In this issue of Blood, Wang et al test the efficacy of a B-cell lymphoma–extra large (BCL-xL) degrading molecule (DT2216) in a distinct subtype of acute myeloid leukemia (AML) that arises from myeloproliferative neoplasms (MPNs).1 Post-MPN AML has a dismal prognosis, where patients are generally refractory to available therapies and have a median survival of ≈6 months.2 As current treatment options for these patients hinge almost entirely on allogeneic stem cell transplantation, this study is a critically important addition to the post-MPN AML therapeutic landscape. Not only do Wang et al offer a novel agent to selectively target BCL-xL, but also one that specifically kills leukemic cells while sparing normal cells, and thus has potential to demonstrate true disease-modifying activity.
Although the biology and underlying mechanisms of post-MPN AML remain unclear, it has been shown that disease evolution is such that the founding MPN driver mutation (JAK2/CALR/MPL), although it is typically retained in the leukemic clone, is not sufficient to promote transformation to AML. Instead, the acquisition of secondary mutations in genes such as TP53, RUNX1, and ASXL1 are required for this transformation to occur.3 How these secondary mutations synergize with driver mutations to promote leukemic transformation is not well understood, but 1 potential mechanism explored by Wang et al at the outset of this study is that specific secondary mutations may cooperate with MPN drivers to upregulate distinct downstream effectors that drive leukemic transformation.
To this end, the authors focus on BCL-xL, which they have previously shown to be upregulated in JAK2-mutated compared with JAK2–wild-type AML cells. Using a combination of approaches including multiomics, they validate that the gene encoding BCL-xL, BCL2L1, is likewise upregulated in JAK2-mutated vs JAK2–wild-type AML cell lines, and in post-MPN AML patient cells compared with those from patients with de novo AML. Last, they show that BCL-xL represents a unique dependency in JAK2-mutated post-MPN AML cells, and note that this dependency is augmented in JAK2 mutant cells that harbor comutations in TP53.
To delve deeper into the role of TP53 in this context, they next performed multiomics on a single-cell level, and found that those post-MPN AML cells harboring an MPN driver mutation (JAK2 or CALR) and a concurrent TP53 mutation display the highest levels of BCL2L1. This suggests that TP53 mutations play an important role in further upregulating and amplifying dependency on BCL2L1 in post-MPN AML cells, and that BCL-xL is thus a promising target for therapy in post-MPN patients with AML with TP53 mutations.
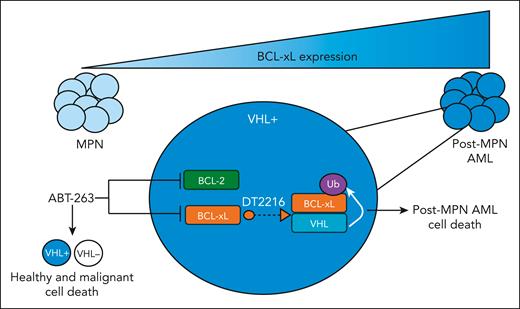
Efforts to target BCL-xL in MPNs using the dual BCL2/BCL-xL inhibitor navitoclax (ABT-263) have been promising, particularly in combination with the JAK inhibitor ruxolitinib.4,5 However, because BCL-xL is critical for healthy platelet survival, ABT-263 has limited clinical tractability because of dose-limiting thrombocytopenia.6,7 To overcome this, Wang et al use their previously developed proteolysis-targeting chimera (PROTAC) platform8 to target BCL-xL to the von Hippel–Lindau (VHL) E3 ligase (DT2216), leading to its ubiquitination and degradation in VHL-expressing cells. Because platelets do not express VHL, DT2216 minimizes the on-target platelet toxicity caused by navitoclax.
Next, the authors tested DT2216 in various post-MPN AML models. Using both induced pluripotent stem cell and JAK2-mutant AML cell lines, they found increased apoptosis of mutant cells compared with wild-type on DT2216 treatment. They further showed that DT2216 is effective in combination with drugs used clinically for patients with MPN and AML, as well as in ruxolitinib-resistant cells, determining that a combination of DT2216 with azacytidine and a mantle cell lymphoma-1 inhibitor shows the best synergy. Finally, they validate that DT2216 reduces post-MPN AML hematopoietic stem and progenitor cell viability and colony formation, as well as suppresses growth of JAK2-mutated AML cells in vivo. In sum, the authors demonstrate the preclinical efficacy of targeting BCL-xL specifically in VHL-expressing post-MPN AML cells using a novel PROTAC (see figure).
BCL-xL expression increases as patients with MPN progress to post-MPN AML. Targeting of BCL-xL and BCL-2 through ABT-263 leads to dose-limiting thrombocytopenia because of the fact that platelets depend on BCL-xL for survival. However, degradation of BCL-xL, mediated by PROTAC DTT2216, only targets VHL-expressing cells, which is not expressed in platelets, thereby killing leukemic cells while minimizing on-target platelet toxicity.
BCL-xL expression increases as patients with MPN progress to post-MPN AML. Targeting of BCL-xL and BCL-2 through ABT-263 leads to dose-limiting thrombocytopenia because of the fact that platelets depend on BCL-xL for survival. However, degradation of BCL-xL, mediated by PROTAC DTT2216, only targets VHL-expressing cells, which is not expressed in platelets, thereby killing leukemic cells while minimizing on-target platelet toxicity.
Resistance and dose-limiting adverse effects of current frontline therapies pose a threat to nearly all malignancies.9 Thus, developing therapies that can target malignant cells while sparing healthy cells is crucial to improving both patient quality of life and survival rates. The development of PROTACs has provided an avenue to target cancer cells while sparing normal cells and, thus, limited on-target and off-target toxicity—the gold standard in cancer drug development. This study is one of the first to investigate PROTACs in MPN and post-MPN AML (a previous study showed promising results with a PROTAC targeting bromodomain and extra-terminal motif10), and the first, to our knowledge, to demonstrate the ability to target BCL-xL without platelet toxicity. As we pave the way toward identifying novel targets and treatment strategies that have true disease-modifying activity and curative potential for this challenging disease, PROTACs represent a key player in the evolving landscape of post-MPN AML therapy.
Conflict-of-interest disclosure: S.E.E. has consulted for Paragon Therapeutics, Swedish Orphan Biovitrum (Sobi), and Incyte. N.S.A. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal