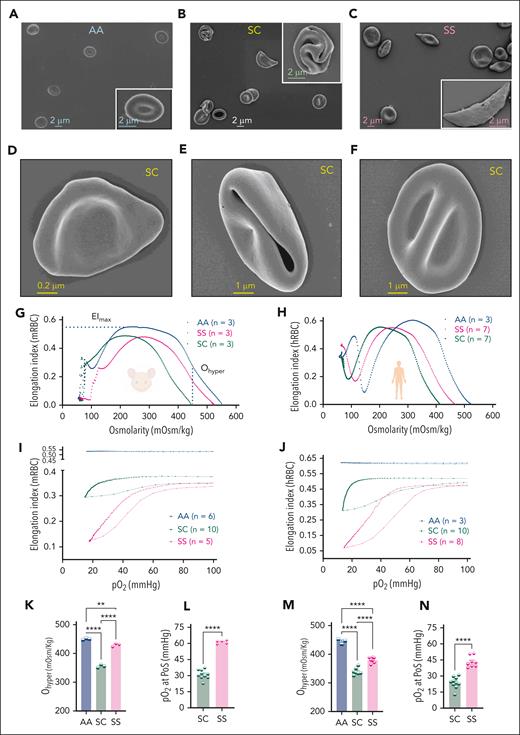
Morphology, hydration, and sickling kinetics of HbSC RBCs. (A-C) SEM images of AA, SC, and SS erythrocytes. Insets show an enlarged version of a classical and representative RBCs in each group. (D-F) Representative SC erythrocytes. Specimens were imaged using a Zeiss FE-SEM (Supra 35 VP) at 2 to 5 keV with a working distance of 3 to 5 mm using the Inlens detector. The magnification scale is shown within the micrographs and insets. (G) Osmoscan curves for mRBCs, showing the RBC deformability under varying osmolarities. The EI is plotted against osmolarity for HbAA, HbSS, and HbSC RBCs, 3 samples per group. The curves illustrate the deformability of RBCs, with maximal EI occurring at iso-osmolar conditions and shifting left for dehydrated cells (xerocytosis). (H) Osmoscan curves for hRBCs under varying osmolar conditions. (I) Oxyscan curves of mRBCs in HbAA (n = 6), HbSC (n = 10), and HbSS (n = 5). (J) Oxyscan curves of hRBCs in HbAA (n = 3), HbSC (n = 10), and HbSS (n = 8). (K) Ohyper in mRBCs indicates the osmolarity in the hyperosmolar region at which the RBCs achieve half of their maximal elongation. (L) The PoS, defined as the pO2 at which a >5% decrease in EI is observed during deoxygenation, indicating the onset of sickling in mRBCs. (M) Ohyper in hRBCs. (N) PoS in hRBCs. For panels K through N: each symbol represents an individual sample, and bars represent mean ± standard error of the mean. Statistics were performed using ANOVA. ∗∗P < .01; ∗∗∗∗P < .00001. mOsm, milliosmoles.
Morphology, hydration, and sickling kinetics of HbSC RBCs. (A-C) SEM images of AA, SC, and SS erythrocytes. Insets show an enlarged version of a classical and representative RBCs in each group. (D-F) Representative SC erythrocytes. Specimens were imaged using a Zeiss FE-SEM (Supra 35 VP) at 2 to 5 keV with a working distance of 3 to 5 mm using the Inlens detector. The magnification scale is shown within the micrographs and insets. (G) Osmoscan curves for mRBCs, showing the RBC deformability under varying osmolarities. The EI is plotted against osmolarity for HbAA, HbSS, and HbSC RBCs, 3 samples per group. The curves illustrate the deformability of RBCs, with maximal EI occurring at iso-osmolar conditions and shifting left for dehydrated cells (xerocytosis). (H) Osmoscan curves for hRBCs under varying osmolar conditions. (I) Oxyscan curves of mRBCs in HbAA (n = 6), HbSC (n = 10), and HbSS (n = 5). (J) Oxyscan curves of hRBCs in HbAA (n = 3), HbSC (n = 10), and HbSS (n = 8). (K) Ohyper in mRBCs indicates the osmolarity in the hyperosmolar region at which the RBCs achieve half of their maximal elongation. (L) The PoS, defined as the pO2 at which a >5% decrease in EI is observed during deoxygenation, indicating the onset of sickling in mRBCs. (M) Ohyper in hRBCs. (N) PoS in hRBCs. For panels K through N: each symbol represents an individual sample, and bars represent mean ± standard error of the mean. Statistics were performed using ANOVA. ∗∗P < .01; ∗∗∗∗P < .00001. mOsm, milliosmoles.
Functional analyses of hydration and sickling were performed on HbSC, HbSS, and HbAA mouse RBCs (mRBCs) and corresponding human RBCs (hRBCs) using osmoscan- and oxygen-gradient ektacytometry. In the osmoscan, RBC deformability (measured as their elongation index [EI]) was determined under fixed shear stress but varying osmolarities. Normal RBCs deform maximally (EImax) in iso-osmolar conditions and have reduced deformability under hyperosmolar (dehydrated) or hypo-osmolar (swollen) conditions. Xerocytosis causes a left-shifted EI curve, with EImax occurring at lower osmolarity. Xerocytosis is quantified by Ohyper, the hyperosmolarity at which EI is half of EImax.
HbSC and HbSS mRBCs and hRBCs had left-shifted osmoscan curves than their normal HbAA counterparts, with the highest shift seen with HbSC RBCs. The corresponding hRBCs had an identical pattern (Figure 2G-H). Quantitatively, the Ohyper of mRBCs and hRBCs was lowest in HbSC, intermediate in HbSS and highest in HbAA RBCs (Figure 2K-M). Hence, the presence of HbC resulted in more severe xerocytosis in HbSC than HbSS RBCs, a hallmark of HbSC-SCD pathobiology.
Sickling kinetics of murine and human HbSC RBCs, along with HbAA and HbSS controls, were determined using oxygen-gradient ektacytometry.41 Here, EI is measured in RBCs subjected to constant shear stress with increasing hypoxia followed by reoxygenation.42 HbSS RBCs retain maximum deformability (EImax) under normoxia until the partial pressure of oxygen (pO2) drops between 40 and 60 mm Hg, when HbS polymerization begins. The pO2 level at which EI drops to 95% of EImax is termed point-of-sickling (PoS). Hypoxia beyond PoS results in rapid HbS polymerization, causing an exponential drop in EI to a minimum (EImin). Reoxygenation results in HbS depolymerization, and HbSS RBCs gradually regain deformability.
PoS was lower, and EImax and EImin higher, in HbSC RBCs compared with HbSS RBCs in both mice and humans (Figure 2I-J,L,N), indicating milder sickling kinetics in HbSC RBCs because of the presence of 50% nonsickling HbC. As expected, HbAA RBCs had the same EI under all oxygen concentrations. Notably, HbSC RBCs (both murine and human) did not regain their baseline deformability upon reoxygenation, indicating some degree of irreversible damage after hypoxia-reoxygenation, conceivably because of a detrimental effect of HbC.
Hypoxia/reoxygenation injury results in RBC xerocytosis
We simulated sickle acute vaso-occlusive crisis (VOC) in HbSC mice with the hypoxia-reoxygenation (H/R) assay,43,44 which has been shown to decrease Hb and platelets, and increase soluble VCAM, a marker of endothelial injury and inflammation (tumor necrosis factor α) in HbSS.45-47 HbSC mice showed a similar drop in RBCs and platelets and a similar increase in soluble VCAM-1 to that in HbSS mice, although their baseline levels were different from HbSS mice (Figure 3A-F). Hence, experimentally induced VOC results in comparable alterations in hematological and endothelial parameters in HbSS and HbSC mice.
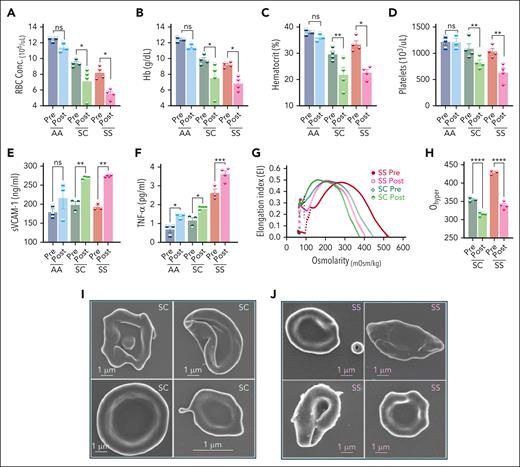
Effects of H/R on hematological features, endothelial activation, inflammation, and HbSC RBC hydration. Mice were exposed to 8% oxygen for 10 hours in a hypoxia chamber, followed by 2 hours of reoxygenation in ambient air. (A) RBC counts (A), Hb concentration (g/dL; B), hematocrit (%; C), platelets (D), sVCAM-1 concentration (ng/mL; E), and Tumor necrosis factor α (TNF-α; picograms per milliliter; F) levels in the plasma, before hypoxia and after H/R. (G) Average osmoscan ektacytometry curves before and after H/R are shown. (H) Ohyper, the osmolarity in the hyperosmolar region at which the RBCs achieve half of their maximal elongation for the data in panel G, is shown. Each symbol in the bar diagrams represents an individual mouse, and the bars represent mean ± standard error of the mean. Statistics were performed using ANOVA. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .00001. Representative SEM images of RBCs after hypoxia in HbSC (I) and HbSS (J). The specimens were imaged using a Zeiss FE-SEM (Supra 35 VP) at 3 keV with a working distance of 2.9 mm using the Inlens detector. The magnification scale is shown within the micrographs and insets. Conc., concentration; mOsm, milliosmoles; ns, not significant; Post, after H/R; Pre, before hypoxia; sVCAM-1, soluble VCAM-1; TNF-α, tumor necrosis factor α.
Effects of H/R on hematological features, endothelial activation, inflammation, and HbSC RBC hydration. Mice were exposed to 8% oxygen for 10 hours in a hypoxia chamber, followed by 2 hours of reoxygenation in ambient air. (A) RBC counts (A), Hb concentration (g/dL; B), hematocrit (%; C), platelets (D), sVCAM-1 concentration (ng/mL; E), and Tumor necrosis factor α (TNF-α; picograms per milliliter; F) levels in the plasma, before hypoxia and after H/R. (G) Average osmoscan ektacytometry curves before and after H/R are shown. (H) Ohyper, the osmolarity in the hyperosmolar region at which the RBCs achieve half of their maximal elongation for the data in panel G, is shown. Each symbol in the bar diagrams represents an individual mouse, and the bars represent mean ± standard error of the mean. Statistics were performed using ANOVA. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .00001. Representative SEM images of RBCs after hypoxia in HbSC (I) and HbSS (J). The specimens were imaged using a Zeiss FE-SEM (Supra 35 VP) at 3 keV with a working distance of 2.9 mm using the Inlens detector. The magnification scale is shown within the micrographs and insets. Conc., concentration; mOsm, milliosmoles; ns, not significant; Post, after H/R; Pre, before hypoxia; sVCAM-1, soluble VCAM-1; TNF-α, tumor necrosis factor α.
H/R caused further xerocytosis of HbSC RBCs, which were the most dehydrated RBCs before H/R (Figure 3G-H). As expected, HbSS RBCs were also significantly dehydrated, after sickling-induced shape change, which promotes cation and water loss.48 SEM after H/R confirmed the severe dehydration in HbSC RBCs, evidenced by pronounced membrane folding due to reduced cytoplasmic volume (Figure 3I-J).
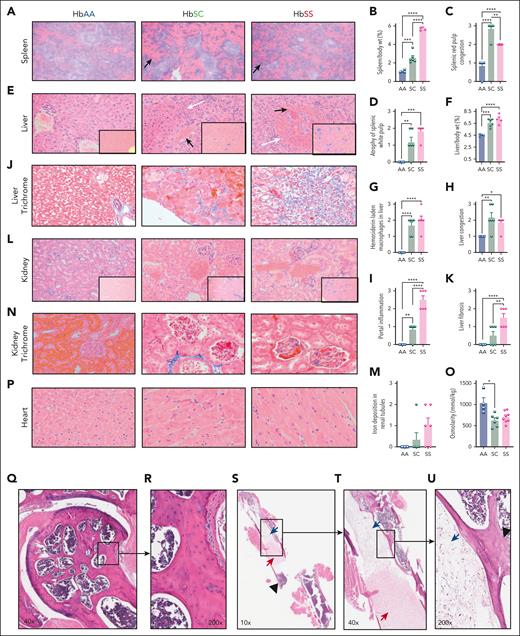
Organ pathology of HbSC mice resembles that of HbSS mice
Despite milder hemolysis and sickling than HbSS mice, significant organ pathology was observed in 7-month-old HbSC mice, which was either comparable with, or slightly milder than, age-matched HbSS mice (Figure 4; supplemental Tables 1-5). The spleen is an organ of extramedullary stress erythropoiesis in mice, unlike humans. Hence, splenomegaly in HbSC mice was less prominent than in HbSS mice, from lower anemia and hemolysis (Figure 4B). Histologically, the spleens of HbSC mice had remarkably more sinusoidal congestion but had less white-pulp atrophy than in HbSS mice (Figure 4A,C-D; supplemental Table 1). Hepatomegaly was comparable in HbSC and HbSS mice, with foci of infarction and congested veins filled with sickled RBCs, extramedullary hematopoiesis, and iron deposition. (Figure 4E-G). Portal inflammation and fibrosis were higher in HbSS, whereas vascular congestion was higher in HbSC livers (Figure 4H-K; supplemental Table 2). Kidneys showed mild glomerular enlargement with congested capillary tufts in HbSC and HbSS mice. There was vascular congestion and hemosiderin deposition in HbSC kidneys, but we did not observe significant fibrosis (Figure 4L-N; supplemental Table 3). Urine osmolarity was reduced in both HbSC and HbSS mice (Figure 4O). Cardiac sections showed hypertrophied cardiomyocytes in HbSC and HbSS mice, although we did not observe evidence of fibrosis (Figure 4P; supplemental Table 4).
Histopathological evaluation of multiple organs of 7-month-old HbSC mice. (A) Representative histological spleen sections stained with hematoxylin and eosin (H&E) of HbAA (right), HbSC (middle), and HbSS (left). Black arrows point to expanded red pulps with congested sinusoids, original magnification ×100. (B) Spleen-to-body-weight ratio. Scoring of splenic red pulp congestion (C) and atrophy of white pulps based on pathology scoring of the spleen (D; supplemental Table 1). (E) Representative H&E pictures of liver sections illustrating congestion (black arrows), and areas of infarcts (blue arrows), original magnification ×200. Insets showing Prussian blue staining for each group. (F) Liver-to-body-weight ratio. Scoring of hemosiderin (G), the liver congestion (H), and portal inflammation (I) based on pathology scoring of the liver (supplemental Table 2). Representative Masson trichrome staining of liver sections (original magnification ×400; J) and liver fibrosis scoring (K), see more details in (supplemental Table 2). (L) Representative H&E pictures of kidney sections with insets showing Prussian blue staining for each group (original magnification ×200). (M) Iron deposition in the renal tubes based on pathology scoring of the kidney (supplemental Table 3). (N) Representative Masson trichrome staining of kidney sections (original magnification ×400). (O) Urine osmolarity, 6 hours; mice were 6 months old in this test. (P) Representative H&E pictures of heart sections (original magnification ×400), with insets showing original magnification ×20. Statistics were performed using ANOVA. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .00001. (Q) Representative H&E-stained bone and femoral sections of 1-year-old HbAA (n = 3), HbSC (n = 3), and HbSS (n = 5) with normal bone architecture and hematopoietic marrow (R) with intact osteocytes occupying the lacunae. (S-U) One abnormal mouse from HbSS group. The SCD mouse exhibits features of bone pathology, including coagulative necrosis of the bone marrow and local bone infarction, characterized by empty osteocyte lacunae (highlighted inset). wt, weight.
Histopathological evaluation of multiple organs of 7-month-old HbSC mice. (A) Representative histological spleen sections stained with hematoxylin and eosin (H&E) of HbAA (right), HbSC (middle), and HbSS (left). Black arrows point to expanded red pulps with congested sinusoids, original magnification ×100. (B) Spleen-to-body-weight ratio. Scoring of splenic red pulp congestion (C) and atrophy of white pulps based on pathology scoring of the spleen (D; supplemental Table 1). (E) Representative H&E pictures of liver sections illustrating congestion (black arrows), and areas of infarcts (blue arrows), original magnification ×200. Insets showing Prussian blue staining for each group. (F) Liver-to-body-weight ratio. Scoring of hemosiderin (G), the liver congestion (H), and portal inflammation (I) based on pathology scoring of the liver (supplemental Table 2). Representative Masson trichrome staining of liver sections (original magnification ×400; J) and liver fibrosis scoring (K), see more details in (supplemental Table 2). (L) Representative H&E pictures of kidney sections with insets showing Prussian blue staining for each group (original magnification ×200). (M) Iron deposition in the renal tubes based on pathology scoring of the kidney (supplemental Table 3). (N) Representative Masson trichrome staining of kidney sections (original magnification ×400). (O) Urine osmolarity, 6 hours; mice were 6 months old in this test. (P) Representative H&E pictures of heart sections (original magnification ×400), with insets showing original magnification ×20. Statistics were performed using ANOVA. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .00001. (Q) Representative H&E-stained bone and femoral sections of 1-year-old HbAA (n = 3), HbSC (n = 3), and HbSS (n = 5) with normal bone architecture and hematopoietic marrow (R) with intact osteocytes occupying the lacunae. (S-U) One abnormal mouse from HbSS group. The SCD mouse exhibits features of bone pathology, including coagulative necrosis of the bone marrow and local bone infarction, characterized by empty osteocyte lacunae (highlighted inset). wt, weight.
Bone histology of the legs in 12-month-old HbSC and HbSS mice showed no evidence of avascular necrosis, although the number of animals in the HbSC group was limited (n = 3; Figure 4Q-R). Of 6 HbSS mice, 1, however, had evidence of infarction of a portion of femoral diaphysis along with coagulative necrosis of the underlying bone marrow (Figure 4S-U; supplemental Table 5).
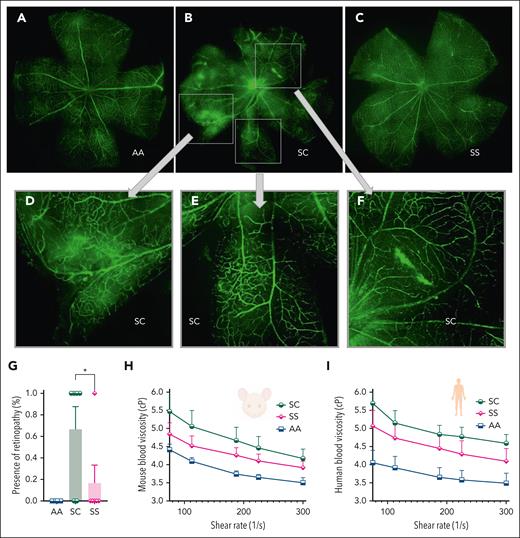
HbSC mice develop proliferative retinopathy more frequently than HbSS mice
Retinal vasculature in 7-month-old HbAA mice was normal. However, 4 of 6 (67%) age-matched HbSC mice showed abnormal vascular growth, vascular leakage, and “comma”-shaped vessels at the distal end of the retinal vessels (Figure 5A-G). Of 6 7-month-old HbSS mice, 1 (17%) showed retinopathy (P < .05).
Sickle retinopathy in HbSC mice. Retinal vasculature was visualized after IV fluorescein isothiocyanate-dextran injections in 7-month-old AA, SC, and SS mice, and images were captured after dissection of the retina by a Nikon C2 confocal laser scanning microscope. Representative images of the retina in AA (A), SC (B), and SS (C) are shown. (D-F) Magnifications of the boxed areas from panel B (HbSC retina) show the neovascularization and abnormal blood vessel growth, “comma”-shaped vessels, and vascular leakage. (G) Percentage of mice with retinopathy (n = 6 in the SC and SS groups, n = 3 in the AA group of mice). No AA mice had retinopathy. Statistics were performed using ANOVA. ∗P < .05. Whole-blood viscosity in mice (H) and human samples (I) is shown at 5 different shear rates. Each line represents the mean + standard error of the mean of 3 to 6 mice or human blood samples per group. cP, centipoise.
Sickle retinopathy in HbSC mice. Retinal vasculature was visualized after IV fluorescein isothiocyanate-dextran injections in 7-month-old AA, SC, and SS mice, and images were captured after dissection of the retina by a Nikon C2 confocal laser scanning microscope. Representative images of the retina in AA (A), SC (B), and SS (C) are shown. (D-F) Magnifications of the boxed areas from panel B (HbSC retina) show the neovascularization and abnormal blood vessel growth, “comma”-shaped vessels, and vascular leakage. (G) Percentage of mice with retinopathy (n = 6 in the SC and SS groups, n = 3 in the AA group of mice). No AA mice had retinopathy. Statistics were performed using ANOVA. ∗P < .05. Whole-blood viscosity in mice (H) and human samples (I) is shown at 5 different shear rates. Each line represents the mean + standard error of the mean of 3 to 6 mice or human blood samples per group. cP, centipoise.
The higher frequency of retinopathy in HbSC-SCD than HbSS-SCD has been attributed to higher blood viscosity conferred by the dense dehydrated HbSC RBC. Indeed, whole-blood viscosity, measured at different shear rates in HbSC, HbSS, and HbAA, showed that both HbSC mice and humans had the highest blood viscosity, followed by HbSS, and then HbAA controls (Figure 5H-I).
In summary, characterization of the hematological parameters, functional analysis of sickling, RBC hydration and VOC, and organ pathology showed that the HbSC mouse model has similar features of HbSC-SCD as human patients except for minor differences in platelets and RBC-turnover rates. Using this model, we studied the mechanism of action by which HU improves clinical symptoms in HbSC-SCD with small increases in HbF and contrasted these with HbSS-SCD.
HbF-independent effects of HU in HbSC and HbSS mice
The humanized Townes HbAA/HbSS mice carry both the HBG and the HBB/HBBE6V genes and regulatory elements. They switch from HBG gene transcription to HBB or HBBE6V gene transcription in midgestation, when hematopoiesis moves from the yolk sac to the fetal liver.29 HbF levels in RBCs start dropping thereafter, with 20% HbF containing RBCs, determined using flow-cytometry (F cells) seen at postnatal day 2 (P4), declining to undetectable levels after P14.49 Efforts at reactivating HbF in adult HbSS mice with HU have failed.28,49 The lack of HbF-induction with HU in adult HbSS (and likely HbSC) mice makes them ideal for studying the non-HbF effects of HU.
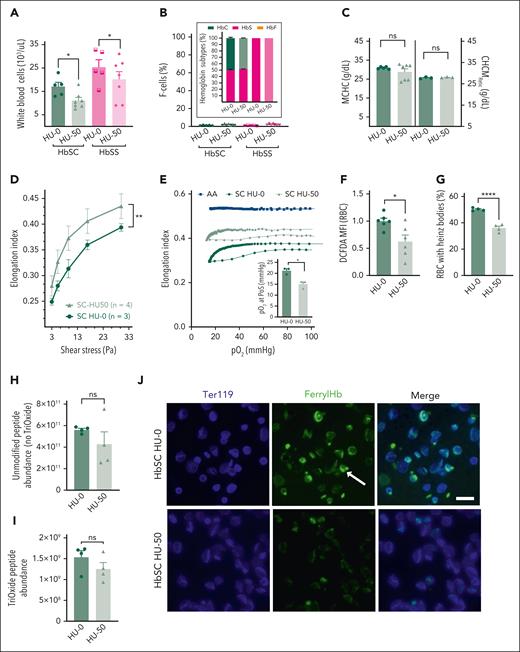
We first titrated the dose of HU in adult HbSS and HbSC mice from 25 to 100 mg/kg BW. HU at >50 mg/kg BW every other day showed significant cytopenia, toxicity, and lethality. Notably, we did not observe higher toxicity in HbSC mice. Adult HbSS and HbSC mice were administered HU 50 mg/kg BW thrice weekly for 4 weeks (HU-50), along with PBS controls (HU-0). Leukocytes and reticulocytes decreased in HU-50 HbSS/HbSC mice, as in patients; MCV decreased from the reduced reticulocytosis caused by HU. However, no upregulation of HbF was seen by HPLC/F-cell analysis (Figure 6A-B; supplemental Figure 4A-G). MCHC and CHCMRetic measurements showed no improvement in HbSC RBC/reticulocyte hydration (Figure 6C). However, shear-stress ektacytometry showed a significant improvement in HbSC RBC deformability (Figure 6D; supplemental Figure 5A). Oxygen-gradient ektacytometry confirmed this improved membrane deformability, reflected in higher EImax in HbSC and HbSS RBCs. Interestingly, PoS was also lower in HbSC/HbSS RBCs (Figure 6E; supplemental Figure 5B).
Non-HbF effects of HU on HbSC RBC parameters and rheology. Adult SC and SS mice, 10 to 12 weeks of age, were treated with HU, 50 mg/kg (HU-50), or PBS (HU-0) intraperitoneally, 3 times a week for 4 weeks, and the peripheral blood was analyzed. (A) White blood cell counts. (B) No upregulation of HbF was observed both by HPLC or F-cell staining. The Hb subtypes, determined by HPLC, are shown in stacked bars (inset), and the F-cell percentage by flow cytometry is also shown. Panels C through J show RBC characteristics of HbSC mice that received HU-0 and HU-50, whereas similar data on HbSS mice are shown in supplemental Figure 5. (C) MCHC in the erythrocytes of HbSC mice is shown in the left panel, and the CHCMRetic in the right panel. (D) RBC deformability (EI), determined at normoxia across a range of shear stresses, is shown. (E) Sickling kinetics of the HbSC RBCs were analyzed using oxygen-scan ektacytometry at constant shear stress, and EI was measured with increasing hypoxia, followed by reoxygenation. The average RBC deformability curves of each group are shown. The pO2 PoS determined from these curves is shown in the inset. (F) Reactive oxygen species in HbSC and HbSS RBCs treated with HU-50 or HU-0 were analyzed by DCFDA staining and flow cytometry. The relative MFI, normalized to HU-0 group, is shown. (G) The percentage of Heinz-body–positive RBCs in HbSC and HbSS RBCs treated with HU-50 or HU-0 is shown. A minimum of 600 RBCs was counted per group. Representative pictures of the original blood smears with RBCs stained for Heinz bodies and controls are shown in supplemental Figure 8C-D. Unmodified peptide abundance (no trioxide; H) and cysteine-93 peptide abundance (I) was measured in 110 000 ghost RBCs from HbSC and HbSS mice treated with HU-50 or HU-0 based on trypsin digestion and comparative profiling by mass spectrophotometry. (J) Immunofluorescence imaging of ferryl Hb in RBCs from HbSC mice HU-0 (top) or HU-50 (bottom). Anti–ferryl Hb (green) and Ter119 (blue) staining were visualized on an EVOS 7000 (ThermoFisher Scientific) using a 60× coverslip-corrected objective (Olympus) and analyzed in the EVOS analysis software (ThermoFisher Scientific). Scale bar, 10 μm. For panels A through C, and F and G: each symbol represents an individual sample, and bars represent mean ± standard error of the mean. Curves in panels D through E represent average values of 3 to 4 mice per group. Statistics were performed using ANOVA. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .00001. DCFDA, 5′,6′-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; MFI, mean fluorescence intensity.
Non-HbF effects of HU on HbSC RBC parameters and rheology. Adult SC and SS mice, 10 to 12 weeks of age, were treated with HU, 50 mg/kg (HU-50), or PBS (HU-0) intraperitoneally, 3 times a week for 4 weeks, and the peripheral blood was analyzed. (A) White blood cell counts. (B) No upregulation of HbF was observed both by HPLC or F-cell staining. The Hb subtypes, determined by HPLC, are shown in stacked bars (inset), and the F-cell percentage by flow cytometry is also shown. Panels C through J show RBC characteristics of HbSC mice that received HU-0 and HU-50, whereas similar data on HbSS mice are shown in supplemental Figure 5. (C) MCHC in the erythrocytes of HbSC mice is shown in the left panel, and the CHCMRetic in the right panel. (D) RBC deformability (EI), determined at normoxia across a range of shear stresses, is shown. (E) Sickling kinetics of the HbSC RBCs were analyzed using oxygen-scan ektacytometry at constant shear stress, and EI was measured with increasing hypoxia, followed by reoxygenation. The average RBC deformability curves of each group are shown. The pO2 PoS determined from these curves is shown in the inset. (F) Reactive oxygen species in HbSC and HbSS RBCs treated with HU-50 or HU-0 were analyzed by DCFDA staining and flow cytometry. The relative MFI, normalized to HU-0 group, is shown. (G) The percentage of Heinz-body–positive RBCs in HbSC and HbSS RBCs treated with HU-50 or HU-0 is shown. A minimum of 600 RBCs was counted per group. Representative pictures of the original blood smears with RBCs stained for Heinz bodies and controls are shown in supplemental Figure 8C-D. Unmodified peptide abundance (no trioxide; H) and cysteine-93 peptide abundance (I) was measured in 110 000 ghost RBCs from HbSC and HbSS mice treated with HU-50 or HU-0 based on trypsin digestion and comparative profiling by mass spectrophotometry. (J) Immunofluorescence imaging of ferryl Hb in RBCs from HbSC mice HU-0 (top) or HU-50 (bottom). Anti–ferryl Hb (green) and Ter119 (blue) staining were visualized on an EVOS 7000 (ThermoFisher Scientific) using a 60× coverslip-corrected objective (Olympus) and analyzed in the EVOS analysis software (ThermoFisher Scientific). Scale bar, 10 μm. For panels A through C, and F and G: each symbol represents an individual sample, and bars represent mean ± standard error of the mean. Curves in panels D through E represent average values of 3 to 4 mice per group. Statistics were performed using ANOVA. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .00001. DCFDA, 5′,6′-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; MFI, mean fluorescence intensity.
Mechanistically, HU lowered RBC but not platelet/white blood cell reactive oxygen species (Figure 6F; supplemental Figures 5A and 7A-B). Furthermore, ferryl Hb, detectable in HU-0 HbSC/HbSS RBCs, was remarkably reduced in the HU-50 group (Figure 6J; supplemental Figure 5G). Consequently, Heinz bodies were also significantly reduced in the HU-50 group, both in HbSC and HbSS RBCs (Figure 6G; supplemental Figures 5D and 8A-F). To assess Hb oxidation, we analyzed the association and the oxidation status of β-globin93Cys (β93Cys; a position in β-globin prone to oxidation) to the RBC membrane by mass spectrometry. RBC membrane ghosts exhibited a higher association of the unmodified β93Cys peptides and trioxidized β93Cys peptides when compared with HbSS mice (Figure 6H-I; supplemental Figure 5E-F). We also observed a trend toward a lower association of the unmodified β93Cys peptides and the trioxidized β93Cys peptides with HU (P = .1). In vitro experiments have shown that HU nitrosylates β93Cys peptides, protecting it from oxidation50; we did not detect β93Cys peptide nitrosylation with in vivo administration of HU to mice, whereas we confirmed the position and site of all detected β93Cys peptide modifications by peptide-fragmentation sequencing (supplemental Figure 6A-B).
Taken together, the HbF-independent effects of HU on HbSC and HbSS RBCs include reduced oxidative stress and oxidized Hb, reduced membrane damage that results in improved membrane deformability, and sickling at lower pO2, but no improvement in HbSC RBC hydration.
HbF effects of HU in HbSC RBCs
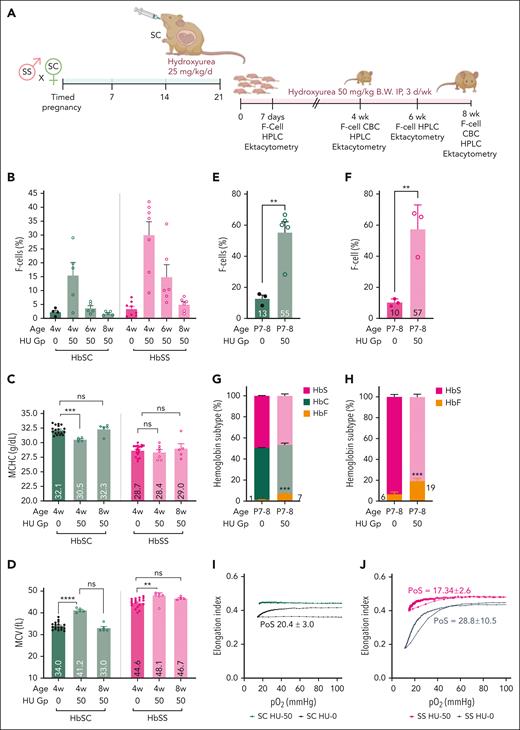
Using the human analogy of initiation of HU early in infancy for high level of HbF expression,51 we exposed pregnant HbSC mice from HbSS×HbSC timed-matings to 25 mg/kg BW per day HU in the last week of gestation to allow HU exposure to unborn mice while HBG gene poised for being switched-off, and continued it postnatally (50 mg/kg, 3 days per week; Figure 7A). We observed heterocellular HbF expression in HbSC and HbSS HU-50 mice at 4 weeks of age, which declined to control levels by age 8 weeks of age. Notably, HbF and F cells in HbSC mice were nearly half compared with those in HbSS, despite the same dose given concurrently to these littermates (Figure 7B). Upregulation of HbF, even at these low HbF levels (∼1% HbF) in the 4-week-old HbSC mice, nevertheless, resulted in significant improvement in HbSC RBC hydration, as evidenced by lower MCHC and higher MCV (Figure 7C-D), which reverted with the decline in HbF at age 8 weeks to levels seen in HU-0 controls. No change in MCHC occurred in 4-week-old HbSS mice. The MCV increased in both HbSC and HbSS RBCs, but the increase was significantly greater in HbSC RBCs than in their respective controls. No significant change in Hb/hematocrit (HCT) in HbSC mice occurred (supplemental Figure 9A-B).
HbF differentially affects HbSC RBC parameters, improving HbSC RBC hydration at low levels and ameliorating sickling at modest levels. (A) Experimental design for panels B through F. HbSC female mice were time-mated with HbSS male mice to get a ∼50:50 ratio of HbSS and HbSC pups. Pregnant females were treated with 25 mg/kg BW HU by oral gavage from day 15 through day 21 of gestation. All pups born from the HU-treated pregnant females were treated with HU 50 mg/kg BW intraperitoneally (IP), 3 days per week for 8 weeks (HU-50 group). Control pregnant females and pups received the same volume of PBS by oral gavage or IP injections, respectively (HU-0 group). A limited amount of blood was collected pragmatically to allow serial analysis on the same set of mice. Hence, microbleeds were done at the indicated time points for quantifying F cells by flow cytometry. When the pups were 4 and 8 weeks old, blood was collected for both F-cell and CBC analyses. The data from these experiments are shown in panels B through F. Experimental design for panels G through I: in 1 experiment, we euthanized pups at P7 to P8 to obtain sufficient blood for HPLC, F cell, and oxygen-gradient ektacytometry. (B) Percent F cells determined using flow cytometry are shown at different ages indicated on the X-axis. (C-D) CBC analysis performed at 4 and 8 weeks is shown. The MCHC (C) and MCV (D)are shown. Percent F cells in 7-to-8-day-old HbSC pups (E) and HbSS (F) treated with the HU-0 and HU-50 groups, determined by flow cytometry, are shown. The HbF, HbC, and HbS percentage by HPLC analysis on the P7 to P8 pups in HbSC (G) and HbSS (H) are shown. Oxygen-gradient ektacytometry performed on the P7 to P8 pups HbSC (I) and HbSS (J) mice treated with HU-0 or HU-50. The PoS is shown on the curve. Each symbol represents an individual mouse, and the bars represent mean ± standard error of the mean. The oxygen-gradient ektacytometry curves represent the means of 3 mice per group. Statistics were performed using ANOVA. ∗∗P < .01; ∗∗∗P < .0001; ∗∗∗∗P < .00001. CBC, complete blood count; d, day; Gp, group; w or wk, week.
HbF differentially affects HbSC RBC parameters, improving HbSC RBC hydration at low levels and ameliorating sickling at modest levels. (A) Experimental design for panels B through F. HbSC female mice were time-mated with HbSS male mice to get a ∼50:50 ratio of HbSS and HbSC pups. Pregnant females were treated with 25 mg/kg BW HU by oral gavage from day 15 through day 21 of gestation. All pups born from the HU-treated pregnant females were treated with HU 50 mg/kg BW intraperitoneally (IP), 3 days per week for 8 weeks (HU-50 group). Control pregnant females and pups received the same volume of PBS by oral gavage or IP injections, respectively (HU-0 group). A limited amount of blood was collected pragmatically to allow serial analysis on the same set of mice. Hence, microbleeds were done at the indicated time points for quantifying F cells by flow cytometry. When the pups were 4 and 8 weeks old, blood was collected for both F-cell and CBC analyses. The data from these experiments are shown in panels B through F. Experimental design for panels G through I: in 1 experiment, we euthanized pups at P7 to P8 to obtain sufficient blood for HPLC, F cell, and oxygen-gradient ektacytometry. (B) Percent F cells determined using flow cytometry are shown at different ages indicated on the X-axis. (C-D) CBC analysis performed at 4 and 8 weeks is shown. The MCHC (C) and MCV (D)are shown. Percent F cells in 7-to-8-day-old HbSC pups (E) and HbSS (F) treated with the HU-0 and HU-50 groups, determined by flow cytometry, are shown. The HbF, HbC, and HbS percentage by HPLC analysis on the P7 to P8 pups in HbSC (G) and HbSS (H) are shown. Oxygen-gradient ektacytometry performed on the P7 to P8 pups HbSC (I) and HbSS (J) mice treated with HU-0 or HU-50. The PoS is shown on the curve. Each symbol represents an individual mouse, and the bars represent mean ± standard error of the mean. The oxygen-gradient ektacytometry curves represent the means of 3 mice per group. Statistics were performed using ANOVA. ∗∗P < .01; ∗∗∗P < .0001; ∗∗∗∗P < .00001. CBC, complete blood count; d, day; Gp, group; w or wk, week.
We terminally bled HbSC and HbSS P7 pups, when HbF levels should be higher than at 4 weeks of age, to obtain sufficient blood for determining HbF and sickling kinetics. Similar proportions of F cells were present in HbSC (55%) and HbSS (57%) HU-50 P7 pups (Figure 7E-F). However, the HbF levels were nearly 3 times higher in HbSS (19%) than HbSC (7%) P7 pups, despite both getting the same dose of HU concurrently (Figure 7G-H).
Furthermore, oxygen-gradient ektacytometry demonstrated complete abrogation of sickling in HbSC RBCs at 7% HbF (PoS occurred at 20 mm Hg at 1% HbF [HU-0] but PoS was not detectable at 7% HbF; Figure 7I). In HbSS HU-0 P7 pups, the PoS occurred at ∼29 mm Hg with 6% HbF (HU-0), and decreased to 17 mm Hg in HU-50 P7 pups with 19% HbF (Figure 7F,H,J).
Discussion
HbSC-SCD has remained a neglected SCD genotype compared with HbSS-SCD, depriving a third of patients with SCD of transformative/disease-modifying therapies because of lack of an animal model. Adults with HbSC have substantial morbidity and mortality from severe disease.8,11 The HbSC mice developed herein exhibited erythrocyte characteristics phenocopying patients with HbSC-SCD: milder anemia, lower RBC turnover, longer RBC half-life, but higher blood viscosity than HbSS-mice, and prominent RBC xerocytosis. HbSC-RBC sickling kinetics resembled those in RBCs of patients with HbSC-SCD. Notably, humanized SCD mice have a more severe RBC phenotype than human patients (HbSS RBC half-life, 2-3 days; 50% reticulocytes). We observe that HbSC mice also have a more severe phenotype than human patients with HbSC-SCD, with ∼20% reticulocytes. Regardless, the HbSC model generated herein and the HbSS models52 are invaluable because they mimic the features of human SCD.
Although HbSC RBCs sickled at lower pO2 than HbSS RBCs, as is expected from the presence of 50% HbS, unlike 100% HbS in HbSS RBCs, HbSC mice showed a similar degree of increase in inflammatory and endothelial activation markers and drop in Hb/platelets as seen in HbSS mice in experimentally induced VOC. Clinically, VOC in patients with HbSC is no less severe than in patients with HbSS. Additionally, HbSC mice showed: (1) marked splenic sinusoidal congestion compared with HbSS mice, because sinusoids have slow vascular flow and therefore congestion is exacerbated by the high blood viscosity in HbSC mice; and (2) sickle retinopathy occurred more frequently in HbSC mice, as seen in patients with HbSC-SCD.
The identical genetic background of the HbSC and HbSS mice, both being knockins for the human HBA and HBB genes, except for the HBB sixth codon difference on 1 allele, allowed us the unique ability to make direct comparisons of the effects of HU in HbSC vs HbSS mRBCs.
HU was first approved for HbSS-SCD in 1997.53,54 However, until very recently,19 only small/retrospective studies of HU in HbSC-SCD had been done,22-27 which showed perplexingly low/modest increases in HbF, unlike much higher HbF induction in patients with HbSS.55,56 The largest retrospective study22 and the recently published PIVOT trial19 (133 and 107 patients with HbSC given HU for 12 months, respectively) also showed modest increases in HbF (2%-4%,22 and 7.6% ± 7.5% with HU at maximum tolerated dose19). However, despite modest HbF induction, there was significant reduction in vaso-occlusive events in both these studies.19,22
Because adult SCD mice do not express HbF with HU,28,49 we were able to uniquely compare the non-F effects on HbSC and HbSS RBCs in this model. We found that HU decreased oxidative damage to Hb by reducing levels of the toxic oxidative transformation of Hb to the ferryl state (HbFe4+). Ferryl Hb irreversibly oxidizes β-globin at Cys-93.57 Oxidized Hb binds to band 3 protein, clustering band 3,50 which then results in Heinz-body formation.58-60 We observed this phenomenon in both HbSC and HbSS RBCs, although a significantly higher proportion of HbSC RBCs had Heinz bodies than HbSS RBCs. This is consistent with studies that show HbC binds more tightly to erythrocyte membrane and alters its structure in HbCC RBCs61; its oxidation results in Heinz-body formation.62,63 Heinz bodies have been similarly shown to form with HbS.63 We show a significant reduction in Heinz bodies in HbSC/HbSS RBCs treated with HU, resulting in improved RBC deformability. Increased Heinz bodies quantitatively correlate with a reduction in RBC deformability.64
We also observed a reduction in PoS with HU in both HbSC and HbSS mice, without HbF upregulation. In vitro exposure of HbS samples to HU was shown to prevent β93Cys oxidation and delay HbS polymerization.65 We observe an increased abundance of the β-globin peptide containing β93Cys on HbSC RBC membrane ghosts, that tends to be reduced with HU treatment, and the same phenomenon is seen with oxidized β93Cys peptides. Finally, our data unequivocally show that HbF is required to improve RBC hydration, because HbSC RBCs show no improvement in hydration with HU when they do not upregulate HbF. Given the known role of band 3 in regulating glycolysis via pyruvate kinase and interactions with Hb, and that pyruvate kinase has 2 cysteines in its catalytic domain, which are prone to oxidation, it is plausible that HU prevents their oxidation, which can be explored in future studies.
It has been known for decades that HU reduces VOC symptoms in patients with HbSS-SCD long before HbF upregulation, which takes 4 to 6 months. The paradigm has been that HU benefits HbSS-SCD because of its HbF-independent effects on RBCs (increased MCV and reducing HbS concentration), leukocytes (reduced leukocytosis and inflammation), and endothelial cells (increasing nitric oxide bioavailability).66 Herein, we show the non-F effects of HU directly on HbSC and HbSS RBCs, which also explain the early benefits of HU.
Additionally, we were able to induce HbF in both HbSC and HbSS mice by starting HU administration around the time the mouse HbF switch occurred. To the best of our knowledge, this is the first demonstration of upregulation of HbF with HU to clinically relevant levels in SCD mice (∼7% in HbSC and ∼19% in HbSS mice). Even a minimal increase in HbF with HU improved HbSC RBC hydration (MCV and MCHC), and a modest increase in HbF by 7% ameliorated sickling of murine HbSC RBCs, explaining the clinical benefits seen with low/modest HbF in patients with HbSC-SCD. In cell-free Hb mixing experiments, HbF reduces HbC crystallization and HbS polymerization.67 Furthermore, F cells were absent in denser fractions when HbSC RBCs were fractionated on a density gradient.8
We further show that, despite getting the same exposure to HU, there is nearly threefold higher induction of HbF in HbSS RBCs than HbSC RBCs, despite similar numbers of F cells. Hence, HbSS mice produce nearly 3 times more HbF/F cells than HbSC mice. These data are very similar to what has been seen clinically in patients with HbSS55 vs those with HbSC who have been given HU.19,22 The fact that this can be modeled in mice makes it possible to study the underlying mechanism in future studies.
Overall, the combined HbF and the non-HbF effects seen in this model explain the remarkable clinical benefit seen with HU in HbSC-SCD.19,22-27
In conclusion, the lack of an animal model of HbSC-SCD has hindered research and translation of therapies for HbSC-SCD. We report, to our knowledge, the first animal model of HbSC-SCD that mimics human HbSC-SCD phenotype and shows unique mechanistic insights into the HbF-dependent and HbF-independent effects of HU in HbSC vs HbSS SCD. This mouse model will allow mechanistic studies and rapid development of translatable therapies for HbSC-SCD.
Acknowledgments
The authors acknowledge the Center for Advanced Microscopy and Imaging at Miami University, Oxford, Ohio, for scanning electron microscopy imaging support; the Research Flow Cytometry Core Facility at Cincinnati Children’s Hospital Medical Center (CCHMC) for providing flow cytometry support; the Erythrocyte Diagnostic Laboratory at CCHMC, specifically Theodosia Kalfa and Jenifer Korpik, for support of hematological tests; the Transgenic Animal and Genome Editing Core Facility, CCHMC, for support with the generation of the sickle cell hemoglobin mice, and Jeffrey Bailey in the Mouse Core for assistance with mouse procedures; and Smith Drew, Lori Miller, and Betsy DiPasquale in the Pathology Core, CCHMC, for organ histopathology staining. They thank the University of Cincinnati, Proteomics Core, including Michael Wydmer and Wendy Haffey, and Rashmi Hegde and her laboratory personnel for allowing the use of, and assistance with, the hypoxia chamber. The authors acknowledge and thank Éva Katona for her contribution to the generation of the ferryl hemoglobin antibody. The visual abstract and Figure 7A were generated with BioRender.com.
This study is partially supported by a grant funded by the Center for Clinical and Translational Science and Training Just-In-Time program and the Research Innovation Pilot award (P.M.). J.B. was supported by HUN-REN-DE (11003) Research Network and Project no. TKP2021-EGA-18; and the National Research, Development and Innovation Fund, Ministry for Innovation and Technology of Hungary, National Institutes of Health Office of the Director S10 Shared Instrumentation Program (grant S10OD026717), which, in part, funded the mass spectrometer used in these studies.
Authorship
Contribution: T.S., M.C., Z.O., M.S., K.S., Z.Z., A.T., H.K., K.D.G., J.B., M.X., K.V., A.S., and T.M.T. performed data acquisition and analyses; Y.-C.H., Z.O., M.S., K.V., K.D.G., T.S., and P.M. performed data plotting/micrography; T.S., M.C., Y.-C.H., and P.M. conducted the experimental methodologies and interpreted data; P.M. conceived this project; T.S. and P.M. designed the experiments, wrote the manuscript; and all authors read and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Punam Malik, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; email: punam.malik@cchmc.org.
References
Author notes
Original data are available on request from the corresponding author, Punam Malik (punam.malik@cchmc.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal