Abstract
The p47phox−/− mouse exhibits a phenotype similar to that of human chronic granulomatous disease (CGD) and, thus, is an excellent model for the study of gene transfer technology. Using the Moloney murine leukemia virus–based retroviral vector MFG-S encoding the human form of p47phox, we performed ex vivo gene transfer into Sca-1+ p47phox−/− marrow progenitor cells without conditioning of donors with 5-fluorouracil. Transduced progenitors were transplanted into moderately irradiated (500 cGy), G-CSF preconditioned sibling p47phox−/− mice. Using the fluorescent probe dihydrorhodamine 123 (DHR), in vivo biochemical correction of the superoxide-generating NADPH oxidase system was detected by flow cytometry in 12.3% ± 0.9% of phorbol myristate acetate–stimulated peripheral blood neutrophils at 4 weeks and 2.6% ± 1.0% at 14 weeks after transplantation. Following gene therapy, mice were challenged with the CGD pathogen Burkholderia (formerly Pseudomonas) cepacia and bacteremia levels were assessed at 24 hours and 7 days after inoculation. At both time points, bacteremia levels in gene corrected p47phox−/− mice were significantly lower than untreated p47phox−/− mice (0.89 ± 0.30 colonies v 237.7 ± 83.6 colonies at 24 hours, P < .02; 4.0 ± 2.0 colonies v 110.2 ± 26.5 colonies at 7 days, P < .0014). More importantly, Kaplan-Meier survival analysis showed a significant survival advantage of gene corrected versus untreated p47phox−/− mice (P < .001). Thus, stem-cell–directed ex vivo gene therapy is capable of restoring phagocyte oxidant-dependent host-defense function in this mouse model of a human immune-system disorder.
CHRONIC GRANULOMATOUS disease (CGD) is an inherited disorder characterized by recurrent life-threatening infections and granuloma formation which affects about five people per million.1,2 The biochemical basis for CGD is a defect in the phagocyte nicotinamide dinucleotide phosphate (NADPH) oxidase, the enzyme complex responsible for producing superoxide (O−⋅2), which in turn supplies microbicidal oxidants determined to be critical for host defense against bacterial and fungal pathogens. The NADPH oxidase is dormant in phagocytes (neutrophils, eosinophils, and monocyte/macrophages) until stimulation with pathogens or any of a group of endogenous mediators of inflammation.3,4 Activation of the NADPH oxidase involves translocation to the phagosome membrane of at least three cytosolic proteins, p47phox (“ph”agocyte “ox”idase), p67phox, and rac2 (a rho-related GTPase).3-6 At the cytoplasmic membrane surface these cytoplasmic oxidase subunits interact with the transmembrane flavocytochrome b558 , composed of gp91phox and p22phox subunits, to form the enzymatically active NADPH oxidase.7 Four genotypes of CGD have been described corresponding to mutations in the genes encoding gp91phox, p47phox, p67phox, and p22phox.8 9
Although prophylactic antibiotics and prophylactic interferon-γ have reduced the frequency of infection, they do not cure the disorder, and the overall mortality is about 2% per year. Allogeneic bone marrow transplantation (BMT) can cure CGD, but this mode of therapy has been used rarely because of the difficulties in finding proper matches and because of the mortality and morbidity associated with this treatment.10-13 However, it shows that hematopoietic progenitor (HPCs) cells are an appropriate target tissue for gene therapy of CGD. The level of correction from gene therapy that would be required for clinical benefit in CGD has not been established. Some estimates have been extrapolated from the observations regarding female carriers of the gp91phox-deficient (X-linked) form of CGD.14 Because extreme skewing of X chromosome gene inactivation (lyonization) can occur, some X-linked female CGD carriers have been identified in whom less than 5% of circulating neutrophils are the normal oxidase positive. Generally, those carrier females with at least 3% normal neutrophils do not appear to have an increased risk of infection (unpublished observations, September 1994), suggesting that clinical benefit from gene therapy of CGD might occur at significantly less than full correction of the defect.
In an ongoing human phase I clinical study of gene therapy of the p47phox-deficient form of CGD, autologous CD34+ HPCs purified from peripheral blood (PB) were transduced ex vivo with retrovirus containing the normal p47phox cDNA and reinfused into patients without any radiation or cytotoxic drug conditioning to enhance engraftment. Preliminary results reported in abstract form indicate that there was a very low-level engraftment of corrected progenitors leading to the appearance of 1 per 2,000 to 1 per 50,000 corrected neutrophils in PB for several months.15 Although encouraging, this very low level of engraftment suggests that conditioning of the recipient might be necessary to achieve a biologically meaningful level of engraftment of gene-corrected cells in humans. Success of this approach in humans might also require infusion of larger numbers of early progenitor cells from PB or marrow and also might require improved methods or conditions for efficient introduction of the therapeutic gene into primitive hematopoietic stem cells.
Recently, we reported development of a p47phox−/− CGD mouse that has a phenotype identical to its human counterpart, including increased susceptibility to spontaneous lethal infections.16 In the present study we use this CGD mouse model to study retrovirus-mediated gene therapy targeting hematopoietic stem cells to address some of the issues raised by the preliminary results from the clinical trial. In contrast to our human trial, HPCs were derived from nonprimed BM rather than mobilized PB, ∼30 times more HPCs were infused relative to body size, and the recipient mice received a nonablative, moderate dose of radiation (500 cGy) as conditioning before transplantation. Although transplantation kinetics in mice likely are not identical to humans, the goal was to determine if conditions of gene therapy that might be feasible in humans could lead to biologically meaningful correction of the genetic defect in CGD mice. Using these conditions, gene therapy in p47phox−/− CGD mice resulted in long-term correction of NADPH oxidase function in a percentage of neutrophils sufficient to restore NADPH oxidase-dependent host-defense function.
MATERIALS AND METHODS
Retroviral vector and producer cell line.The open reading frame of human p47phox cDNA was inserted into the Nco I cloning site of MFG-S (vector backbone provided by Somatix Therapy Corp) as described previously.17 Plasmid DNA containing the MFG-S p47phox recombinant retroviral vector was introduced by transfection18 into the ecotropic producer line Ψ-cre19 and high-titer clones were selected for use in this study.
Sca-1+ cell purification.BM from sibling p47phox−/− mice were obtained by flushing tibias and femurs. The donors were not pretreated with 5-fluorouracil (5-FU) or any other agent before harvest. Purification of the Sca-1+ cells was performed on the pooled marrow as described previously.20 This purification was carried out to increase multiplicity of infection and thereby enhance transduction efficiency. Others have shown that the Sca-1+ population contains significant HPC activity as measured by radioprotection and long-term multi-lineage repopulation.21 The Sca-1+ cells were placed in the serum-free media X-Vivo 10 (BioWhitaker, Walkersville, MD), with 1% human serum albumin containing the growth factors recombinant murine interleukin-3 (rmIL-3) (20 ng/mL; R&D Systems, Minneapolis, MN), rmIL-6 (10 ng/mL; R&D), rm stem cell factor (rmSCF ) (100 ng/mL; R&D), and recombinant human granulocyte colony-stimulating factor (rhG-CSF ) (10 ng/mL; R&D). The cell culture was then placed in a humidified incubator overnight.
Animals.p47phox−/− mice were produced as previously reported.16 Both mutant and wild type (WT) were 129/Sv × C57BL/6 incrossed. All animals were fed autoclaved Rat & Mouse Ration formula (Zeigler Bros, Gardners, PA) and housed in autoclaved cages under specific pathogen-free conditions. In addition, all p47phox−/− mice were maintained on Bactrim prophylaxis (30 mg/kg), except during and after bacterial challenge studies (see below).
Gene transfer.MFG-S p47phox-containing supernatant was obtained after overlaying serum-free media onto confluent Ψ-cre producer cells for 6 hours the day before each set of transductions. To remove any contaminating producer cells, the supernatant was centrifuged at 1,000 rpm for 5 minutes and decanted into a fresh tube. The retroviral supernatant was then stored at 4°C for no more than 24 hours before use. Cells underwent 3 consecutive days of transductions beginning the day after BM harvest. For each, retroviral supernatant was diluted to 50%, growth factors added as described above with protamine (6 μg/mL; BIOFLUIDS, Rockville, MD), and then admixed with the Sca-1+ cells (0.1 × 106 cells/mL of retroviral supernatant) and placed into 6-well plates. Plates were then centrifuged at 2,500 rpm (1,200g) for 1 hour at 32°C.22 After centrifugation, the retroviral supernatant and nonadherent cells were removed from the plates and spun down at 1,500 rpm for 5 minutes. The retroviral supernatant was then decanted and nonadherent cells resuspended in fresh retroviral supernatant and placed back into the 6-well plates. A second 1-hour spin transduction was then performed as described above. After the second spin transduction, the plates were placed in a humidified incubator for 5 hours. At this point, the retroviral supernatant was removed as described above and nonadherent cells were resuspended in fresh media with growth factors and placed back into the 6-well plates. Cells were then incubated overnight. After the third day of transductions, the Sca-1+ cells were washed once in X-Vivo 10, counted, resuspended in phosphate-buffered saline (PBS) 0.1% bovine serum albumin, and loaded into syringes in preparation for transplantation (500 μL per mouse). Some cells were also maintained in liquid culture or plated in 0.18% agarose for in vitro analysis of oxidase activity. Cell viability was determined by Trypan Blue exclusion.
Sca-1+ cell transplant.Nine recipient p47phox−/− mice (two separate experiments of four and five mice, respectively) were preconditioned with twice daily rhG-CSF (4 μg/20 g mouse) subcutaneously (SC) for 4 days before transplant as described previously.20 On the fifth day, mice received a single SC injection of rhG-CSF followed by 500 cGy total body irradiation (TBI) 1 hour later. Four hours after TBI, recipients were transplanted with 2.5 × 106 transduced Sca-1+ donor cells by tail-vein injection. Nongene-therapy–treated control p47phox−/− mice and WT control mice were not irradiated.
Biochemical analyses of NADPH oxidase activity.Transduced p47phox−/−, control naive p47phox−/−, and WT Sca-1+ progenitor cells were differentiated in suspension culture and assayed for O−⋅2 production at 10 and 14 days using a luminol-enhanced chemiluminescence assay of O−⋅2 production.23 24 Briefly, aliqouts of 100,000 cells were placed in 100 μL of saline in triplicate wells of a 96-well flat-bottom, white plate (Labsystems, Helsinki, Finland) to which was added 100 μL of Diogenes luminol solution (National Diagnostics, Atlanta, GA) containing phorbol myristate acetate (PMA) at 5 μg/mL. Chemiluminescence was read in every well for 0.5 seconds, every minute for 40 minutes, in a Luminoskan luminometer (Labsystems). This was sufficient time in each case to reach a peak rate of photon emission. Total photon emission over this period was taken as the measure of O−⋅2 output.
Nitroblue tetrazolium (NBT) dye (Sigma, St Louis, MO) reduction to formazan precipitate was used as a measure of O−⋅2 production by granulocyte monocyte (GM) and granulocyte erythrocyte monocyte megakaryocyte (GEMM) colony-forming unit (CFU) after gene transfer in agarose at 10 days of culture, as described previously.14 Briefly, 1 mL of PBS containing 0.1% NBT and PMA at 1 μg/mL was layered over the culture. After 1 hour, cells were fixed by adding 1.5% paraformaldehyde for 5 minutes and colonies scored for NBT reduction using an inverted microscope. Naive nontransduced p47phox−/− and WT Sca-1+ clonogenic progenitors were used as negative and positive controls, respectively. In vivo analysis of NADPH oxidase activity was performed by flow cytometric analysis of PB neutrophils using the dihydrorhodamine 123 (DHR) assay as described previously.25 Briefly, gene-corrected p47phox−/−, untreated control p47phox−/−, and WT mice were bled by tail venisection. Two hundred microliters of whole blood was placed in polypropylene tubes and lysed with prewarmed ammonium chloride lysis buffer (pH 8.0). Cells were then washed once with and then resuspended in 400 μL of Hanks' Buffered Saline Solution (HBSS, without Ca, Mg, or phenol red), 0.5 g albumin (human fraction V), and 1 mL of 0.5 mol/L EDTA (pH 8.0). 1.8 μl of 29 mmol/L DHR and 5 μL of catalase (1,400 U/μL) was added to each tube, which were then incubated for 5 minutes in a 37°C shaking water bath. After 5 minutes, 100 μL of 3.2 × 103 nmol/L PMA was added to each reaction tube and the tubes were returned to the water bath for an additional 14 minutes. After incubation, all samples were immediately analyzed by flow cytometry using a FACSort (Becton Dickinson Immunocytometry System [BDIS], San Jose, CA) with CellQuest software (BDIS). Neutrophils were identified based on forward and side scatter characteristics.26 However, with mouse blood it was not possible to establish a gate including most neutrophils that completely excluded lymphocytes. For this reason, the data for experimental p47phox−/− mice are adjusted to reflect the results with WT mice. Each sample was run in the setup mode until a neutrophil acquisition gate was established, at which point only events in this gate were acquired. At least 10,000 events were collected in this gate in all studies. Analysis of neutrophil DHR fluorescence was performed by constructing a side scatter/FL2 dot plot and DHR-positive cells were identified by gating based on negative (untreated p47phox−/−) and positive (WT) control samples. The experimental mice were bled and evaluated 1 week before transplantation (baseline analysis), 1 month after transplantation, and every 2 weeks thereafter.
Burkholderia cepacia infection.At 12 to 14 weeks posttransplantation, experimental gene-corrected p47phox−/− mice were challenged along with age-matched control p47phox−/− and WT mice with an intraperitoneal (ip) dose previously demonstrated to kill 100% of p47phox−/− mice and not kill WT mice (105 CFU/mouse; unpublished observations, March 1996). The challenge was performed at 14 weeks (Exp. 1) and 12 weeks (Exp. 2) after gene therapy. Bactrim prophylaxis was discontinued in both experimental and control p47phox−/− mice 1 week before bacterial challenge. Tail venisection to determine bacteremia was performed 24 hours and 1 week after injection with B cepacia. Blood was diluted in sterile water at 1:10 to lyse blood cells; further 10-fold serial dilutions of the lysed blood were plated in semi-soft agar (Becton Dickinson, Cockeysville, MD) and colonies enumerated 48 hours after incubation at 37°C. Data are expressed as number of colonies per 10 μL of undiluted blood (the amount of undiluted blood in the diluted sample used per plate unless colonies was too numerous to count in the 10 μL plates, in which case the plates containing 1 μL of blood were used for scoring and that number was multiplied by 10). Despite extensive cleansing of the tail site of venisection, when unchallenged WT mice were used to assess background bacterial contamination, an average of 4.5 ± 2.1 colonies was seen in plates to which 10 μL of blood was added. As will be shown in the Results section, bacteremia in challenged control p47phox−/− mice averaged more than 100 colonies per 10 μL of blood plated in all experiments, an amount that is over 20-fold higher than background contamination.
Statistical analysis.Estimates of survival were calculated by the Wilcoxon scores (rank sum) for the variable survival with a Bonferroni correction (SAS System, Cary, NC) and by the product-limit (Kaplan-Meier) method for right-censored data (JMP, version 3.1; SAS Institute, Cary, NC). For bacteremia data, differences between groups were determined by Student's t-test comparing the control p47phox−/− mice with the experimental gene-corrected p47phox−/− mice.
RESULTS
Transplantation of genetically modified p47phox−/− Sca-1+ progenitors.In two separate experiments, Sca-1+ hematopoietic progenitor cells21,27-29 were purified from the BM of adult p47phox−/− mice and cultured in serum-free media with growth factors to induce cell cycling and proliferation. During the first 3 days of ex vivo culture, the cells were transduced with ecotropic envelope packaged MFG-S retroviral vector carrying the human p47phox cDNA under the control of the Moloney murine leukemia virus LTR promotor.17 Correction of the enzymatic defect was evaluated in vitro on cells in suspension cultures with the chemiluminescence assay and on clonogenic progenitors using the NBT dye reduction assay.14,23 24 After 14 days of differentiation, suspension cultures of both WT control and experimental p47phox−/− Sca-1+ cells contained myeloid cells that produced O−⋅2 when stimulated with PMA as measured by chemiluminescence, whereas similarly cultured nontransduced control p47phox−/− cells did not. MFG-S-p47phox retroviral transduction of the p47phox−/− Sca-1+ cells increased chemiluminescence of the differentiated myeloid cells significantly to 47.73% (Exp. 1) and 83.46% (Exp. 2) of that seen in the WT myeloid cell cultures, compared with 0.01% of WT chemiluminescence in the untreated p47phox−/− cell cultures (P < .001; Student's t-test). For the Exp. 2, cells were also plated in agarose and correction of clonogenic progenitors determined by the NBT assay. At day 10 of incubation, 76 of 106 (30%) of colonies were NBT+ from cultures of transduced p47phox−/− progenitors compared with 129 of 160 (81%) in WT cultures. No NBT+ colonies (0 of 114) were detected in naive p47phox−/− colonies.
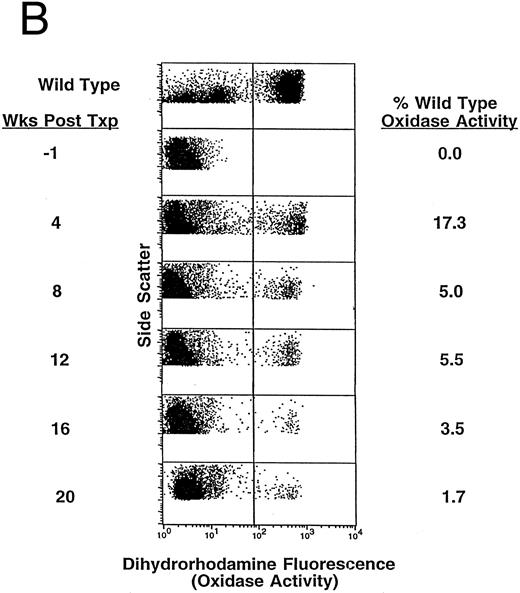
The majority of the transduced Sca-1+ cells (2.5 million per mouse) were transplanted into four (Exp. 1) and five (Exp. 2) moderately irradiated (500 cGy) p47phox−/− mice immediately after the final transduction cycle. The recipients were treated with high-dose rhG-CSF for 4 days before TBI, as we have previously shown that such treatment enhances engraftment at low-dose TBI.20 Others have demonstrated a radioprotective effect in mice pretreated with high-dose rhG-CSF.30 The experimental p47phox−/− mice were bled biweekly beginning 4 weeks posttransplant and in vivo correction of the neutrophil NADPH oxidase system was evaluated at each time point by flow cytometry using the fluorescent probe dihydrorhodamine 123.25 The average number of PB neutrophils demonstrating high-level correction of NADPH oxidase activity in the experimental p47phox−/− mice was 12.3% ± 0.9% at 4 weeks after transplantation and 2.6% ± 1.0% at 14 weeks after transplantation. Nonspecific DHR activity in untreated p47phox−/− mice evaluated in parallel was always less than 0.05% of WT activity. Table 1 shows all of the data points relating to the percent of NADPH oxidase–corrected PB neutrophils (DHR assay) both before and after bacterial challenge until time of death in all animals that underwent gene therapy. The mean DHR values ± SEM derived from Table 1 are plotted in Fig 1A. As can be seen, there was a rapid decrease in the percentage of corrected cells between 4 and 8 weeks posttransplant followed by a much slower rate of decline thereafter. Of the two animals that continue to survive after challenge, DHR expression has decreased but has remained detectable even after 40 weeks (see animal 2, Table 1). Figure 1B shows dot plots of neutrophil DHR fluorescence over time for a representative mouse (animal 2) after gene therapy. As demonstrated, significant short-term and low-level long-term expression has been achieved in this mouse after insertion of the human p47phox gene. It is notable that the mean fluorescence of DHR+ neutrophils in mice undergoing gene transfer was similar to that seen in WT mice, suggesting a high level of NADPH oxidase function in individual corrected neutrophils.
Percent of PB Neutrophils Oxidase Positive by DHR Assay After Gene Therapy
| . | Animal No. . | Experiment 1 . | Experiment 2 . | Mean ± SE . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | . |
| Wk Posttransplant | Baseline | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 4 | 13.9 | 17.3 | 8.4 | 10.5 | 12.5 | 14.0 | 12.1 | 11.2 | 10.5 | 12.3 ± 0.86 | |
| 6 | 3.7 | 7.0 | 5.6 | 2.7 | 4.4 | 10.7 | 9.1 | 3.7 | 6.3 | 5.9 ± 0.89 | |
| 8 | 2.6 | 5.0 | 3.1 | 1.9 | — | — | — | — | — | 3.2 ± 0.66 | |
| 10 | 4.5 | 5.7 | 4.2 | 1.5 | 5.0 | 10.0 | 2.5 | 2.3 | 1.7 | 4.2 ± 0.89 | |
| 12 | 3.2 | 5.5 | 1.7 | 1.1 | 1.9* | 10.8* | 3.2* | 1.8* | 0.9* | 3.4 ± 1.04 | |
| 14 | 3.1* | 2.8* | 1.7* | 0.8* | 1.3 | 9.9 | 1.6 | 1.3 | 0.9 | 2.6 ± 0.95 | |
| 16 | 2.1 | 3.5 | 1.0 | 0.6 | 0.1 | 4.9 | 0.4 | 4.3 | Died | 2.1 ± 0.67 | |
| 18 | 1.3 | 0.8 | 1.0 | Died | Died | 3.9 | 0.3 | 1.1 | 1.4 ± 0.52 | ||
| 20 | Died | 1.7 | Died | Died | 0.5 | 1.0 | 1.1 ± 0.35 | ||||
| 22 | 0.8 | 0.1 | Died | 0.5 ± 0.35 | |||||||
| 24 | 0.5 | 0.6 | 0.6 ± 0.05 | ||||||||
| 28 | 0.4 | Alive | 0.4 | ||||||||
| 40 | 0.1 | 0.1 | |||||||||
| Alive | |||||||||||
| . | Animal No. . | Experiment 1 . | Experiment 2 . | Mean ± SE . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | . |
| Wk Posttransplant | Baseline | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 4 | 13.9 | 17.3 | 8.4 | 10.5 | 12.5 | 14.0 | 12.1 | 11.2 | 10.5 | 12.3 ± 0.86 | |
| 6 | 3.7 | 7.0 | 5.6 | 2.7 | 4.4 | 10.7 | 9.1 | 3.7 | 6.3 | 5.9 ± 0.89 | |
| 8 | 2.6 | 5.0 | 3.1 | 1.9 | — | — | — | — | — | 3.2 ± 0.66 | |
| 10 | 4.5 | 5.7 | 4.2 | 1.5 | 5.0 | 10.0 | 2.5 | 2.3 | 1.7 | 4.2 ± 0.89 | |
| 12 | 3.2 | 5.5 | 1.7 | 1.1 | 1.9* | 10.8* | 3.2* | 1.8* | 0.9* | 3.4 ± 1.04 | |
| 14 | 3.1* | 2.8* | 1.7* | 0.8* | 1.3 | 9.9 | 1.6 | 1.3 | 0.9 | 2.6 ± 0.95 | |
| 16 | 2.1 | 3.5 | 1.0 | 0.6 | 0.1 | 4.9 | 0.4 | 4.3 | Died | 2.1 ± 0.67 | |
| 18 | 1.3 | 0.8 | 1.0 | Died | Died | 3.9 | 0.3 | 1.1 | 1.4 ± 0.52 | ||
| 20 | Died | 1.7 | Died | Died | 0.5 | 1.0 | 1.1 ± 0.35 | ||||
| 22 | 0.8 | 0.1 | Died | 0.5 ± 0.35 | |||||||
| 24 | 0.5 | 0.6 | 0.6 ± 0.05 | ||||||||
| 28 | 0.4 | Alive | 0.4 | ||||||||
| 40 | 0.1 | 0.1 | |||||||||
| Alive | |||||||||||
ip bacterial challenge.
Kinetics of appearance of NADPH oxidase–positive blood neutrophils after gene therapy in p47phox−/− mice. Mice undergoing gene therapy were bled at 1 month after transplant and biweekly thereafter. Gene expression in PMA-activated neutrophils was detected by flow cytometry using the fluorescent probe dihydrorhodamine-123 (DHR). Data shown are the percentage of NADPH oxidase–positive blood neutrophils in experimental mice adjusted for the percentage determined in WT mice. (A) Mean percent of PB neutrophils that are NADPH oxidase positive in the DHR assay for nine experimental p47phox−/− mice beginning at 4 weeks after gene therapy. The vertical axis is the mean percentage ± SEM of DHR+ circulating neutrophils in experimental mice. The horizontal axis represents time in weeks. This figure is derived from the data set shown in Table 1. (B) DHR flow cytometric dot-plot analysis of PB NADPH oxidase–positive neutrophils from one of the experimental p47phox−/− mice included in the averaged data presented in (A). The vertical axis is neutrophil side scatter and the horizontal axis is neutrophil DHR fluorescence as a measure of NADPH oxidase activity. In each dot plot, 10,000 events (cells) are indicated with DHR+ neutrophils in a cluster to the right of the vertical line. The top dot plot displays the neutrophil DHR fluorescence from a representative WT mouse and is followed by sequential dot plots of the gene corrected p47phox−/− mouse beginning 1 week before gene therapy (baseline) and then followed at 4-week intervals after gene therapy out to 20 weeks.
Kinetics of appearance of NADPH oxidase–positive blood neutrophils after gene therapy in p47phox−/− mice. Mice undergoing gene therapy were bled at 1 month after transplant and biweekly thereafter. Gene expression in PMA-activated neutrophils was detected by flow cytometry using the fluorescent probe dihydrorhodamine-123 (DHR). Data shown are the percentage of NADPH oxidase–positive blood neutrophils in experimental mice adjusted for the percentage determined in WT mice. (A) Mean percent of PB neutrophils that are NADPH oxidase positive in the DHR assay for nine experimental p47phox−/− mice beginning at 4 weeks after gene therapy. The vertical axis is the mean percentage ± SEM of DHR+ circulating neutrophils in experimental mice. The horizontal axis represents time in weeks. This figure is derived from the data set shown in Table 1. (B) DHR flow cytometric dot-plot analysis of PB NADPH oxidase–positive neutrophils from one of the experimental p47phox−/− mice included in the averaged data presented in (A). The vertical axis is neutrophil side scatter and the horizontal axis is neutrophil DHR fluorescence as a measure of NADPH oxidase activity. In each dot plot, 10,000 events (cells) are indicated with DHR+ neutrophils in a cluster to the right of the vertical line. The top dot plot displays the neutrophil DHR fluorescence from a representative WT mouse and is followed by sequential dot plots of the gene corrected p47phox−/− mouse beginning 1 week before gene therapy (baseline) and then followed at 4-week intervals after gene therapy out to 20 weeks.
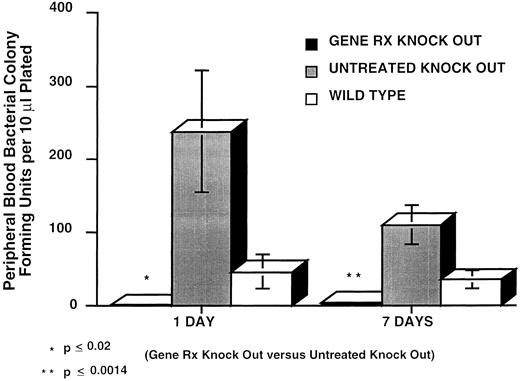
In vivo response of gene-corrected p47phox−/− mice to infection challenge with B cepacia. To determine whether gene correction could restore in vivo host-defense function of p47phox−/− mice, we injected 2.5 × 105 CFU of plateau phase B cepacia, ip, into three groups of mice: the 9 experimental gene-corrected p47phox−/− mice; 11 age-matched control p47phox−/− mice; and 8 WT mice. Figure 2 shows the levels of bacteremia. At 24 hours the mean bacteremia levels of experimental gene-corrected p47phox−/− mice, control p47phox−/− mice, and WT mice were 0.89 ± 0.30 bacterial colonies/10 μL blood, 237.7 ± 83.6 bacterial colonies/10 μL blood, and 45.8 ± 22.9 bacterial colonies/10 μL blood, respectively. The difference between experimental p47phox−/− mice and control p47phox−/− mice at 24 hours was significant (P < .02, Student's t-test). At 7 days, the mean bacteremia levels of experimental gene-corrected p47phox−/− mice, control p47phox−/− mice, and WT mice were 4.0 ± 2.0 bacterial colonies/10 μL blood, 110.2 ± 26.5 bacterial colonies/10 μL blood, and 34.9 ± 11.5 bacterial colonies/10 μL blood, respectively. The difference between experimental p47phox−/− mice and control p47phox−/− mice at 7 days was highly significant (P < .0014, Student's t-test).
Bacteremia in mice after ip injection of 2.5 × 105 CFU B cepacia. PB was collected 24 hours and 1 week after challenge. The vertical axis is PB B cepacia in CFU per 10 μL blood plated at the indicated times after challenge. The bars, left to right, respectively, for day 1 and day 7 correspond to the mean ± SEM bacterial colony counts per 10 μL blood plated from nine gene corrected p47phox−/− mice (Gene RX Knock Out, closed bars), 11 uncorrected control p47phox−/− mice (Untreated Knock Out, shaded bars), and 8 WT control mice (Wild Type, open bars).
Bacteremia in mice after ip injection of 2.5 × 105 CFU B cepacia. PB was collected 24 hours and 1 week after challenge. The vertical axis is PB B cepacia in CFU per 10 μL blood plated at the indicated times after challenge. The bars, left to right, respectively, for day 1 and day 7 correspond to the mean ± SEM bacterial colony counts per 10 μL blood plated from nine gene corrected p47phox−/− mice (Gene RX Knock Out, closed bars), 11 uncorrected control p47phox−/− mice (Untreated Knock Out, shaded bars), and 8 WT control mice (Wild Type, open bars).
In experiments preliminary to this study, p47phox−/− mice challenged with 105 to 107 CFU of B cepacia survived an average of 7.6 ± 3 days whereas WT mice survived indefinitely, suggesting that WT mice are capable of eradicating experimental infection with B cepacia. We compared survival in the experimental gene-corrected p47phox−/− group of mice with the control p47phox−/− and WT groups of mice following B cepacia challenge. A Kaplan-Meier plot of survival after bacterial challenge is shown in Fig 3. As shown, only 50% of control p47phox−/− mice survived for 2 weeks after challenge and all were dead by day 30. In contrast, ∼50% of experimental p47phox−/− mice survived past day 30 and two of these animals appear to have cleared their infection (alive >100 days postchallenge). The survival advantage of the gene-corrected experimental p47phox−/− mice compared with uncorrected control p47phox−/− mice was highly significant (P < .001). This survival advantage was present despite the fact that experimental gene-corrected p47phox−/− mice had been previously irradiated while control p47phox−/− mice had not been irradiated, and despite the fact that the number of gene-corrected neutrophils were continuing to decrease over time in the gene-corrected animals.
Survival after ip challenge with 2.5 × 105 CFU B cepacia plotted as a Kaplan-Meier survival analysis. The plot demonstrates the survival of gene corrected p47phox−/− mice (Gene Rx Knock Out), uncorrected control p47phox−/− mice (untreated Knock Out), and WT control mice (Wild Type) after bacterial infection challenge. The vertical axis is the proportion of mice surviving and the horizontal axis is time after challenge in days. The value for “n” equals the number of mice alive in each group at the time of challenge. All WT mice survived the challenge and remain alive, while two of the Gene Rx Knock Out group survived the challenge long term and remain alive at the time of this report.
Survival after ip challenge with 2.5 × 105 CFU B cepacia plotted as a Kaplan-Meier survival analysis. The plot demonstrates the survival of gene corrected p47phox−/− mice (Gene Rx Knock Out), uncorrected control p47phox−/− mice (untreated Knock Out), and WT control mice (Wild Type) after bacterial infection challenge. The vertical axis is the proportion of mice surviving and the horizontal axis is time after challenge in days. The value for “n” equals the number of mice alive in each group at the time of challenge. All WT mice survived the challenge and remain alive, while two of the Gene Rx Knock Out group survived the challenge long term and remain alive at the time of this report.
DISCUSSION
In the recently issued “Report and Recommendation of the Panel to Assess the NIH Investment in Research on Gene Therapy” chaired by Stuart Orkin and Arno Motulsky,31 the committee noted that, “Confidence in current approaches to somatic gene therapy would rise if a genuine genetic deficiency in an animal were unequivocally corrected.” The report further noted that, “Although genetic defects in animals have been corrected by introducing transgenes into the germline (or by interbreeding with transgenic animals), somatic gene transfer has not permanently corrected a genetic disease in an animal (eg, a mouse model of a single-gene disorder).” We have shown that it is possible to use retrovirus-mediated gene therapy to achieve prolonged correction of the NADPH oxidase defect in the p47phox−/− mouse model of CGD. Although the level of correction at 14 weeks after transplant resulted in a phenotype with an average of 2.6% of circulating neutrophils being NADPH oxidase positive, this was sufficient to provide significant protection from early mortality usually associated with experimental ip B cepacia infection. This provides important evidence that even a low level of correction of the CGD phenotype by gene therapy would provide clinical benefit. It also shows that this benefit occurs even where the number of corrected circulating neutrophils is slowly decreasing over time.
Although most hematopoietic cell gene transfer studies in mice use 5-FU treatment of donors to increase the number of early progenitors entering the replication cycle, we used donor Sca-1+ hematopoietic progenitor cells from donor p47phox−/− CGD mice that had not received such conditioning. Current retrovirus vectors approved for clinical use are all based on modifications of murine leukemia or sarcoma viruses. Efficient integration of these vectors into the genome of target cells appears to require cycling of the cells, possibly because breakdown of the nuclear membrane during cell division allows access of the provirus into the nucleus.32,33 New types of vectors in development based on lentiviruses, such as human immunodeficiency virus 1, may not require cell cycling for integration.34 35 However, efficient integration of murine retrovirus vectors into uncommitted pluripotent hematopoietic progenitors capable of long-term reconstitution has been difficult to achieve. This problem relates in part to the fact that most stem cells appear to be in G0 and there is a lack of understanding of the factors regulating entry of these cells into the replication cycle without triggering terminal differentiation.
Many investigators have advocated 5-FU treatment of donor mice before BM harvest to enhance the number of cycling stem cells and, thus, the transduction efficiency of retrovirus vectors into long-term repopulating cells.36-40 However, conditioning donors with 5-FU or other agents may fail to enhance transduction efficiency while reducing the engraftment potential of these cells.41-43 In addition, because some level of conditioning of the recipient before transplant may be required for engraftment, it is important to know if cytotoxic treatment before stem cell harvest can be avoided.44-48 For these reasons, we procured marrow cells without conditioning the donor.
The need for pretransplantation recipient conditioning to achieve significant levels of engraftment of autologous stem cells (or syngeneic in the case of the mouse study) is controversial.20,42,44-50 Using a range of TBI dosing as the conditioning regimen in a murine stem cell transplantation model, we recently reported that low-level TBI is necessary and sufficient to achieve significant engraftment of purified stem cells.20 In that model system, with a limit of detection of donor cells of 0.1%, no engraftment was detected in the absence of any conditioning. Over 90% donor cell chimerism was achieved with a dose of 550 cGy, and almost 14% chimerism could be achieved at only 120 cGy TBI. These doses are significantly below doses generally used for marrow ablation. Regimens using higher doses of TBI to achieve 100% chimerism do so at an unnecessary cost of added mortality and morbidity and actually may be detrimental to the function of marrow micro-environment required for stem cell homing and survival. In the current study we found that the dose of 500 cGy TBI resulted in transient neutropenia, but was well tolerated by the p47phox−/− CGD mice and was not associated with any apparent increased mortality in any of the study animals. We are currently exploring lower doses of TBI in this model because it would be best to use the lowest TBI dose possible in consideration of application of this model to human studies.
At 1 month after gene therapy in the p47phox−/− CGD mice the level of chimerism as measured by NADPH oxidase activity in PB neutrophils ranged from 8% to 17% in individual animals. There was a rapid decrease over the next month and then a slow drift downward. The level of normal neutrophil chimerism, in the animal whose neutrophil NADPH oxidase analysis is shown in Fig 1B, has continued to decrease slowly over time and is currently at a level of 0.1% at 40 weeks post gene therapy. Preliminary to the current study, we explored engraftment using congenic mice differing at the Ly5 locus as a stable marker20 rather than gene transfer marking, but progenitors were cultured and recipients conditioned as in the current gene transfer study. In those studies, stable high-level (>70%) long-term chimerism after transplantation was achieved. Thus, the kinetics shown here are consistent with a high level of gene transduction of committed progenitors and a much lower transduction efficiency of longer-term repopulating progenitors. This highlights the importance of developing improved methods for gene targeting the most primitive stem cells.
Dinauer et al51 have developed a “knock out” mouse model of the X-linked, gp91phox-deficient form of chronic granulomatous disease. Recently they have demonstrated a high level of in vitro correction of the oxidase defect in myeloid cells differentiated in culture from transduced BM progenitors derived from these mice using a murine stem cell virus vector encoding the human gp91phox.52 As in our studies, the human oxidase component was capable of correcting the CGD defect in murine myeloid cells. This X-linked CGD mouse model and our autosomal recessive CGD mouse model provide complementary systems for testing the gene-therapy methods that will need to be developed to achieve high-level permanent correction of the two most common forms of CGD, together accounting for over 90% of patients.
In our current study the increased survival after experimental ip bacterial challenge in the gene-therapy group of p47phox−/− CGD mice was associated with lower levels of average bacteremia for this group relative to the control p47phox−/− CGD mice at both 24 hours and 7 days. We cannot be certain that the lower average level of bacteremia was responsible for the prolonged survival of the gene-therapy group of p47phox−/− CGD mice relative to the control group of p47phox−/− CGD mice because there was no statistically significant correlation between bacteremia levels and length of survival in individual mice. B cepacia, a catalase positive and Gram-negative phytopathogen, used as the challenge organism in this study, is a significant pathogen for human CGD patients and is responsible for considerable morbidity and mortality.53,54 However, it is rarely a pathogen in immunocompetent human hosts and, in studies preliminary to the current gene-therapy study, was not fatal to WT mice when injected at doses 100-fold higher than those used in the present study. Because B cepacia is resistant to some oxygen-independent phagocytic killing mechanisms, these results also highlight the critical role of the NADPH oxidase in oxygen-dependent killing of this microorganism.55 Even the limited number of NADPH oxidase–positive neutrophils present in the gene-therapy group of p47phox−/− CGD mice appears to have provided significant protection from the fatal effects of this organism. These studies provide a rationale for continued efforts to develop gene therapy for patients with CGD using retrovirus vectors to target hematopoietic stem cells.
ACKNOWLEDGMENT
We thank Dr David W. Alling for his statistical analysis, Dr John I. Gallin for his encouragement and enthusiastic support, and Dr Andrea Barnes and others at the NIH 14B South animal facility for their animal care and technical assistance.
M.M. and S.H.J. contributed equally to this project.
Address reprint requests to Harry L. Malech, MD, Laboratory of Host Defenses, NIAID, NIH, Bldg 10, Room 11N113, 10 Center Dr, MSC 1886, Bethesda, MD 20892-1886.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal