Abstract
Mutations in the DNA mismatch repair (MMR) gene hMSH2 underlie a novel pathway of tumorigenesis for some cancers of epithelial origin. Mice deficient in MSH2 are susceptible to lymphomas but defects in this gene have not been identified in human lymphoid tumors. To determine if the lymphomas these mice develop are related to a particular subtype of human lymphoma we evaluated 20 clinically ill homozygous MSH2−/− mice ranging in age from 2 to 13 months. The murine tumors comprised a single histopathologic entity representing the malignant counterpart of precursor thymic T cells and closely resembled human precursor T-cell lymphoblastic lymphoma (LBL). Evaluation of the expression of three T-cell malignancy associated genes showed that Rhombotin-2 (RBTN-2 also known as Lmo-2), TAL-1 (also known as SCL), and HOX-11 were expressed in 100%, 40%, and 0% of the murine tumors, respectively. The MSH2−/− murine model of precursor T-cell LBL was substantiated by the finding of a nearly identical expression profile of RBTN-2, TAL-1, and HOX-11 in 10 well-characterized cases of human LBL. Direct evidence for MSH2 abnormalities in human LBL was established by sequence analysis of exon 13 of hMSH2, which revealed coding region mutations in 2 of 10 cases. Our findings implicate defects in the MMR system with the aberrant expression of T-cell specific proto-oncogenes and define a new pathway of human lymphomagenesis.
CANCER IS the end point of cumulative genetic changes that confer proliferative, invasive, or metastatic potential on normal cells. As an example, in colon cancer, mutations in at least five to six specific genes are required for full expression of the malignant phenotype.1 However, a background mutation rate of approximately 1.4 × 10−10 mutations/bp/cell generation can only account for, at most, 2 or 3 mutations in each tumor. Consequently, it is realistic to suppose that a mutation could be selected for on the basis of it conferring an increased mutation rate, especially during the early stages of tumorigenesis.2 3
The DNA mismatch repair (MMR) genes serve to maintain fidelity of genomic replication. Defects in any of the known genes that comprise this system, hMSH2, hMLH1, hPMS1, hPMS2, and GTBP, can result in an increased rate of mutation.4,5 The relationship between defects in MMR genes and tumorigenesis is best defined in kindreds with hereditary nonpolyposis colon cancer (HNPCC) where defects in hMSH2 and hMLH1 account for 50% and 30% of these cancers, respectively.6-8 Defects in MMR genes have also been found in some sporadic tumors of epithelial origin9-13 but have not been directly implicated in the development of human hemato-lymphoid neoplasms.
However, there are data to suggest that defects in MSH2 may be involved in lymphomagenesis. In previous studies, we and others showed that mice carrying a targeted germline disruption of the MSH2 gene are viable and susceptible to lymphoid tumors.14,15 In these studies, the pathologic characterization, which was limited to a few lymphoid tumors, suggested histologic homogeneity consistent with lymphoma of thymic T-cell origin. As well, these lymphomas were found to evolve from a background of apparently normal hematopoietic progenitors, as thymic immunophenotypic profiles were normal before the onset of lymphoma.14 These findings suggest that following MSH2 deficiency, molecular activating events occur which lead to lymphocyte transformation and expression of a malignant phenotype. Formal evidence linking MMR gene defects with oncogene activation is lacking; hence, these mice represent an excellent model to investigate activating events as a consequence of MSH2 deficiency.
The aberrant expression of three proto-oncogenes, Rhombotin-2 (RBTN-2), HOX-11, and TAL-1, which normally are expressed in most tissues but not in T cells, have been identified as activating events in the development of human precursor T-cell neoplasms.16 Chromosomal rearrangements, deletions, or point mutations are thought to underlie the aberrant expression of RBTN-2, HOX-11, and TAL-1 in some human precursor T-cell neoplasms.16 17 However, the mechanism responsible for the development of these genetic alterations remains poorly defined. Specifically, defects in the genes that comprise the MMR system have not been linked to the aberrant expression of these proto-oncogenes.
We now report the histologic and molecular characterization of 20 murine lymphomas arising in MSH2−/− mice and show that they comprise a single histopathologic entity and represent the murine counterpart of human precursor T-cell lymphoblastic lymphoma (LBL). The homogeneous nature of the murine tumors suggested that specific recurring activating events in precursor T-cell neoplasms may be selected for following MSH2 deficiency. Evaluation of RBTN-2, TAL-1, and HOX-11 showed expression in 100%, 40%, and 0% of the murine tumors, respectively. The MSH2−/− murine model of precursor T-cell LBL was substantiated by the finding of a nearly identical expression profile of RBTN-2, TAL-1, and HOX-11 in 10 well-characterized cases of human LBL. Direct evidence for MSH2 abnormalities in human LBL was established by sequence analysis of exon 13 of hMSH2, which revealed coding region mutations in 2 of 10 cases. Our findings implicate defects in the MMR system with the aberrant expression of T-cell–specific proto-oncogenes and define a new pathway of human lymphomagenesis.
MATERIALS AND METHODS
Specimens.Autopsies from 20 MSH2−/− mice were performed after killing for failure to thrive. Details for the generation of MSH2−/− mice have been previously reported.14 Ten human biopsy specimens were obtained from a large bank of well-characterized archival lymphomas after a computer search was performed using the entry phrase “precursor T-cell leukemia/lymphoma.” All human cases had lymphoblastic morphology, expressed terminal deoxynucleotidyl transferase (TdT), expressed cytoplasmic and/or surface CD3, and had clonal rearrangements of the T-cell receptor (TCR) β and δ genes. In all instances, human LBL cases were negative for bone marrow (BM) infiltration as assessed by light microscopy, thereby fulfilling criteria for the diagnosis of precursor T-cell lymphoblastic lymphoma.18 Human and murine specimens were divided and fixed in 10% buffered formalin and embedded in paraffin or snap frozen in liquid nitrogen and stored at −70°C for DNA and RNA extraction.
Histology.Sections of formalin-fixed, paraffin-embedded tissue obtained from MSH2−/− mice and from human biopsy specimens were cut at 5-μm thickness and stained with hematoxylin and eosin (H&E) and examined by light microscopy.
Immunohistochemistry.A three-stage avidin-biotin immunoperoxidase method was used to immunophenotype murine lymphomas and evaluate RBTN-2 protein expression. Anti-CD3, anti-CD4, anti-CD8, and anti-B220 antibodies were used for immunophenotyping on acetone-fixed frozen sections. The anti–RBTN-2 antibody, a rabbit polyclonal IgG, was a kind gift of Drs G.A.M. Neale and R. Goorha (St Jude Children's Research Hospital, Memphis, TN). The details of the generation of this antibody have been previously published.19 Formalin-fixed, paraffin-embedded sections were microwaved at high power for 8 to 10 minutes in 0.01 mol/L citric acid solution, pH 6.0. Sections were overlaid with 200 μL of anti-RBTN-2 antibody diluted 1:1,000 in phosphate-buffered saline (PBS)/bovine serum albumin (BSA) and incubated for 1 hour at room temperature. Sections were then washed in PBS and overlaid with 200 μL of biotin-conjugated swine antirabbit Ig antibody diluted 1:200 in PBS and incubated for 1 hour at room temperature. Sections were then washed repeatedly in PBS and incubated with 200 μL of a 1:400 dilution of streptavidin-horseradish peroxidase conjugate for 1 hour at room temperature. Slides were stained with 0.2 mg/mL 3,3′ diaminobenzidine tetrahydrochloride in PBS containing 0.01% H2O2 for 5 minutes. Sections were washed and counterstained with 2% Methylgreen (Fisher Scientific, Montreal, PQ, Canada) that had been chloroform extracted.
Southern blot analysis.DNA was extracted from snap-frozen tissue, digested with restriction enzymes as recommended by the supplier (New England BioLabs, Beverly, MA), electrophoresed through 0.7% agarose gels, and transferred to nylon membranes (Hybond N+; Amersham, Arlington Heights, IL). All membranes were prehybridized, hybridized, and washed as recommended by the supplier. All 32P-labeled probes were prepared by the random priming method. The mouse Jβ2 and Cδ probes were used to assess TCR gene rearrangements.20 21
Reverse transcriptase-polymerase chain reaction (RT-PCR).Total cellular RNA was isolated by the RNAzol B method according to the manufacturers instructions (Cinna/Biotecx, Friendswood, TX). Single-stranded cDNA was then prepared from total cellular RNA by reverse transcription according to the manufacturer's recommendations (Perkin-Elmer, Branchburg, NJ). The cDNA was amplified in the presence of 1 U of Taq polymerase (Cetus, Emeryville, CA) and the appropriate 5′ and 3′ primers in a total volume of 50 μL using standard buffers. A touchdown PCR method was used to evaluate the expression of human and murine RBTN-2, TAL-1, and HOX-11. The cycling conditions were 95°C for 1 minute, 65 → 55°C for 1 minute, and 72°C for 1 minute for a total of 35 cycles (25 cycles at an annealing temperature of 55°C). For visualization of the products, 10% of the PCR mixture was electrophoresed in a 1.5% agarose gel containing ethidium bromide. All experiments were performed in duplicate. Primers 5′-GGATCCTCGGCCATCGAAAGGAAGAGC-3′, corresponding to nucleotides (nt) 1-27 and 5′-ATCCCATTGATCTTGGTCCACTC-3′, corresponding to nt 448-470, allowed amplification of a 470-bp product of mouse RBTN-2 (Genbank Accession No. M64360); and 5′-GGATCCTGCCGGAGAGACTATCTC-3′, corresponding to nt 539-562 and 5′-GAATTCAGTGAACACCTCCGCAAA-3′, corresponding to nt 706-729, allowed amplification of a 289-bp product of human RBTN-2(X61118); 5′-AACCGGGTGAAGAGGAGGCCCTCC-3′, corresponding to nt 3182-3205 within exon 5 and 5′-AAGCACGTCCTGTAGAAGGTC-3′, corresponding to nt 3571-3591 within exon 6, allowed amplification of a 356-bp product of mouse TAL-1 (U01530); 5′-AATCGAGTGAAGAGGAGACC-3′, corresponding to exon 5 nt 55-74 and 5′-TGGTCATTGAGCAGCTT-3′, corresponding to exon 6 nt 188-204, allowed amplification of a 246-bp product of human TAL-1 (M63584); 5′-GTAACCGCAGATACACAAAGG-3′, corresponding to exon 1 nt 556-576 and 5′-GTGATTTTGGTGGAGTCGTCAG-3′, corresponding to exon 3 nt 184-205, allowed amplification of a 460-bp product of mouse HOX-11 (L21164); 5′-GTAACCGCAGATACACAAAGG-3′, corresponding to nt 913-933 within exon 1 and 5′-GTGATTTTGGTCGAGTCGTCAG-3′, corresponding to nt 1424-1445 within exon 3, allowed amplification of a 424-bp product of human HOX-11 (M75952). For these sets of experiments primers were synthesized according to sequences obtained from Genbank and analyzed with a primer selection program (Amgen Institute, Toronto, Ontario, Canada). Primers SL5′ (5′-GAAGGCCATCCGTGTAGATC-3′) and SL3′ (5′-GGTTCAATGTAGTCCAGTC-3′) were used to amplify murine cDNA for the expression of TdT, as previously described.22
Amplification and sequence analysis of hMSH2 from genomic DNA.DNA was extracted from human LBL biopsy specimens using DNAzol (GIBCO-BRL, Bethesda, MD) according to the manufacturer's instructions. PCR was performed using approximately 100 ng of genomic DNA, with primers and conditions previously described. 7 The amplification conditions were 95°C for 1 minute, 46°C for 1 minute, and 72°C for 1 minute for a total of 35 cycles. For single-strand conformation polymorphism (SSCP) analysis, equal volumes of PCR reaction products and STOP solution (Sequenase kit Version 2.0; USB, Cleveland, OH) were mixed, boiled, and placed on ice. Two microliters of the mixture was loaded onto a 4.5% polyacrylamide nondenaturing gel containing 5% glycerol, and electrophoresed at 4°C for 7 to 10 hours at 35 W. Products were transferred to prewetted nylon membranes and the DNA was fixed and denatured with 0.4 N NaOH. Visualization of conformers was accomplished by simultaneous hybridization to end-labeled sense and anti-sense primers and exposure to x-ray film. SSCP analysis was performed on replicate PCR experiments and yielded identical results. For sequence analysis, PCR products were first cloned using the TA cloning system (Invitrogen, San Diego, CA). Ten clones from two separate PCR experiments were sequenced by the dideoxy method using the Sequenase Kit, Version 2.0 (USB). The M13 universal primers were used as primers for sequencing and both sense and antisense strands were sequenced.
RESULTS
Histopathologic characterization of MSH2−/−murine lymphomas.To determine if the lymphomas these mice develop represent a single histopathologic entity, or display heterogeneity with respect to lineage and postulated normal counterpart in lymphocyte ontogeny, we evaluated 20 clinically ill mice ranging in age from 2 to 13 months, median 3.8 months. All animals had thymic lymphomas while none had neoplasms of nonlymphoid origin. The lymphomas arising in MSH2−/− mice were found to be uniform in their primitive morphologic appearance and all displayed a lymphoblastic morphology (Fig 1). All murine lymphomas expressed CD3, were negative for B220, and showed clonal TCR gene rearrangements by Southern hybridization, indicating T-cell origin (Table 1). These murine T-cell lymphomas showed the full spectrum of immunophenotypes characteristic of lymphoblastic lymphoma: CD4+ or CD8+ single positive, CD4+CD8+ double positive, and CD4−CD8− double negative. To confirm that these lymphomas represent monoclonal expansions of precursor and not postthymic T lymphocytes, we evaluated them for expression of TdT. The MSH2−/− murine lymphomas uniformly expressed TdT, thereby establishing their origin from precursor T cells.18 22 Thus, these lymphomas comprise a single histopathologic entity representing the malignant counterpart of precursor thymic T cells and closely resemble the human entity of human LBL.
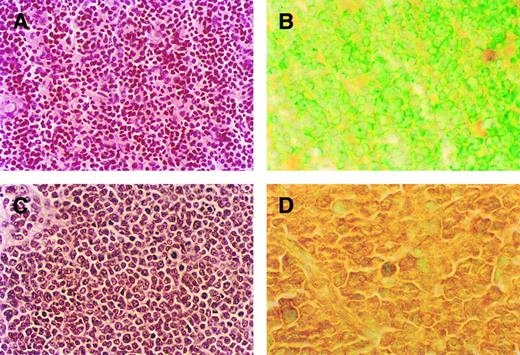
Comparison of thymic histology and RBTN-2 expression in MSH2 −/− mice before the development of lymphoma and MSH2 −/− mice with thymic lymphoblastic lymphoma (A-D). (A) Thymic histology from 8-week-old MSH2 −/− mouse before the development of lymphoma, original magnification (OM) × 500. (B) Thymic tumor histology from 4-month-old MSH2 −/−, OM × 500. Note the uniform morphologic appearance of the tumor cells with fine nuclear chromatin, inconspicuous nucleoli, scant cytoplasm, and numerous mitotic figures consistent with lymphoblastic lymphoma. (C) RBTN-2 protein expression by immunohistochemistry in thymus of MSH2 −/− mouse before the development of lymphoma, OM × 1,000. (D) MSH2 −/− thymic tumor, OM × 1,000. Immunohistochemistry showed strong RBTN-2 protein expression in thymic tumor only.
Comparison of thymic histology and RBTN-2 expression in MSH2 −/− mice before the development of lymphoma and MSH2 −/− mice with thymic lymphoblastic lymphoma (A-D). (A) Thymic histology from 8-week-old MSH2 −/− mouse before the development of lymphoma, original magnification (OM) × 500. (B) Thymic tumor histology from 4-month-old MSH2 −/−, OM × 500. Note the uniform morphologic appearance of the tumor cells with fine nuclear chromatin, inconspicuous nucleoli, scant cytoplasm, and numerous mitotic figures consistent with lymphoblastic lymphoma. (C) RBTN-2 protein expression by immunohistochemistry in thymus of MSH2 −/− mouse before the development of lymphoma, OM × 1,000. (D) MSH2 −/− thymic tumor, OM × 1,000. Immunohistochemistry showed strong RBTN-2 protein expression in thymic tumor only.
Lymphoma Characterization in MSH2−/− Mice
| Mouse . | Histology . | Immunophenotype . | TdT . | TCR Status β/δ . | RBTN-2 . | Tal-1 . |
|---|---|---|---|---|---|---|
| D8 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | +/+ | +ve | +ve |
| L12 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | +/+ | +ve | +ve |
| R15 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | +/+ | +ve | −ve |
| H27 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | −/+ | +ve | −ve |
| K7 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | −/+ | +ve | −ve |
| P21 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | +ve |
| T9 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | +ve |
| L15 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | −ve |
| F3 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | −ve |
| B20 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | −ve |
| S7 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | −/+ | +ve | −ve |
| C31 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | −/+ | +ve | +ve |
| F1-C2 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | +/+ | +ve | +ve |
| E5 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | +/+ | +ve | −ve |
| H59 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | +/+ | +ve | −ve |
| E38 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | +/+ | +ve | −ve |
| J3 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | −/+ | +ve | +ve |
| E22 | Lymphoblastic | B220−, CD3+, CD4−, CD8+ | +ve | +/+ | +ve | −ve |
| K1 | Lymphoblastic | B220−, CD3+, CD4−, CD8+ | +ve | +/+ | +ve | −ve |
| F1-C3 | Lymphoblastic | B220−, CD3+, CD4−, CD8+ | +ve | −/+ | +ve | +ve |
| Mouse . | Histology . | Immunophenotype . | TdT . | TCR Status β/δ . | RBTN-2 . | Tal-1 . |
|---|---|---|---|---|---|---|
| D8 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | +/+ | +ve | +ve |
| L12 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | +/+ | +ve | +ve |
| R15 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | +/+ | +ve | −ve |
| H27 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | −/+ | +ve | −ve |
| K7 | Lymphoblastic | B220−, CD3+, CD4−, CD8− | +ve | −/+ | +ve | −ve |
| P21 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | +ve |
| T9 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | +ve |
| L15 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | −ve |
| F3 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | −ve |
| B20 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | +/+ | +ve | −ve |
| S7 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | −/+ | +ve | −ve |
| C31 | Lymphoblastic | B220−, CD3+, CD4+, CD8+ | +ve | −/+ | +ve | +ve |
| F1-C2 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | +/+ | +ve | +ve |
| E5 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | +/+ | +ve | −ve |
| H59 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | +/+ | +ve | −ve |
| E38 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | +/+ | +ve | −ve |
| J3 | Lymphoblastic | B220−, CD3+, CD4+, CD8− | +ve | −/+ | +ve | +ve |
| E22 | Lymphoblastic | B220−, CD3+, CD4−, CD8+ | +ve | +/+ | +ve | −ve |
| K1 | Lymphoblastic | B220−, CD3+, CD4−, CD8+ | +ve | +/+ | +ve | −ve |
| F1-C3 | Lymphoblastic | B220−, CD3+, CD4−, CD8+ | +ve | −/+ | +ve | +ve |
TdT refers to terminal deoxynucleotidyl transferase activity. TCR status refers to the T-cell receptor β and δ genes, and the (+) and (−) refers to the presence or absence of T-cell receptor gene rearrangements. For TCR δ the (+) includes both rearrangements and deletions.
Proto-oncogene expression profiles in MSH2−/−murine lymphomas and human lymphoblastic lymphomas.The activation of three genes, TAL-1, RBTN-2, and HOX-11, has been implicated in the development of T-cell malignancy in humans.16 To investigate if events resulting from MSH2 deficiency in the mice could be associated with the activation of these genes, we used RT-PCR to evaluate their expression in the thymus of −/− animals at a time when there was no histologic evidence of malignancy, and when there was gross involvement of the thymus by lymphoma. The murine tumors all showed expression of RBTN-2 (Fig 2) that was confirmed by immunohistochemistry (Fig 1). RT-PCR analysis also showed that 40% of the MSH2−/− murine tumors expressed TAL-1 while none expressed HOX-11. RT-PCR analysis of histologically normal thymuses of −/− and +/+ animals was negative for RBTN-2, TAL-1, and HOX-11 expression.
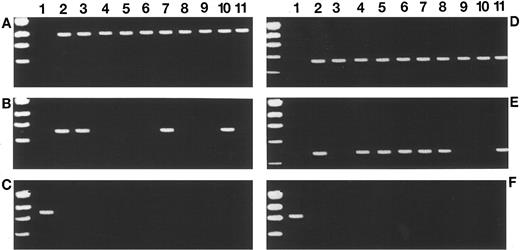
Proto-oncogene expression profiles in MSH2 −/− mice and human LBL by RT-PCR (A-F). Results of RBTN-2, TAL-1, and HOX-11 expression in 10 representative MSH2 −/− thymic tumors are shown in (A), (B), and (C), respectively. Lane 1 in (A) and (B) represent amplification from MSH2 −/− thymic tissue before the development of lymphoma (negative control) and lane 1 in (C) represents a positive control with amplification using cDNA from day 9 ES cells. RBTN-2 expression is detected in all MSH2 −/− thymic tumors, TAL-1 is detected in 40%, while none express HOX-11. (D), (E), and (F) show the expression profiles of RBTN-2, TAL-1, and HOX-11, respectively, in 10 cases of human LBL. Lane 1 in (D) and (E) represent amplification using normal human lymphocyte cDNA (negative control). Lane 1 in (F) is a positive control from SiL-1, a human T-cell acute lymphoblastic leukemia (ALL) cell line. Expression of RBTN-2, TAL-1, and HOX-11 is detected in 100%, 70%, and 0% of the cases, respectively.
Proto-oncogene expression profiles in MSH2 −/− mice and human LBL by RT-PCR (A-F). Results of RBTN-2, TAL-1, and HOX-11 expression in 10 representative MSH2 −/− thymic tumors are shown in (A), (B), and (C), respectively. Lane 1 in (A) and (B) represent amplification from MSH2 −/− thymic tissue before the development of lymphoma (negative control) and lane 1 in (C) represents a positive control with amplification using cDNA from day 9 ES cells. RBTN-2 expression is detected in all MSH2 −/− thymic tumors, TAL-1 is detected in 40%, while none express HOX-11. (D), (E), and (F) show the expression profiles of RBTN-2, TAL-1, and HOX-11, respectively, in 10 cases of human LBL. Lane 1 in (D) and (E) represent amplification using normal human lymphocyte cDNA (negative control). Lane 1 in (F) is a positive control from SiL-1, a human T-cell acute lymphoblastic leukemia (ALL) cell line. Expression of RBTN-2, TAL-1, and HOX-11 is detected in 100%, 70%, and 0% of the cases, respectively.
To further substantiate the histopathologic evidence that MSH2−/− murine lymphomas recapitulate human LBL, 10 well-characterized cases of human T-cell LBL were evaluated for expression of TAL-1, RBTN-2, and HOX-11. The human LBL cases showed a nearly identical proto-oncogene expression profile as the murine lymphomas because all cases expressed RBTN-2, 70% expressed TAL-1, and none expressed HOX-11 (Fig 2). Thus, the murine lymphomas and human LBL are virtually identical with respect to histopathologic features, cell of origin, and pattern of proto-oncogene expression.
hMSH2 gene mutations in human lymphoblastic lymphomas.Because of the striking similarities between the murine lymphomas and human LBL we screened for abnormalities in exon 13 of hMSH2, including both intron-exon boundaries, by SSCP analysis (Fig 3). Exon 13 encodes a region highly conserved within all MutS homologs that forms part of the putative DNA binding domain.7,23 24 Five of the 10 human LBL cases displayed an abnormal SSCP pattern and were further studied by sequencing. Two of these five cases contained mutations in the coding region of exon 13 not previously reported in human malignancies. In one instance, a heterozygous frameshift mutation resulted from a 1-bp deletion at codon 672-673 which created a new termination codon 34 bp downstream. In another case, a heterozygous missense C → A mutation at codon 724 occurred which resulted in a substitution of lysine for threonine at a position perfectly conserved in all mutS homologs (Fig 4). In addition, all five LBL cases with abnormal SSCP patterns showed a T → C change at the −6 intronic splice acceptor site. Cases of human LBL not showing SSCP band shifts and control placental DNA were also sequenced and revealed wild-type sequences. Corresponding normal tissue from the LBL patients was not available for study, consequently, microsatellite instability and loss of heterozygosity for hMSH2 could not be determined.
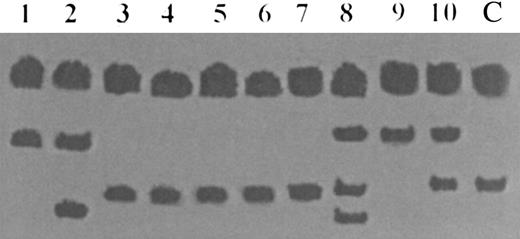
SSCP analysis of exon 13 of the hMSH2 gene in 10 cases of human LBL. Lanes 1 through 10 represent cases of human LBL and lane 11 represents normal human DNA. Conformational band shifts representing mutational change are seen in lanes 1, 2, and 8 through 10.
SSCP analysis of exon 13 of the hMSH2 gene in 10 cases of human LBL. Lanes 1 through 10 represent cases of human LBL and lane 11 represents normal human DNA. Conformational band shifts representing mutational change are seen in lanes 1, 2, and 8 through 10.
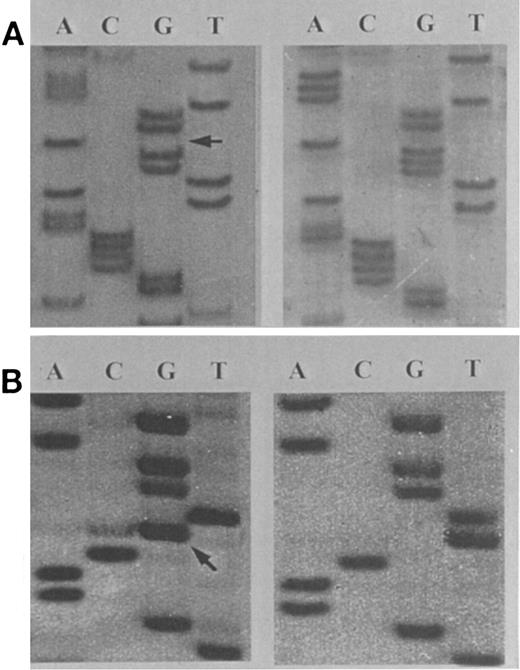
Sequence analysis of amplification products of exon 13 of the hMSH2 gene in human LBL (A and B). (A) A patient with a heterozygous frameshift mutation resulting from a 1-bp deletion at codon 672-673. (B) A patient with a heterozygous missense C → A mutation at codon 724, displayed in an antisense configuration. The locations of the mutations are indicated with arrows.
Sequence analysis of amplification products of exon 13 of the hMSH2 gene in human LBL (A and B). (A) A patient with a heterozygous frameshift mutation resulting from a 1-bp deletion at codon 672-673. (B) A patient with a heterozygous missense C → A mutation at codon 724, displayed in an antisense configuration. The locations of the mutations are indicated with arrows.
DISCUSSION
The results presented here show that hMSH2, one of the critical genes within the MMR system, is mutated in human precursor T-cell LBL. Defects in the genes that comprise the MMR system can cause the microsatellite instability (ie, destabilization of tracts of simple repetitive DNA) observed in many hereditary colorectal cancers and in some sporadic cancers of the colon, ovary, pancreas, and endometrium.6,7,9-13,25,26 Microsatellite instability has not been shown to be a consistent feature of hemato-lymphoid malignancies and consequently it has been assumed that defects of the MMR system do not contribute to the development of these tumors.27 However, because of our observations that lymphomas arising in MSH2−/− mice represent a single histopathologic entity closely resembling human precursor T-cell LBL and have similar patterns of proto-oncogene expression as the human counterpart, we reasoned that mutations in hMSH2 may be present in human LBL.
In our study, hMSH2 coding region mutations were identified in 2 of 10 human LBL cases. In studies of HNPCC, hMSH2 mutations have been recognized in 10% to 40% of kindreds and have been found to be dispersed throughout exons 5-14 with no mutational ‘hot spots’ identified.8,28 In the majority of cases, mutations leading to premature termination codons have been described. In approximately 20% of HNPCC kindreds, missense mutations have been detected that are considered significant because they segregate with disease and occur in conserved domains. In one of our LBL cases, we found a not previously described premature termination codon. We believe the missense mutation identified in the other LBL case is also significant because it results in the substitution of a basic amino acid for a neutral amino acid and occurs within a domain conserved in all MutS homologs that is thought to mediate binding of MSH2 to heteroduplex DNA.7,23 24 Our evaluation of mutations in hMSH2 focused exclusively on exon 13 and the flanking regions of the genomic DNA containing the intron/exon junctions. Thus, it is likely that our study underestimates the frequency of hMSH2 mutations in human LBL because other exons, the promoter, intronic, and 3′ untranslated regions of the hMSH2 gene were not investigated.
The finding of the T → C substitution at the −6 position of the splice acceptor site of exon 13 of hMSH2 in 5 of 10 human LBL cases may be significant. This substitution is a recognized polymorphism that is found in the germline of approximately 8% of normal individuals.29 In HNPCC this substitution has been reported to segregate with disease and has also been associated with mutations in the coding region of hMSH2.7 In the evaluation of patients with chronic ulcerative colitis this substitution was found at a significantly increased frequency in the germline of those patients who progressed to high-grade dysplasia or carcinoma.29 This substitution has also been reported as an acquired change in repair-defective sporadic colon cancers whose matching constitutional DNA was wild type.7 Transitions within the donor acceptor splice sites may result in the attenuation of transcription, or the utilization of alternate splice acceptor sites, to produce aberrant proteins.30 31
Remarkably, the neoplasms that developed in all 20 MSH2−/− mice by 13 months of age were exclusively of lymphoid origin and comprised a single histopathologic entity. This histopathologic uniformity is unlike that observed in the knockout murine model of another well-known tumor suppressor gene. Mice deficient in p53 are susceptible to lymphomas, but also develop a variety of malignancies of nonlymphoid origin at a similar age.32 Perhaps the reason for histopathologic uniformity in the tumors of MSH2-deficient mice is that in the developing thymus of newborn mice, the high turnover of maturing T cells offers a window in which MSH2 deficiency can accelerate the accumulation of transforming events.
We evaluated the murine lymphomas for the expression of RBTN-2, TAL-1, and HOX-11, three proto-oncogenes implicated in the development of human precursor T-cell malignancies.16 We found aberrant expression of RBTN-2 and TAL-1 in 100% and 40% of the murine tumors, respectively, while none expressed HOX-11. These proto-oncogenes were not expressed in histologically normal thymuses of MSH2−/− mice. These findings show an association between MSH2 deficiency and the aberrant expression of specific oncogenes and support that RBTN-2 and TAL-1 expression is important in the development of LBL. The molecular mechanism(s) by which these genes become activated is not known. The nearly identical proto-oncogene expression profiles observed in the murine lymphomas and human LBL provides additional evidence that the MSH2−/− mouse is a relevant model of human LBL. This finding, taken together with the observation of codon region mutations in 2 of 10 of the human cases, further strengthens the contention that defects in hMSH2 are implicated in the development of human LBL.
Our finding that 40% of the lymphomas arising in MSH2-deficient mice co-express RBTN-2 and TAL-1 is in keeping with a recent study which showed that protein dimerization between RBTN-2 and Tal-1 altered thymocyte development and potentiated T-cell tumorigenesis in double transgenic mice made to coexpress both of these genes.33 Hence, Tal-1 represents one likely candidate DNA binding protein that promotes precursor T-cell neoplasia in MSH2−/− mice in concert with RBTN-2.33-35 Therefore, we hypothesize that the aberrant expression of the cooperating oncogenes RBTN-2 and TAL-1 represent secondary events in the development of malignancy as a result of MSH2 deficiency. Conversely, the finding that not all of the murine tumors expressed TAL-1 suggests that RBTN-2 interacts with other proteins to induce lymphomagenesis. Therefore, these mice represent a valuable model for the identification of other proteins that may interact with RBTN-2 in the development of T-cell neoplasms.
We have shown that the lymphoblastic lymphomas arising in MSH2-deficient mice are the counterpart of human precursor T-cell lymphoblastic lymphoma and represent an excellent model for investigating the role of mismatch repair in lymphomagenesis. Furthermore, the identification of mutations in hMSH2, one of the critical genes of the MMR system, in human lymphoblastic lymphoma defines a new pathway of human lymphomagenesis.
R.L. and J.F.D. contributed equally and are recipients of Medical Research Council of Canada Awards.
Address reprint requests to Mark D. Minden MD, PhD, FRCPC, Professor of Medicine, University of Toronto, Princess Margaret Hospital, 610 University Ave, Toronto, Ontario, Canada, M5G-2M9.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal