In this issue of Blood, Wang et al1 describe a way forward to novel small molecule remedies for sickle cell disease (SCD). The authors employed structure-aided drug design to develop potent new reversible LSD1 inhibitors (LSD1i), resulting in robust γ-globin expression in vitro and in an SCD mouse model. Furthermore, combined treatment with a BRD4 degrader rescued the erythroid-to-myeloid lineage conversion that accompanies LSD1 inhibition. These findings may offer novel therapeutic prospects for the treatment of SCD.
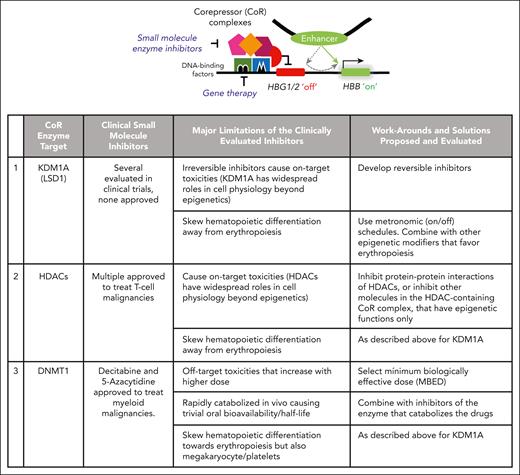
SCD has an especially high prevalence in pediatric populations in low-income countries, afflicting tens of millions.1 Practical, accessible oral medication solutions are hence demanded, ideally remedying root-cause pathophysiology for maximum impact. This root-cause pathophysiology is well understood: inheritance of mutated adult β-globin genes (HBB) and outputting less soluble sickle hemoglobin (HbS), which polymerizes and precipitates when deoxygenated, thus damaging and destroying red blood cells, in a manner that also causes vaso-occlusion. Also well understood is that erythroid progenitors in all patients contain genes for unmutated fetal β-globin family genes (γ-globin, HBG1, and HBG2). If permitted, HBG1/2 can produce the most powerful known natural disease-modifying molecule, fetal hemoglobin (HbF, α2γ2), which intercalates with and inhibits HbS polymerization.2 The switch from fetal to adult globin gene transcription that occurs in human development (“developmental switch”) is recapitulated every time an erythroid progenitor in a human child or adult differentiates or matures (the “maturational switch”) (see figure).3 This maturational switch involves the exchange of activating with repressing epigenetic marks at HBG1/2 (see figure).3 Although coordinated by DNA-binding factors such as BCL11A, ZBTB7A, and DRED, the work of placing repressive epigenetic marks requires and is executed by enzymes (corepressors [CoRs]) (see figure).4,5 CoR enzymes, designed by evolution to manipulate and be manipulated by small molecules, are druggable and moreover scientifically validated treatment targets for HbF elevation, including by inherited loss-of-function polymorphisms in these genes that cause hereditary persistence of fetal hemoglobin production (reviewed in reference6). Of the CoR enzymes recruited by BCL11A etc, clinical-stage small molecule inhibitors are available for histone deacetylases (HDACs), DNA methyltransferase (DNMT1), and lysine-specific demethylase 1A (KDM1A, LSD1) (see figure).
A switch from fetal-globin gene (γ-globin, HBG1/2) to adult β-globin gene (HBB) transcription occurs during erythropoiesis (“maturational switch”) and requires the repression of HBG1/2 by druggable repressing epigenetic enzymes (CoRs). Gene therapy targets the DNA-binding factors that recruit the CoR enzymes. An alternative approach is to inhibit the enzyme elements in the hub (eg, LSD1, HDACs, DNMT1) with small molecules. Limitations of available clinical-stage CoR enzyme inhibitors are shown, including lessons learned for moving forward. Small molecule inhibitors for other CoR enzymes in the hub, CHD4 and CBX5, are in a preclinical stage of development.
A switch from fetal-globin gene (γ-globin, HBG1/2) to adult β-globin gene (HBB) transcription occurs during erythropoiesis (“maturational switch”) and requires the repression of HBG1/2 by druggable repressing epigenetic enzymes (CoRs). Gene therapy targets the DNA-binding factors that recruit the CoR enzymes. An alternative approach is to inhibit the enzyme elements in the hub (eg, LSD1, HDACs, DNMT1) with small molecules. Limitations of available clinical-stage CoR enzyme inhibitors are shown, including lessons learned for moving forward. Small molecule inhibitors for other CoR enzymes in the hub, CHD4 and CBX5, are in a preclinical stage of development.
However, no clinical-stage CoR enzyme inhibitors are presently approved to treat SCD, because all have major limitations that need to be addressed (see figure): Wang et al usefully demonstrate work-arounds and solutions for these limitations. For example, a problem with clinical-stage LSD1 inhibitors evaluated to date is their irreversible mechanism of action, problematic when the targeted enzyme has cell physiology roles beyond epigenetic, such that irreversible inhibition causes cytotoxicity that undermines an overall goal of sustainable disease modification. Wang et al therefore pursued a structure-guided medicinal chemistry campaign to develop reversible LSD1 inhibitors, which bound noncovalently to the amine oxidase-like domain of LSD1. In vitro comparisons to a previously evaluated clinical-stage irreversible LSD1 inhibitor showed that the lead reversible inhibitors were more potent and produced larger increases in fetal hemoglobin expression. In preclinical in vivo studies in SCD mice (University of Alabama Birmingham mice in which the endogenous globin gene loci were replaced with human globin, HBG, and sickle-mutated HBB genes), their lead LSD1 inhibitors produced large increases in HbF and total hemoglobin, without major decreases in platelet or neutrophil counts, and with other evidence of decreased SCD pathophysiology.
Wang et al then addressed a second major limitation inherent to small molecule inhibitors of epigenetic enzymes: these enzymes are recruited into lineage master transcription factor hubs, which regulate lineage-commitment decisions. Thus, all small molecule CoR inhibitors can be expected to produce shifts in lineage-differentiation patterns. Moreover, different lineage master transcription factors use different CoR enzymes, so different CoR inhibitors may produce different patterns of shift. For example, histone deacetylase and LSD1 inhibitors skew blood cell production toward granulomonopoiesis and away from erythromegakaryopoiesis, and DNMT1 inhibitors skew in the opposite direction.7 One method for restraining lineage skewing within bounds of clinical safety, while also producing intended clinical pharmacodynamic benefits such as HbF elevation, is to use on-off (metronomic) schedules of drug administration (see figure).7,8 Another is to combine drugs that produce off-setting shifts: Wang et al combined LSD1 inhibitors with a degrader of bromodomain containing chromatin-remodeling enzymes. Enzymes that remodel chromatin for gene activation often contain bromodomains. The bromodomain degrader alleviated the LSD1 inhibitor-mediated shift away from erythropoiesis. Another combination used in previous work was to combine LSD1 inhibitors with DNMT1 inhibitors that produce off-setting lineage-commitment shifts.9
Hydroxyurea, the drug most widely used for SCD modification, can successfully increase HbF, but does so indirectly, via antimetabolite cytotoxic effects that accelerate erythropoiesis (“stress” erythropoiesis), forcing red blood cells to mature with an enhancer still at HBG1/2 (see figure). However, cytotoxicity should be avoided if possible, since >10-fold higher erythropoietic output is demanded lifelong just to maintain hemoglobin at already low levels, and attrition of hematopoietic reserves and hence less capacity to meet these demands, seen for example by an absolute reticulocyte count <10-fold higher than normal together with hemoglobin <9 g/dL, is a powerful predictor of early death.10 Further work will therefore be needed to show that the novel reversible LSD1 inhibitors do not produce major cytotoxic effects. In sum, Wang et al introduce a novel class of small molecule CoR enzyme inhibitor candidates, and in addition, teach broader lessons relevant to all efforts aiming for accessible, molecular-targeted root-cause remedy of SCD.
Conflict-of-interest disclosure: Y.S. holds patents around tetrahydrouridine/decitabine/5-azacytidine, ISWI family inhibition, and cancer differentiation inducers and has equity and board interest in EpiDestiny and Treebough Therapies. D.L. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal