Abstract
To investigate the role of bcl-2 in lymphohematopoiesis, a long-term bone marrow reconstitution system was established. Transplantation of 1,000 c-Kit+ Sca-1+ and lineage markers negative cells from bcl-2−/− mouse bone marrow resulted in long-term reconstitution of nonlymphoid cells. However, T cells were totally absent and B-lymphocyte development was severely impaired at a very early stage of differentiation in the chimeric mouse. On the other hand, transplantation of day 14 fetal liver cells from bcl-2−/− mice resulted in generation of both T and B cells in the recipient, albeit transiently. These data suggest that bcl-2 plays a critical role in the development of lymphoid progenitor cells from the hematopoietic stem cell (HSC), but is not essential for the development of nonlymphoid cells and the self-renewal of HSC. In addition, lymphopoiesis from fetal liver HSC appears to be less dependent on bcl-2 than adult bone marrow HSC.
AN INTEGRAL MEMBRANE protein that functions to repress programmed cell death,1 25-kD bcl-2 is widely expressed during embryogenesis.2,3 In an adult, however, endogenous bcl-2 protein is normally restricted to long-lived cells such as mature B and T lymphocytes, neurones, early hematopoietic progenitors, stem cell zones of intestine, epidermis, and hormonally regulated epithelium.4,5 Within the thymus, bcl-2 is expressed in mature thymocytes in the medulla and is present only in scattered cells in the cortex.6 Cell sorting experiments have shown that bcl-2 is expressed in nearly all CD4+ and CD8+ single-positive thymocytes, but the vast majority of CD4+CD8+ double-positive thymocytes lack bcl-2 expression. In the very immature CD4−CD8− double-negative cells, bcl-2 expression was variable, with the CD44+CD25− subset demonstrating a high percentage of positivity.7,8 Reverse transcription polymerase chain reaction (RT-PCR) studies have shown that CD4−CD8−TCRαβ−CD25+ cells in the thymus express high levels of bcl-2 mRNA.9
In B-lineage cells, bcl-2 is expressed in the earliest stage, then at very low levels throughout the immature B-cell stage and finally at higher levels in the mature (IgD+) B-cell stage.10 This stage-specific expression suggests a role of bcl-2 in T- and B-cell differentiation. The physiologic role for bcl-2 in lymphocyte maturation, however, remains unknown. Recently, using somatic and germ-line transmitted bcl-2 knock-out mice, we demonstrated that bcl-2 is necessary to maintain a stable lymphoid system but is not required for phenotypic lymphocyte maturation. The mature T cells that lacked bcl-2 had shorter life-spans and increased sensitivity to apoptotic stimuli such as glucocorticoids or γ-irradiation. Furthermore, mature T and B cells without bcl-2 disappeared from the periphery by 6 to 8 weeks postnatally. In addition to the disappearance of mature cells, immature cells including thymocytes and immature B lineage cells also disappeared with time.11-13
Several issues, however, remain to be elucidated. First, the shortened life-span of mature lymphocyte does not explain the decreased number of immature cells. Since the expression of bcl-2 is not limited to mature B cells and single positive T cells, progenitor cells such as double negative T cells could also be affected by the absence of bcl-2. Second, it is not clear why lymphocytes develop normally during the first 1 to 2 weeks after birth. The majority of bcl-2 deficient mice die between 2 to 6 weeks after birth due to uremia caused by polycystic kidney disease. This makes it difficult to perform a long-term analysis of the lymphohematopoietic system in bcl-2−/− mice.
MATERIALS AND METHODS
Mice.The bcl-2 targeted mice13 were maintained by heterozygous matings in our animal facility. PCR screening was performed on the litters at 2 weeks of age by using two sets of primers in which the first primers were located in the neo gene and the second primers were located in the endogenous bcl-2 gene. First set, 5′-TGCTAAAGCGCATGCTCCAGACTG-3′ and 5′-ATTCGTTCTCTTTATACTACCAAGG-3′, second set, 5′-CGTCCCGCCTCTTCACCTTTCAGC-3′ and 5′-ATCCTCCCCCAGTTCACCCCATCC-3′. Genomic DNA (200 ng) was used as the template for the PCR (94°C for 30 seconds, 62°C for 30 seconds, 72°C for 1 minute and 30 seconds). C57BL/6J-Ly5.1:Pep3b (B6-Ly5.1) mice were maintained in our animal facility and used for these studies at between 4 and 6 weeks of age.
Antibodies.For cell sorting, biotinylated rat monoclonal antibodies (MoAb), RA-6B2 (anti-B220),16 M1/70 (anti-Mac-1),17 RA3-8C5 (anti-Gr-1),18 Gk1.5 (anti-L3T4),19 53-6.7 (anti-Ly 2),20 and TER11921 were used as lineage markers. Allophycocyanin (APC) conjugated ACK-2 (anti-c-kit)22 and phycoerythrin conjugated Sca-1 (anti-Ly 6A/E)23 were used as stem cell markers. B220, Mac-1, GK1.5 were purified by affinity chromatography on immobilized protein A and were derivatized with biotin by standard techniques.24 Ly 2 and Gr-1-biotin was purchased from Pharmingen (San Diego, CA). TER119-biotin was obtained from Dr Tatsuo Kina (University of Kyoto, Kyoto, Japan). Rat MoAb c-kit was obtained from Dr Shinichi Nishikawa (University of Kyoto), and conjugated with APC by standard method24 in our facility. Sca-1-PE was purchased from Pharmingen. All biotinylated reagents were visualized by using Streptavidin-Texas Red second step reagent (GIBCO-BRL, Tokyo, Japan).
For analysis of reconstituted cells, mouse MoAbs A20.1 (anti-Ly 5.1) obtained from Dr Yumiko Saga (RIKEN, Tsukuba, Japan) and 104.2 (anti-Ly 5.2) obtained from Dr Hidetaka Yakura (Tokyo Metropolitan Institute for Neuroscience, Tokyo, Japan) were derivatized with Texas Red or biotin, respectively. B220 and Ly 2 were labeled with APC in our facility. PE-labeled Gr-1 and GK1.5, and FITC-labeled TCRαβ (57H) were purchased from Pharmingen. Rat MoAb APC labeled Thy1.2 and PE labeled Mac-1 was purchased from Dainippon Seiyaku (Tokyo, Japan).
Cell preparation.Thymus glands were surgically excised and pressed through a 100-gauge stainless steel mesh. Bone marrow cells were flushed from femurs with staining medium (phosphate buffered saline with 3% fetal calf serum and 0.05% sodium aside). The suspensions were filtered through a 200-gauge nylon mesh to remove debris.
Staining with antibodies.Total bone marrow cells from two or three bcl-2 knockout mice or their littermates were stained with a cocktail of biotinylated rat MoAbs specific for mouse differentiation antigens Gr-1, Mac-1, B220, TER119, CD4, and CD8 (see above for antibody designations) for 20 minutes at 4°C. After washing the cells three times with staining medium, cells were treated with streptavidin conjugated magnetic beads (BioMag; PerSeptive Diagnostics, Cambridge, MA), for 10 minutes at 4°C to remove lineage marker high positive cells. Unreacted cells were collected and stained with c-kit-APC, Sca-1-PE and SAV-Texas Red at 4°C for another 20 minutes. After a second wash, the cells were resuspended in staining medium at a final concentration of 1 × 106 cells/mL supplemented with propidium iodide (PI, 1 μg/mL).
FACS cell sorting.Stained cells were analyzed by FACStarplus or FACS vantage (Becton Dickinson) equipped with a 488 nm argon laser and a 599 nm dye laser. Data from 50,000 cells was collected and analyzed. Computer-assisted data analysis of results was done on a MicroVAX computer (Digital Equipment Corp, Maynard, MA) with FACS/DESK software (version 5.5-2) made available through the FACS development group at Stanford University. After analysis, c-kit+ Sca-1+ Lin−/low cells were sorted. Residual erythrocytes, debris, doublets, and dead cells were excluded by forward scatter, side scatter, and PI gating. Fluorescence intensity of individual cells was measured as relative fluorescence units.
In vitro colony assay (CFU-C and HPP-CFC).Methylcellulose culturing was carried out using a modification of the published technique.25 One milliliter of culture medium contained an adequate number of sorted c-kit+ Sca-1+ Lin−/low bone marrow cells, 1.2% methylcellulose (Fisher Scientific, Norcross, GA), alpha-medium (Flow Laboratories, North Ryde, Australia), 30% fetal calf serum (Flow Laboratories), 1% deionized bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO), 0.1 mmol/L 2-mercaptoethanol (Eastman Organic Chemical, Rochester, NY), and IL-3 (100 U/mL) and Epo (1 U/mL). In the case of high proliferative potential colony forming cell (HPP-CFC) assay, IL-1 (2 ng/mL), IL-3 (10 ng/mL), M-CSF (10 U/mL), GM-CSF (10 ng/mL), and stem cell factor (SCF, 10 ng/mL) were added. The cultures were prepared in 35-mm non-tissue culture dishes (Falcon Labware, Oxnard, CA) and incubated at 37°C in a humidified atmosphere of 5% CO2 . The number of colonies was counted after 14 days of culture using an inverted microscope.
In vivo colony assay.A spleen colony assay was performed using a standard method.26 Ten to 14-week-old B6-Ly 5.2 female mice were lethally irradiated at a dose of 9.5 Gy total body irradiation. Sorted c-kit+ Sca-1+ Lin−/low bone marrow cells were injected into the irradiated mice intravenously via the retro-orbital plexus. After injection, the spleens were removed at day 12, fixed in Bouin's solution, and macroscopically visible spleen colonies were counted.
Long-term reconstitution assay.Two thousand sorted c-kit+ Sca-1+ Lin−/low cells from bcl-2 knockout or litter mate mice were injected into the irradiated B6-Ly 5.1 mice (9.5 Gy). After injection, peripheral blood cells were collected periodically and stained in four colors with donor-specific anti-Ly 5.2 (FITC) and recipient-specific anti-Ly 5.1 (Texas Red), PE conjugated anti-Gr-1 and Mac-1, and APC conjugated anti-B220 and Thy-1. Four color FACS analysis was performed on the FACStarplus as described above.
Intra-thymic injection.Intra-thymic injections of sorted c-kit+ Sca-1+ Lin−/low bone marrow cells were performed under nembutal anesthesia.27 Four to 5-week-old female B6-Ly 5.1 mice were irradiated (6.5 Gy) within 6 hours before surgery. Two thousand sorted c-kit+ Sca-1+ Lin−/low bone marrow cells from 4-week-old bcl-2 knockout or litter mate mice were injected into one thymic lobe of each recipient mouse in a volume of 5 μL. Mice were sacrificed by neck dislocation at 24 days after injection and a cell suspension was prepared from each thymic lobe. The suspensions were stained in four colors with anti-Ly 5.2 (FITC), anti-Ly5.1 (TR), anti-CD4 (PE), and anti-CD8 (APC). Four color FACS analysis was performed as described above.
PCR studies on TCRβ gene rearrangement.PCR analysis of TCRβ gene rearrangement was carried out as previously described.28 Briefly, 0.5 μg of genomic DNA prepared from indicated thymocytes were PCR-amplified by 5 U of EX Taq polymerase (Takara Biomedicals, Shiga, Japan) in the presence of oligonucleotide primers which are specific for Dμ-5′ and Jμ-3′ for detection of D-Jβ rearrangement or Vβ8.2-5 and Jμ-3′ for V-DJ rearrangement. The sequences are Vβ8.2-5 (5′-CRACCCCCTCTCAGACATCA-3′), Dμ-5′ (5′-ACAAGCTTCAAAGCACAATGCTGGCT-3′), and Jμ-3′ (5′-GGGTCTAGACTCTCAGCCGGCTCCCTCAGGGG-3′). Amplified DNA products were electrophoresed on 5% polyacrylamide gel, denatured in 0.4 mol/L NaOH, and were electrotransferred to Gene Screen Plus membranes (Dupont, Boston, MA). Membranes were hybridized with biotinylated probes, and hybridization was visualized by the Phototope chemiluminescence detection reagents (New England Biolab, Beverly, MA). Jβ2 primer was used for the detection of TCRβ gene rearrangements.29
RT-PCR studies on the expression of bcl-2.Total RNA was extracted from 2 × 104 sorted cells using an ISOGEN total RNA isolating kit (Wako, Tokyo, Japan). After Ethanol Precipitation, the RNA pellet was dissolved in 12 μL of DEPC-treated water. After addition of 1 μL of 0.5 ng/mL oligo-dT primer, the reaction was incubated at 70°C for 10 minutes, and placed on ice for 2 minutes. The following reagents were then added: 4 μL of 5× PCR reaction buffer (500 mmol/L KCL, 200 mmol/L Tris-HCl, 25 mmol/L MgCl2); 2 μL of 0.1 mol/L DTT, 1 μL of a 10-mmol/L mixture of all four deoxynucleotide triphosphates and 1 μL of M-MLV reverse transcriptase at 200 U/mL (GIBCO-BRL). The reaction was incubated at 42°C for 60 minutes and then 180 μL of TE buffer was added.
PCR reactions were performed in 50-μL volumes containing 10 μL of cDNA sample, 1× PCR buffer, 100 mmol/L of each of four deoxynucleotide triphosphates, 2 mmol/L of each sense and antisense primers, and 5 U of Taq polymerase (Takara). After an initial 5-minute incubation at 95°C, the 35 cycles of PCR reactions were carried out using the following conditions: denaturation at 95°C for 45 seconds, annealing at 60°C for 45 seconds, and polymerization at 72°C for 1 second. To verify that equal amounts of RNA were added to each PCR reaction, the “house-keeping gene” elongation factor 1 alpha (EF1α) was also amplified. The forward and reverse primers for bcl-2 were F(5′-GCTGGGGATGACTTCTCTCG-3′) and R(5′-CAGCCAGGAGAAATCASAACA-3′); for EF1α F(5′-CTGGCTTCACTGCTCAGGTG-3′) and R(5′-TAGCCTTCTGAGCTTTCTGG-3′).
RESULTS
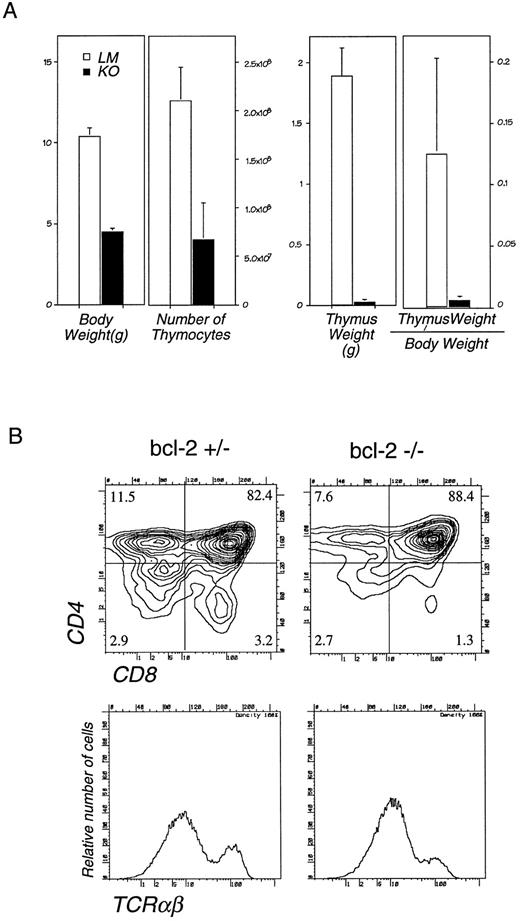
Lymphocyte development in bcl-2−/− mice.Analysis of lymphoid tissues from 4-week-old bcl-2−/− mice revealed a significant decrease in cell number both in the thymus and lymph nodes. In the thymus, cell number was decreased to , thymus weight to th, and the ratio of thymus weight/total body weight to th that of wild type mice (Fig 1A). Absolute number of cells in the c-Kit+Pgp-1+ fraction that contains earliest T precursors is 12.0 ± 7.4 × 104 in litter mate (LM) and 0.7 ± 0.6 × 104 in bcl-2−/− thymus. As reported previously,13 bcl-2+/+ mice and +/− mice were not significantly different from each other. In spite of greatly diminished thymocyte cell numbers, FACS analysis did not show a loss of any thymocyte subpopulations (Fig 1B). Thus, the lack of bcl-2 did not result in the blockage of lymphocyte development at certain differentiation steps. The decrease in thymocyte cell number may be explained by either the death of thymocytes due to apoptosis at all developmental stages or a decrease in the supply of progenitor cells from BM to the thymus.
(A) Thymic cellularity and weight in bcl-2–deficient mice. The bcl-2−/− mice (closed bars) and their LM (open bars) were killed at 4 weeks of age, and the total cell number in the thymus, total body weight, and the thymus weight were measured. The average and standard deviation of 12 bcl-2−/− and 15 LMs are shown. (B) Flow cytometric analysis of thymocytes from 4-week-old bcl-2−/− and +/− mice. Single cell suspension of thymocytes from a bcl-2+/− mouse (left) and a bcl-2−/− mouse (right) were stained with anti-CD4, CD8, and TCRαβ MoAb. Representative two-color immunofluorescence contour plots of CD4 and CD8 expression (upper) and histograms of TCRαβ expression (lower) are shown.
(A) Thymic cellularity and weight in bcl-2–deficient mice. The bcl-2−/− mice (closed bars) and their LM (open bars) were killed at 4 weeks of age, and the total cell number in the thymus, total body weight, and the thymus weight were measured. The average and standard deviation of 12 bcl-2−/− and 15 LMs are shown. (B) Flow cytometric analysis of thymocytes from 4-week-old bcl-2−/− and +/− mice. Single cell suspension of thymocytes from a bcl-2+/− mouse (left) and a bcl-2−/− mouse (right) were stained with anti-CD4, CD8, and TCRαβ MoAb. Representative two-color immunofluorescence contour plots of CD4 and CD8 expression (upper) and histograms of TCRαβ expression (lower) are shown.
The severity of polycystic kidney (PCK) disease varied in each bcl-2–deficient mouse. Most mice died at 2 to 6 weeks of age due to uremia, but some survived longer. Interestingly, the long lived mice tended to have mild uremia and a less significant decrease in peripheral lymphocyte number including thymocyte cell number. Consequently, we speculated that the decrease in lymphocyte cell number was not intrinsic to stem cells, but secondary to other changes in the environment such as uremia caused by PCK disease. To rule out this possibility, we attempted a stem cell transplantation from bcl-2−/− mice into irradiated normal mice.
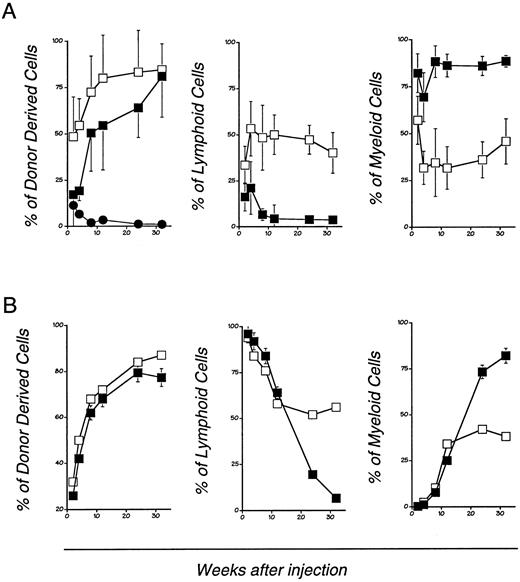
Long-term reconstitution ability of bcl-2 deficient hematopoietic stem cells.One thousand cells positive for c-kit and Sca-1 but negative for lineage markers (Lin−) obtained from bcl-2−/− or bcl-2+/− LM mouse BM were injected into irradiated C57BL/6-Ly5.1 recipient mice. After transplantation, peripheral blood was collected periodically and analyzed for donor-derived myeloid and lymphoid cells using antibodies specific for Ly5.1, Ly5.2, and other MoAbs. In the transplantation of LM-derived stem cells, successful lymphohematopoietic reconstitution was seen in all cases, whereas for the bcl-2−/− derived stem cells only 8 of 31 cases succeeded. However, when C57BL6 × 129F1 mouse was used as a recipient, in 6 of 6 cases, the graft was accepted (data not shown). Once engrafted, HSCs from bcl-2−/− mice maintained hematopoiesis for more than 32 weeks in all cases (Fig 2A). An in vitro colony formation assay did not reveal any significant defect in HSCs from the bcl-2−/− mice. On the other hand, CFU-S assay revealed that those from bcl-2−/− had significantly reduced CFU-S activity (Table 1). This may account for delayed and decreased chance of engraftment by the HSC derived from bcl-2−/− mice.
Long-term lymphohematopoietic reconstitution by bcl-2–deficient mouse BM stem cells (A) and fetal liver cells (B). Transplantation was performed as described in experimental procedures. Peripheral blood mononuclear cells from the recipient mice transplanted with bcl-2−/− (closed squares and circles) or +/− (open squares) mouse-derived HSC were analyzed at each experimental period. The cells were stained with donor type common leukocyte antigen specific MoAb (anti-Ly5.2), recipient specific (anti-Ly5.1) MoAb, Thy-1/B220 mixture and Gr-1/Mac-1 mixture. Four-color flow cytometry analysis was performed. The percentage of Thy-1/B220 positive cells or Gr-1/Mac-1 positive cells in donor-derived cells (Ly5.2 positive fraction) were calculated. Kinetics of donor derived cells (left), and the relative percentage of donor derived lymphoid cells (middle), and myeloid cells (right) are shown. Each data point represents mean ± SD from 16 individual experiments.
Long-term lymphohematopoietic reconstitution by bcl-2–deficient mouse BM stem cells (A) and fetal liver cells (B). Transplantation was performed as described in experimental procedures. Peripheral blood mononuclear cells from the recipient mice transplanted with bcl-2−/− (closed squares and circles) or +/− (open squares) mouse-derived HSC were analyzed at each experimental period. The cells were stained with donor type common leukocyte antigen specific MoAb (anti-Ly5.2), recipient specific (anti-Ly5.1) MoAb, Thy-1/B220 mixture and Gr-1/Mac-1 mixture. Four-color flow cytometry analysis was performed. The percentage of Thy-1/B220 positive cells or Gr-1/Mac-1 positive cells in donor-derived cells (Ly5.2 positive fraction) were calculated. Kinetics of donor derived cells (left), and the relative percentage of donor derived lymphoid cells (middle), and myeloid cells (right) are shown. Each data point represents mean ± SD from 16 individual experiments.
In Vivo and In Vitro Colony Forming Activity of bcl-2 Knockout Hematopoietic Stem Cells
| . | HPP-CFU* . | CFU-C† . | CFU-S‡ . |
|---|---|---|---|
| . | /100 Cells . | /100 Cells . | /1,000 Cells . |
| bcl-2−/− | 12.5 ± 1.5 | 16.2 ± 7.6 | 4.6 ± 1.3† |
| bcl-2+/− | 14.5 ± 2.5 | 19.0 ± 1.1 | 7.7 ± 1.7 |
| . | HPP-CFU* . | CFU-C† . | CFU-S‡ . |
|---|---|---|---|
| . | /100 Cells . | /100 Cells . | /1,000 Cells . |
| bcl-2−/− | 12.5 ± 1.5 | 16.2 ± 7.6 | 4.6 ± 1.3† |
| bcl-2+/− | 14.5 ± 2.5 | 19.0 ± 1.1 | 7.7 ± 1.7 |
The c-kit+ Sca-1+ Lin− cells from BM were sorted on the basis of staining analysis. In vitro colony assays were performed. Mean ± SD for n = 6.
Numbers of colonies in the presence of IL-1, IL-3, M-CSF, GM-CSF, and SCF(KL) at day 14.
Number of colonies in the presence of IL-3 and Epo at day 14.
Cells were injected into irradiated B6 mice intravenously and the number of spleen colonies were counted at day 12.
ρ P < .01.
In the recipients of LM HSC, donor-derived lymphoid and myeloid cells were present in an approximately equal ratio. On the other hand, in recipients of bcl-2−/− mouse-derived HSCs, myeloid cells predominated over lymphocytes (Fig 2A). The small number of lymphoid cells that were observed in the early posttransplantation period were B220 positive B lineage cells, but T cells were not seen (data not shown).
To compare the adult BM and fetal liver (FL)-derived HSC, we performed transplantation of day 14 FL cells. As shown in Fig 2B, FL-derived HSCs reconstituted the BM of irradiated normal mice just as LM-derived HSC. They differed from adult BM-derived HSCs, however, in that (1) a large number of lymphoid cells appeared in the first 10 weeks, which was then replaced by myeloid cells (Fig 2B); and (2) both T and B cells were present in the lymphoid fraction (data not shown).
Thus, HSCs from bcl-2−/− mice, either adult BM or FL-derived, supported the generation of nonlymphoid cells for up to 8 months. The generation of donor-derived T and B lymphocytes, however, was significantly impaired in the recipients. The lymphoid specific decrease in cell number in normal irradiated hosts clearly indicates that this defect is intrinsic to the hematopoietic cells of the bcl-2−/− mice and that expression of bcl-2 is required for the continuous supply of lymphoid cells.
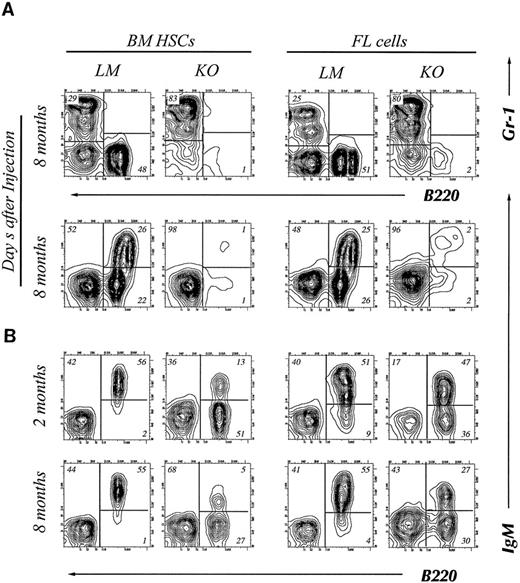
Bcl-2 is required for early B-cell differentiation.Eight months after transplantation, recipient mice were killed and B-cell development in the bone marrow was analyzed by FACS. In the recipients of bcl-2−/− BM HSC, Gr-1 positive myeloid cells predominated with B220 positive cells being significantly reduced. The recipients of LM HSC, on the other hand, showed equal numbers of myeloid and B-lineage cells (Fig 3A). Within the B220 positive population, we found small numbers of both IgM+ and IgM− cells (Fig 3A, left). Similar results were obtained from the recipients of day 14 FL cells (Fig 3A, right).
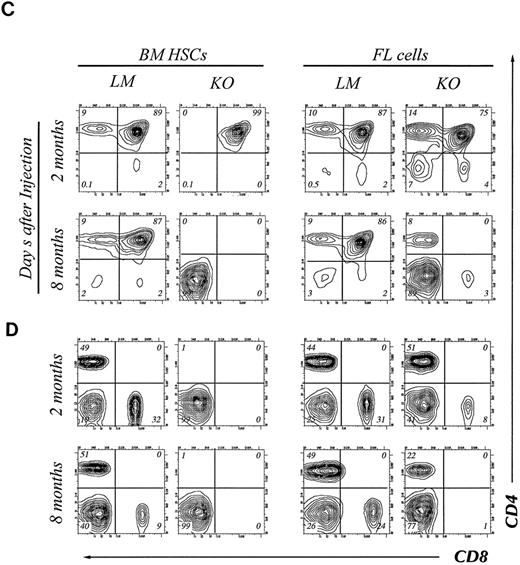
Four-color flow cytometry analysis of T and B lymphocyte subpopulations in the thymus, BM, and lymph node of chimeric mice. The mice were killed at 2 or 8 months after injection of HSCs. Gate used to define donor-derived cells were set according to the expression of Ly5.2 and Ly5.1. Results shown are representative of eight experiments. B cell subpopulations in BM (A) and lymph node (B) of chimeric mice. Single cell suspensions of BM cells were stained with donor-specific MoAb (anti-Ly5.2), B220, Gr-1, and IgM. The two-color contour plots of the expression of B220 and Gr-1 are shown (A, upper) and expression of B220 and IgM is shown (A, lower). Two color contour plots of B220 and IgM expression analyzed at 2 months (upper) and 8 months (lower) are shown in B. CD4 and CD8 expression on donor-derived cells in thymus (C) or lymph node (D). Two-color contour plots from analysis at 2 months after injection (upper) and 8 months after injection (lower) are shown.
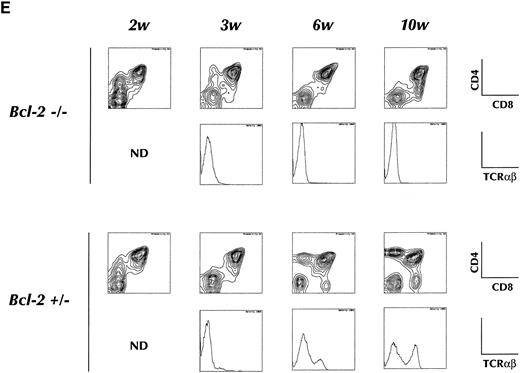
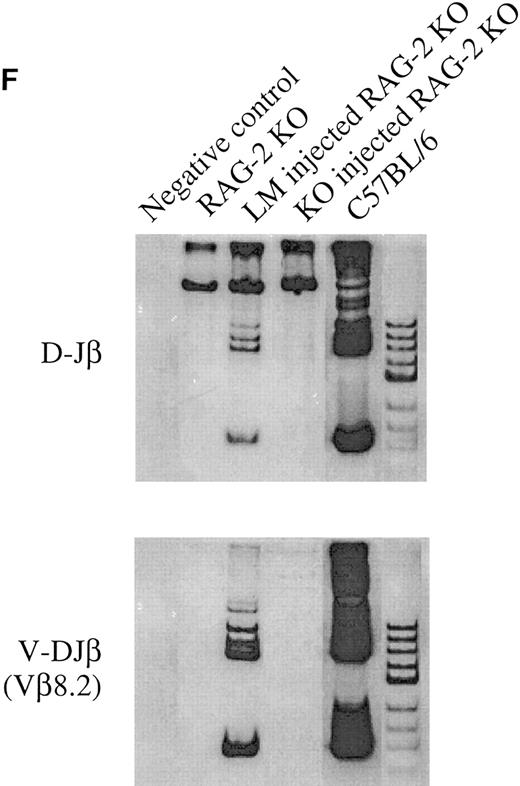
(E) Short-term T-cell development in the thymus of recipient animals. Purified HSCs from bcl-2−/− or +/− BM are injected into 6.5 Gy irradiated RAG-2 KO recipients, and analyzed for CD4, CD8, or TCRαβ expression on donor-derived cells in thymus at indicated periods after transplantation. Two-color contour plots of CD4 and CD8 expression (upper) or single color histogram of TCRαβ expression (lower) are shown. (F ) PCR analysis of D-Jβ and V-DJβ gene rearrangements in the thymocytes. Genomic DNA prepared from thymocytes of indicated recipient animals was subject to the gene rearrangement analysis using indicated PCR primers and probes, as describe in Materials and Methods. In all experiments measuring DNA rearrangements, equal amounts (0.5 μg/reaction) of genomic DNA were used for PCR amplification to minimize possible deviation among multiple PCR reactions. The sensitivity for every PCR detection was normalized by pre-titrating PCR cycle numbers. For the analysis of D-Jβ rearrangement, unrearranged DNA gives 1.8 kb signals corresponding to germline D-Jβ2, whereas the rearrangement gives six discrete signals corresponding to rearranged D-J genes using six Jβ2 segments. For the analysis of Vβ8.2-DJβ, unrearranged DNA gives no signals, whereas the rearrangement gives discrete signals corresponding to rearranged genes. Biotinylated Hinf I fragments of pX174 DNA (726-24 bp; GIBCO-BRL) were used as a molecular weight marker.
Four-color flow cytometry analysis of T and B lymphocyte subpopulations in the thymus, BM, and lymph node of chimeric mice. The mice were killed at 2 or 8 months after injection of HSCs. Gate used to define donor-derived cells were set according to the expression of Ly5.2 and Ly5.1. Results shown are representative of eight experiments. B cell subpopulations in BM (A) and lymph node (B) of chimeric mice. Single cell suspensions of BM cells were stained with donor-specific MoAb (anti-Ly5.2), B220, Gr-1, and IgM. The two-color contour plots of the expression of B220 and Gr-1 are shown (A, upper) and expression of B220 and IgM is shown (A, lower). Two color contour plots of B220 and IgM expression analyzed at 2 months (upper) and 8 months (lower) are shown in B. CD4 and CD8 expression on donor-derived cells in thymus (C) or lymph node (D). Two-color contour plots from analysis at 2 months after injection (upper) and 8 months after injection (lower) are shown.
(E) Short-term T-cell development in the thymus of recipient animals. Purified HSCs from bcl-2−/− or +/− BM are injected into 6.5 Gy irradiated RAG-2 KO recipients, and analyzed for CD4, CD8, or TCRαβ expression on donor-derived cells in thymus at indicated periods after transplantation. Two-color contour plots of CD4 and CD8 expression (upper) or single color histogram of TCRαβ expression (lower) are shown. (F ) PCR analysis of D-Jβ and V-DJβ gene rearrangements in the thymocytes. Genomic DNA prepared from thymocytes of indicated recipient animals was subject to the gene rearrangement analysis using indicated PCR primers and probes, as describe in Materials and Methods. In all experiments measuring DNA rearrangements, equal amounts (0.5 μg/reaction) of genomic DNA were used for PCR amplification to minimize possible deviation among multiple PCR reactions. The sensitivity for every PCR detection was normalized by pre-titrating PCR cycle numbers. For the analysis of D-Jβ rearrangement, unrearranged DNA gives 1.8 kb signals corresponding to germline D-Jβ2, whereas the rearrangement gives six discrete signals corresponding to rearranged D-J genes using six Jβ2 segments. For the analysis of Vβ8.2-DJβ, unrearranged DNA gives no signals, whereas the rearrangement gives discrete signals corresponding to rearranged genes. Biotinylated Hinf I fragments of pX174 DNA (726-24 bp; GIBCO-BRL) were used as a molecular weight marker.
Interestingly, although B220 positive cells were barely detectable in BM at 8 month posttransplantation, B-lineage cells were present in peripheral lymph nodes (Fig 3B). In contrast to B220+ cells derived from LM HSC, many of those derived from bcl-2−/− HSC were B220 positive but IgM negative. These B220+ IgM-cells in the peripheral lymph node may represent immature B cells, NK cells, or surviving plasma cells.
A significant decrease in B-lymphoid cells in the BM and the presence of B lymphocytes in the peripheral lymphoid organs may suggest that bcl-2 expression is more important for early B-cell development than for the survival of peripheral B lymphocytes.
T-cell differentiation in the thymus.Although transplantation of BM HSC from bcl-2−/− mice generated a small number of B cells throughout the experimental period, T cells were barely detectable. We therefore analyzed carefully T-cell differentiation in the thymus. As shown in Fig 3C, transplantation of HSC from adult bcl-2−/− mouse BM resulted in the generation of CD4CD8 double positive cells but not of single positive cells at 2 months after transplantation. These double positive cells do not express detectable amounts of TCR up to 3 weeks after transplantation of either bcl-2−/− or bcl-2+/− HSC. Normal T-cell development was then observed in the recipient of bcl-2+/− HSC but not in those of bcl-2−/− HSC (Fig 3E). Indeed, PCR analysis revealed a total absence of TCR β-chain gene rearrangement (Fig 3F ). The emergence of such double positive cells after irradiation has been reported elsewhere.30 31 In contrast, injection of day 14 FL cells resulted in normal thymocyte differentiation at 2 months after transplantation (Fig 3C, right). In the peripheral lymph nodes, mature T cells were found in the recipients of bcl-2−/−FL cells but not of adult BM HSC.
At 8 months, the total thymocyte cell number in the recipient of KO HSC was reduced to (LM, 2.1 ± 0.7 × 108; KO, 1.5 ± 0.3 × 106) leaving predominantly double negative cells in both adult and fetal HSCs transplanted animals (Fig 3C). These double negative cells were Mac-1+ non T lineage cells (data not shown). Thus, adult bcl-2−/− BM HSC are not capable of supporting T lymphopoiesis, but, those from fetal liver can for at least the first 2 to 3 months. In the recipients of FL cells, some CD8 positive T cells were detected 2 months after transplantation in the lymph node. However, by 8 months, CD8+ cells were no longer detectable. These data support the notion that CD8 positive T cells are more dependent on bcl-2 expression for their survival in the periphery.13 FACS analysis of the CD4+ T cells remaining in lymph nodes revealed Pgp-1hi Mel-14−, so-called memory phenotype.
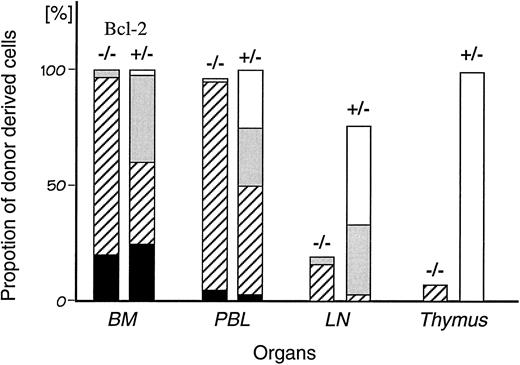
Intrathymic injection of bcl-2 deficient hematopoietic stem cells.In contrast to the high level of chimerism (>90%) in bone marrow and peripheral blood, less than 20% of cells in lymph nodes and thymus were donor bcl-2−/− HSC derived (Fig 4). Because myeloid cells in BM and peripheral blood were supplied normally over an 8-month period, transplanted HSCs were present and functioning. However, T-cell differentiation was not detectable in the recipient's thymus. Therefore, it was likely the supply of T progenitor cells from BM was defective in bcl-2−/− HSC recipients. In order to test this hypothesis, we attempted to bypass this step in the pathway by directly injecting HSCs into the recipient's thymic lobe.
Comparison of the chimerism in individual hemato/lymphoid organs of the recipient chimeric mice. The percentage of donor-derived cells (Ly5.2 positive cells) at 8 months after transplantation in each organ was analyzed by flow cytometry. The chimerism in BM was defined as 100. Percent chimerism was calculated as (percentage of the population in each organ)/(percentage of the population in BM). The average proportion of each cell lineage in individual organs is shown as T lymphocytes (□), B lymphocytes (), myeloid (▨), others (▪). Experiments were performed using seven bcl-2−/− chimeric mice and four control chimeric mice from which the means were calculated.
Comparison of the chimerism in individual hemato/lymphoid organs of the recipient chimeric mice. The percentage of donor-derived cells (Ly5.2 positive cells) at 8 months after transplantation in each organ was analyzed by flow cytometry. The chimerism in BM was defined as 100. Percent chimerism was calculated as (percentage of the population in each organ)/(percentage of the population in BM). The average proportion of each cell lineage in individual organs is shown as T lymphocytes (□), B lymphocytes (), myeloid (▨), others (▪). Experiments were performed using seven bcl-2−/− chimeric mice and four control chimeric mice from which the means were calculated.
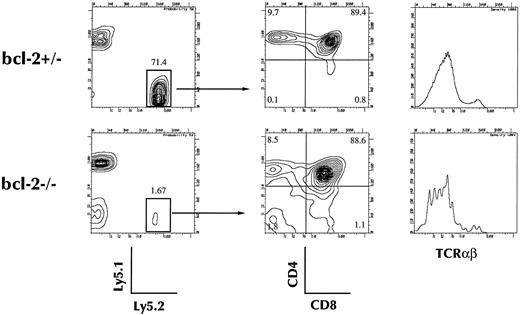
Four weeks after injection of 2 × 103 of c-Kit+ Sca-1+ Lin− cells into the thymic lobes of a sublethally irradiated Ly5.1 congenic mouse, 4-color FACS analysis was performed. As shown in Fig 5, the thymus repopulating ability of the bcl-2−/− HSC was significantly lower than that of LM HSC. Although the number of donor-derived thymocytes was small (LM, 8.4 ± 5.1 × 106; KO, 0.6 ± 0.5 × 106), they showed a normal pattern of thymocyte differentiation as evidenced by CD4, CD8, and TCR expression. Thus, the major reason for the absence of thymocyte differentiation in bcl-2−/− HSC recipients appears to be in the migration process.
Thymocyte reconstitution by bcl-2–deficient BM stem cells after intrathymic injection. Twenty-one days after intrathymic injection, mice were killed and single cell suspensions were prepared from thymuses. Cells were analyzed for the expression of Ly5.1 and Ly5.2, CD4, and CD8, or Ly5.2, CD4, CD8, and TCRαβ. Expression of CD4 and CD8 on donor-derived (Ly5.2+, Ly5.1− cells) and TCRαβ expression in chimeric mice of bcl-2−/− HSCs or bcl-2+/− control chimeric mice are shown. Results shown are representative of five separate experiments.
Thymocyte reconstitution by bcl-2–deficient BM stem cells after intrathymic injection. Twenty-one days after intrathymic injection, mice were killed and single cell suspensions were prepared from thymuses. Cells were analyzed for the expression of Ly5.1 and Ly5.2, CD4, and CD8, or Ly5.2, CD4, CD8, and TCRαβ. Expression of CD4 and CD8 on donor-derived (Ly5.2+, Ly5.1− cells) and TCRαβ expression in chimeric mice of bcl-2−/− HSCs or bcl-2+/− control chimeric mice are shown. Results shown are representative of five separate experiments.
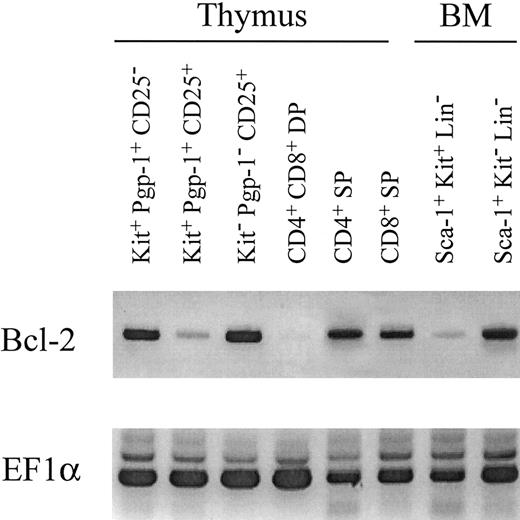
Expression of bcl-2 in normal lympho-hemopoietic cells.The above data show that HSCs from bcl-2−/− mice can reconstitute BM with the exception of the lymphoid compartments. Both T and B lymphopoiesis were severely disturbed at a very early progenitor cell level. We therefore analyzed bcl-2 expression at various stages of differentiation from HSCs to T lymphocytes by RT-PCR (Fig 6). Sca-1+ c-kit+ Lin− BM HSCs expressed only a trace amount of bcl-2, whereas c-kit+ Pgp-1+ CD25− Lin−, the common lymphoid precursor cells,32 were found to express a large amount of bcl-2. As reported previously, expression of bcl-2 is decreased at the double positive stage but upregulated in single positive thymocytes. Interestingly, bcl-2 was highly expressed in Sca-1+ c-kit− Lin− cells in BM. The cells in this fraction formed neither myeloid nor erythroid colonies. We are currently in the process of characterizing the cells in this fraction.
Expression of bcl-2, EF1α was examined by RT-PCR in total cellular RNA isolated from various cell fractions in thymus and BM of C57BL/6 mice. Four-color combinations of reagents specific for c-Kit, CD44, CD25, and CD4+CD8 mixture were used to discriminate three fractions in CD4/CD8 double negative thymocytes. In BM, three-color combinations with c-Kit, Sca-1, and a lineage mixture were used to discriminate two fractions associated with Kit and Sca-1 expression in Lin-cells. Similar results were obtained in three other experiments.
Expression of bcl-2, EF1α was examined by RT-PCR in total cellular RNA isolated from various cell fractions in thymus and BM of C57BL/6 mice. Four-color combinations of reagents specific for c-Kit, CD44, CD25, and CD4+CD8 mixture were used to discriminate three fractions in CD4/CD8 double negative thymocytes. In BM, three-color combinations with c-Kit, Sca-1, and a lineage mixture were used to discriminate two fractions associated with Kit and Sca-1 expression in Lin-cells. Similar results were obtained in three other experiments.
DISCUSSION
Adult and fetal HSC are different in bcl-2 dependency.We have previously reported that lymphoid cells develop normally for 1 to 2 weeks after birth in bcl-2−/− mice.13 In this report, however, we describe the absence of T lymphopoiesis and significantly retarded B lymphopoiesis after transplantation of bcl-2−/− BM HSC into normal mice. This discrepancy may be explained by a difference in the requirement of bcl-2 between embryonal and adult HSC for development of lymphoid cells. An alternative explanation is the difference in the embryonal and adult microenvironment. It is known that the serum steroid level is low in the embryo, but increases rapidly after birth. Lymphoid progenitor cells may die by apoptosis in the presence of a high concentration of steroid in the absence of bcl-2. To clarify this issue, we transplanted bcl-2−/− FL cells into lethally irradiated adult mice. Donor-derived T lymphocytes developed normally for at least the first 2 months, suggesting that lymphopoiesis from FL HSC is less dependent on bcl-2 than that from adult BM HSCs.
There are several possibilities to account for age associated change in bcl-2 dependency. First, fetal lymphoid progenitor cells may use a molecule(s) other than bcl-2 for their survival. Bcl-x, for example, is one possible candidate. Second, as demonstrated in other systems, HSCs themselves may change as they age.21 Such a change could be brought about by the accumulation of various substances as cells age that render HSC or progenitor cells more susceptible to apoptosis.33 Third, lymphoid progenitors in day 14 FL may take a shorter time to generate mature T and B lymphocytes compared with the adult BM HSC (our unpublished observation). The bcl-2 dependent time period of FL HSCs may also be shorter, and because of this, the lymphoid progenitor cells may have a better chance of survival.
Role of bcl-2 in early lymphoid progenitors.We, and others, have previously shown that the most immature cells in the thymus are capable of differentiating into T, B, NK, and dendritic cells but not myeloid or erythroid cells.34-36 It is of note that although development of both T and B cells was affected by the lack of bcl-2, its effect was more profound on T rather than B lineage cells. Furthermore, the results of the intrathymic injection experiments indicate that the development of T cells is disturbed at a pre-thymus level. These data suggest that lack of bcl-2 impedes lymphopoiesis at a very early stage common to both T and B lineages. This notion is further supported by RT-PCR studies that show higher expression of bcl-2 in committed progenitors than in primitive BM HSCs.
As a small number of thymocytes developed after direct injection of HSCs in the thymic lobes, these HSCs do have the potential to differentiate into T cells albeit at low efficiency. It is therefore assumed that the absolute number of common lymphoid progenitor cells is decreased in the thymus of these mice. These common lymphoid progenitor cells may die of apoptosis before they reach the thymus, while B progenitors stay in the BM and some of them differentiate into mature B cells. This difference in the requirement for migration may have resulted in the observed difference in T and B lymphopoiesis.
Recently, it was reported that expression of bcl-2 not only suppresses apoptosis, but induces cell proliferation.37 As reported for erythroid cells,38 the supply of lymphocytes may be regulated, at least in part, at the common lymphoid progenitor level by way of cytokine dependent apoptosis. An increase of lymphoid progenitor cells in BM should increase the chance of those cells reaching the thymus for maintenance of T lymphopoiesis. Based on these data, we believe that the supply of progenitor cells to the thymus is mediated by lymphoid committed progenitor cells but not by the HSCs themselves.
Generation of abberant CD4+CD8+TCR− T cells.We observed appearance of CD4+CD8+TCR− T cells in the recipients of both bcl-2+/− and −/− HSC shortly after transplantation. The difference is that in normal mice, these cells are eventually replaced by normally developing DP TCRdull thymcytes, whereas in SCID or bcl-2−/− mice, they remain in the thymus for several months. Appearance of such abberant DP cells have been reported in SCID or RAG KO studies.30 31 It is not yet clear how CD4+CD8+TCR negative cells are generated after irradiation. Radiation to T cell progenitors may somehow stimulate them to express CD4 and CD8 independent of TCR-mediated signals. However, this is clearly not the case, since nonirradiated T-cell progenitors can become double positive in the irradiated host as shown in this study. Irradiation cause changes in cytokine/hormone production in the thymic or extrathymic environment that may in turn render T progenitors express CD4CD8 by as yet unknown mechanisms.
Are memory T cells bcl-2 independent?In the recipients of FL cells, we found CD4+ T cells in the lymph node even 8 months after transplantation. Thus, these T cells may have survived because they were stimulated through their T-cell receptors, or they may be memory T cells. There have been reports showing that antigen stimulation of peripheral T cells generates two subgroups. In one group, there is an increase of bcl-2 and the cells survive and turn into memory cells, while cells in the other group fail to increase bcl-2 expression and consequently die of apoptosis.39,40 On the other hand, it is known that CD4+ cells are relatively resistant to apoptosis even without bcl-2. Moreover, it was shown that antigen stimulation further rendered the cells from bcl-2−/− mice even more resistant to cell death.11 Thus, increased bcl-2 expression may not necessarily indicate differentiation into memory T cells.
Susceptibility of bcl-2−/− HSC to allograft rejection. During the transplantation experiments, we noticed that HSCs from bcl-2−/− mice had a significantly lower probability of successful engraftment (25.8% in bcl-2−/− and 100% in LM HSC). Due to the difficulty in obtaining large numbers of bcl-2−/− mice, we prepared HSCs from a donor mice singly. Two interesting features were found. First, the engraftment was either as good as that of LM controls or a complete graft failure. Second, the probability of engraftment varied from donor to donor. These features lead us to assume that this resulted from immunologic rejection due to differences in genetic background. As our bcl-2−/− mice had a mixed genetic background of C57BL/6 and 129 mouse strains, the transplantation of HSCs from these mice into C57BL/6 may have been an allogenic transplantation. Compared with LM, bcl-2−/− mouse derived HSCs appeared to be more susceptible to immunologic rejection. In fact, when these HSC were transplanted into C57BL/6x129 F1 recipients, successful engraftment was observed in all cases. These data may indicate that bcl-2 expressed in BM HSCs has a role in resisting attacks by host cytotoxic T cells or NK cells.
ACKNOWLEDGMENT
We thank Tomoko Toyoshima for FACS operation, Michie Ito for secretarial assistance, Cathy Tarlinton for reading the manuscript, Dr Yosuke Takahama for helpful discussion, and Shoko Tsuda for TCR rearrangement study.
Supported by grants from Fujisawa Pharmaceutical Co, Ltd, The Ministry of Education, Science and Culture, and Yokohama Foundation for Advancement of Medical Science. Y.M. is supported by research fellowship of the Japan Society for the Promotion of Science for Young Scientists.
Address reprint requests to Hiromitsu Nakauchi, MD, Institute of Basic Medical Sciences and Center for TARA, University of Tsukuba, Tsukuba Science-city, Ibaraki 305, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal