Abstract
To examine the safety and efficacy of recombinant-methionyl human stem cell factor (r-metHuSCF), 38 patients with intermediate-grade or immunoblastic high-grade non-Hodgkin's lymphoma who were eligible for autologous transplantation were randomized to receive r-metHuSCF (5, 10, 15, or 20 μg/kg/d) plus Filgrastim (10 μg/kg/d) or Filgrastim (10 μg/kg/d) alone to mobilize peripheral blood progenitor cells. Subcutaneous administration of r-metHuSCF was well tolerated in conjunction with a multi-agent pre-medication regimen; local injection site reactions were the most commonly seen adverse event. The total mononuclear cell count, CD34+ cell content, granulocyte-macrophage colony-forming cells (GM-CFC), and burst-forming units-erythroid (BFU-E) per kilogram in the apheresis product was similar when all patients were analyzed by treatment cohort and mobilization regimen (Filgrastim or r-metHuSCF in combination with Filgrastim); however, when prior chemotherapy was taken into account in a supplementary analysis, clinically important differences were observed. Extensive prior therapy was defined as the amount of exposure to specific stem cell toxic chemotherapeutic agents that patients received. These agents include procarbazine, nitrogen mustard, melphalan, nitrosoureas (≥2 cycles of any of these drugs) or greater than 7.5 g of cytosine arabinoside. In these patients, there was an increased number of CD34+ cells (1.76 v 0.28 × 106/kg), GM-CFC (20.5 v 5.0 × 104/kg), and BFU-E (36.9 v 8.9 × 104/kg) in patients receiving r-metHuSCF and Filgrastim (N = 18) compared with Filgrastim alone (N = 5). These patients also had a decreased time to an untransfused platelet count of 20 × 109/L that was 10.5 days shorter in the patients who received r-metHuSCF and Filgrastim (12.5 v 23 days). These differences were not found to be statistically significant, possibly because of small size, but are clinically important.
PERIPHERAL BLOOD progenitor cell (PBPC) transplantation has been found to be safe and effective in accelerating hematopoetic reconstitution after myelosuppresive or myeloablative therapy. Collection of PBPCs is more efficient and engraftment occurs more rapidly in patients who have mobilized PBPCs than in patients who have PBPCs collected at steady state.1 The most common methods for PBPC mobilization are by the use of cytotoxic chemotherapy,2,3 specific cytokines,4,5 or both chemotherapy and cytokines.6,7 However, the optimal method for mobilizing PBPCs into the circulation has not yet been defined. Chemotherapy-induced mobilization is associated with a risk of neutropenic fever and infection, and can create difficulty scheduling the apheresis procedures due to variable rates of recovery. In contrast, cytokines provide predictable kinetics of mobilization with minimal morbidity. The use of Filgrastim-mobilized PBPCs after myeloablative therapy is associated with the following: (1) more rapid recovery of neutrophils (defined as days to absolute neutrophil count [ANC] >0.5 × 109/L), (2) faster engraftment of platelets, with an associated decrease in platelet transfusions, (3) a decrease in the number of red blood cell (RBC) transfusions, and (4) a decrease in the number of days of hospitalization compared with bone marrow (BM) autografts.8
Soluble human stem cell factor (SCF) is a naturally occurring 36-kD homodimeric glycoprotein that contains 165 amino acids per monomer and acts on lineage-committed and non–lineage-committed hematopoietic progenitor cells.9-11 In culture, recombinant SCF stimulates proliferation of primitive undifferentiated blast cells and synergizes with other hematopoietic growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF ), G-CSF, erythropoietin (EPO), interleukin-3 (IL-3), and IL-6 to stimulate proliferation of erythroid, megakaryocytic, granulocytic, mast, and basophil progenitor cells.12 13 The SCF receptor (c-kit) is present on many tissues of neuroectodermal, epithelial, and hematopoietic origin; to date, no B- or T-cell non-Hodgkin's lymphoma (NHL) tumor specimens have stained positive for c-kit.14 15
In rodents,16 dogs,17 and baboons,18,19 species-specific recombinant SCF produces leukocytosis with modest increases in the number of erythroid and lymphoid cells in the PB and marked increases in the number of stem cells both in the BM and PB. The combination of low doses of PEGylated recombinant rat-SCF (rrSCF) plus optimal doses of Filgrastim caused a synergistic increase in PBPCs in mice compared to treatment with either of these hematopoietic growth factors alone.20 In lethally irradiated mice, PBPCs mobilized with the combination of rrSCF plus Filgrastim provided faster and more efficient engraftment than PBPCs mobilized by rrSCF or Filgrastim alone.20 This observation was confirmed in a canine model where administration of recombinant canine G-CSF (rcGCSF) for 7 days led to a 5.4-fold increase in colony-forming unit GM (CFU-GM) but the combination of rcSCF and rcG-CSF led to a 21.6-fold increase.21 In the canine study, the PBPCs collected following the administration of rcSCF and rcG-CSF were able to rescue lethally irradiated dogs. Baboons administered low-dose recombinant-methionyl human SCF plus Filgrastim had a 1.8-fold increase in PB mononuclear cells and a 14-fold increase in PBPCs compared with baboons mobilized by Filgrastim alone.22 All of the baboons received a lethal dose of irradiation and then were transplanted with their growth-factor–mobilized PBPCs. All animals engrafted; however, the time to platelet count of 20 × 109/L was 8 days for the animals receiving PBPCs mobilized with recombinant methionyl human SCF (r-metHuSCF) and Filgrastim versus 42 days for animals receiving PBPCs mobilized with Filgrastim alone. This encouraging data provided the framework for human clinical trials with r-metHuSCF.
Phase I studies of r-metHuSCF have been conducted in patients with non–small cell lung cancer23 (Crawford J., et al, manuscript in preparation) and advanced breast cancer24 (Demetri G., et al, manuscript in preparation). These studies examined the safety of repetitive administration of r-metHuSCF at doses of either 5, 10, 25, or 50 μg/kg/d administered before and after chemotherapy. Virtually all patients experienced mild to moderate dermatologic reactions at the injection site including edema, erythema, pruritus, skin hyperpigmentation, and/or urticaria. Thirteen of 56 patients in phase I trials exhibited moderate to severe multisystem allergic-like reactions which included respiratory symptoms (cough, sore throat, throat tightness, and/or dyspnea) with or without generalized urticaria. All of these reactions were transient and reversible. Approximately 50% of these patients were not premedicated with antihistamines and some also had a previous history of atopy. These reactions are believed to be mast cell–mediated and appear to be dose related, occurring primarily in those patients who received ≥25 μg/kg/d of r-metHuSCF. The hematologic effects of r-metHuSCF included increases in neutrophils as well as a trend to more rapid recovery of neutrophils and platelets postchemotherapy. Increased numbers of PBPCs were also noted in the PB of patients treated with r-metHuSCF, although it did not appear that r-metHuSCF used as a single agent was able to mobilize sufficient progenitors for PBPC transplantation.
Based on these results in animals and patients, we have examined the use of r-metHuSCF in combination with Filgrastim in an attempt to further improve the quality and quantity of PBPCs collected and in turn reduce the duration of myelosuppression after myeloablative therapy. In this phase I/II study we evaluated r-metHuSCF in combination with Filgrastim versus Filgrastim alone, as a control group, for PBPC mobilization in patients with intermediate-grade and immunoblastic high-grade NHL. In addition, we assessed the ability of PBPCs collected via these two mobilization strategies to promote engraftment after myeloablative therapy. A premedication regimen designed to reduce the toxicity of r-metHuSCF administration was used.
PATIENTS AND METHODS
Patient Eligibility
Patients with histologically confirmed intermediate-grade or immunoblastic high-grade NHL in second remission or with chemosensitive disease in first relapse were enrolled at one of the four study centers. Chemosensitive disease was defined as at least a partial response to standard-dose salvage chemotherapy. Eligible patients were between the ages of 18 and 65, had a Karnofsky performance status greater than 80%, and had a life expectancy of at least 6 months. Patients were required to have an ANC >1.5 × 109 cells/L, platelet count >100 × 109/L, hemoglobin level >9 g/dL, adequate major organ function as defined by serum creatinine and bilirubin of <2 mg/dL, forced expiratory volume (FEV1) and forced vital capacity (FVC) greater than 60%, and a cardiac ejection fraction (LVEF) within the institution's normal range.
Patients who had received hematopoietic growth factor therapy within 2 weeks before study entry, prior radiotherapy to ≥25% of the BM, and those with BM or central nervous system (CNS) involvement by lymphoma were excluded. Patients with other malignancies within the past 5 years, with the exception of surgically cured basal cell carcinoma of the skin or in situ carcinoma of the cervix were excluded, as were any patients who had undergone a prior BM transplant. Other exclusion criteria included current or recent symptoms of asthma occurring within the last 3 years, significant IgE-mediated hypersensitivity (including allergic rhinitis, allergic eczema, known history of anaphylactic reactions, chronic recurrent urticaria, or acquired or congenital angioedema), active infection or fever greater than 38.2°C, human immunodeficiency virus (HIV) seropositivity, known allergy to Escherichia coli–derived products, pregnancy or breast feeding, or significant nonmalignant disease. Patients could not have received an investigational drug within 4 weeks of study entry. The concurrent use of β adrenergic blocking agents, or concurrent use or use within the past 2 weeks of a monoamine oxidase inhibitor (MAO), was prohibited due to potential interactions with the r-metHuSCF premedications. Patients with psychiatric, addictive, or any disorder which compromised their ability to give truly informed consent were also excluded.
Study Design
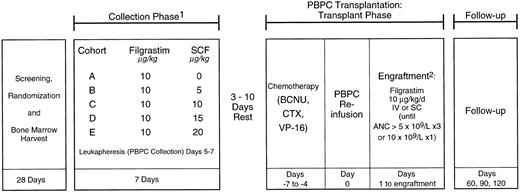
Written informed consent was obtained from all patients who were enrolled on this phase I/II study approved by the Institutional Review Boards of all participating centers. Patients were randomized to receive either r-metHuSCF (r-metHuSCF; Amgen Inc, Thousand Oaks, CA) in combination with Filgrastim (r-metHuSCF; Amgen Inc) or Filgrastim alone (see Fig 1). All patients had a BM harvest performed after randomization, but before hematopoietic growth factor treatment began, which was cryopreserved as a back-up progenitor cell source should the PBPCs not engraft. This study was divided into two parts: collection phase (mobilization) and transplant phase.
Study schema. NHL patients were randomized to receive Filgrastim 10 μg/kg/d or Filgrastim and one of four doses of r-metHuSCF (5, 10, 15, or 20 μg/kg/d) for 7 days. All patients were leukapheresed on days 5, 6, and 7 of the collection phase. After a 3- to 10-day rest period, patients received the transplant chemotherapy regimen followed by PBPC infusion and Filgrastim 10 μg/kg/d until ANC ≧5.0 × 109/L for 3 days or 10 × 109/L for 1 day. 1Premedications consisting of diphenhydramine and ranitidine were administered beginning 12 to 24 hours before r-metHuSCF and continuing for at least 48 hours after the last injection. In addition, pseudoephedrine and albuterol were administered 30 to 60 minutes before each injection. 2BM backup to be given if failure to engraft by day 28.
Study schema. NHL patients were randomized to receive Filgrastim 10 μg/kg/d or Filgrastim and one of four doses of r-metHuSCF (5, 10, 15, or 20 μg/kg/d) for 7 days. All patients were leukapheresed on days 5, 6, and 7 of the collection phase. After a 3- to 10-day rest period, patients received the transplant chemotherapy regimen followed by PBPC infusion and Filgrastim 10 μg/kg/d until ANC ≧5.0 × 109/L for 3 days or 10 × 109/L for 1 day. 1Premedications consisting of diphenhydramine and ranitidine were administered beginning 12 to 24 hours before r-metHuSCF and continuing for at least 48 hours after the last injection. In addition, pseudoephedrine and albuterol were administered 30 to 60 minutes before each injection. 2BM backup to be given if failure to engraft by day 28.
Collection Phase
During the collection phase, patients randomized to receive r-metHuSCF received prophylaxis designed to prevent or minimize symptoms potentially caused by the release of mast cell mediators by being treated with the following premedications: diphenhydramine 50 mg every 6 hours, ranitidine 150 mg every 12 hours, pseudoephedrine 120 mg orally 60 minutes before injection of r-metHuSCF, and albuterol 2 puffs 30 to 60 minutes before injection of r-metHuSCF. Diphenhydramine and ranitidine were started 12 to 24 hours before the first injection of r-metHuSCF and continued for at least 48 hours after the last injection. Patients who had mild to moderate distant skin reactions despite this prophylactic regimen had the dose of diphenhydramine doubled. One of four dosages of r-metHuSCF (5, 10, 15, and 20 μg/kg/d) were administered in combination with Filgrastim at 10 μg/kg/d to each patient to determine the optimal dosage of r-metHuSCF. The doses were selected based on results from earlier phase I studies23 24 (Crawford J., et al, manuscript in preparation and Demetri G., et al, manuscript in preparation).
Treatment with r-metHuSCF and Filgrastim or Filgrastim alone (10 μg/kg/d) was started on day 1 of the collection phase and continued for 7 days (Fig 1). Filgrastim was used as the comparative group to evaluate its potential synergy with an earlier acting cytokine, r-metHuSCF, to mobilize PBPC in patients undergoing myeloablative chemotherapy and PBPC transplantation. Patients were randomized (1:2) between the control group A (Filgrastim alone) and whichever of the sequential r-metHuSCF and Filgrastim cohorts (B, C) was open at the time of each patient's enrollment. After observing an acceptable safety profile of r-metHuSCF in cohorts B and C, two additional cohorts (cohorts D, 15 μg/kg; and E, 20 μg/kg) were added to this study. At the time that these additional cohorts were added, a statistical review of the patient sample size requirements indicated that it was no longer necessary to maintain the same ratio of randomization between the combination cytokine cohort and the Filgrastim cohort to obtain an adequate number of patients receiving Filgrastim alone. Randomization for these two cohorts was therefore done in a 1:4 ratio between Filgrastim and the combination of r-metHuSCF and Filgrastim. r-metHuSCF and Filgrastim were administered as separate subcutaneous injections. Filgrastim was administered as the first injection to patients who received both hematopoietic growth factors and the injection of r-metHuSCF that followed was administered in a different area of the body. The first injection of r-metHuSCF was administered to patients on an inpatient basis. Subsequent injections were given in the clinic and were followed by 4 hours of observation by qualified healthcare personnel.
On days 5 through 7 of the collection phase of the study, leukapheresis was performed through a hemodialysis-quality central venous catheter using a Cobe Spectra leukapheresis machine (CS3000; Baxter Healthcare Corp, Deerfield, IL). Ten liters of blood was processed over a 3- to 6-hour period for each procedure. The minimum target collection was 4 × 108 mononuclear cells (MNC)/kg, which was based on standard procedures used in 1993. However, the number of CD34+ cells, GM colony-forming cells (GM-CFC), and burst-forming units-erythroid (BFU-E) were also assessed at a centralized lab. All patients were required to undergo 3 days of leukapheresis regardless of the yield achieved on days 1 and 2. If the minimum yield was not reached after 3 days of leukapheresis, a fourth leukapheresis was performed to obtain the target level of mononuclear cells. Patients requiring four aphereses were considered mobilization failures but were permitted to continue on to transplant if the target number of MNC was obtained. All leukapheresis products were cryopreserved in 10% dimethylsulfoxide (DMSO) and were viably frozen and stored at less than −135°C. Complete blood counts were done daily. If the white blood cell (WBC) count was >100 × 109/L, the dose of Filgrastim was to be decreased to 1 μg/kg/d.
Transplant Phase
Three to 10 days postleukapheresis, patients began a 4-day myeloablative regimen consisting of cyclophosphamide (6,000 mg/m2), carmustine (450 mg/m2), and etoposide (1,600 mg/m2), given on days −7 to −4. PBPC infusion occurred on day 0 of the treatment phase. All patients received prophylactic antibiotics including trimethoprim-sulfamethoxazole until day −2 and norfloxacin until the first fever (≥38°C); patients who tested seropositive for herpes simplex virus received acyclovir. All patients received Filgrastim 10 μg/kg/d subcutaneously or intravenously beginning on day 0 and continuing until the ANC was >5.0 × 109/L for 3 days or 10.0 × 109/L for 1 day. Infusion of the back-up BM was to be performed if the ANC was still <0.5 × 109/L on day 28.
Progenitor Cell Assays
All progenitor cell assays (MNC, GM-CFC, and BFU-E) and CD34+ cell enumeration were done in the Department of Developmental Hematology at Amgen. PB assays were performed on samples collected daily on days 1, 4, 5, 6, and 7 for all patients. In cohorts A and B, assays were also done on samples collected on days 8, 11, and 14. One-milliliter aliquots from each of the apheresis products were obtained daily during apheresis for progenitor cell assays.
An MNC fraction was prepared from all samples by density centrifugation on a Ficoll-hypaque gradient (Pharmacia Biotech, Uppsala, Sweden). The low-density fraction was collected, and the total WBC count and MNC number was determined using a nuclear stain (1% crystal violet in acetic acid) with scoring on a hemocytometer.
MNC were cultured at 100,000 and 10,000 cells per plate in a double-layer agarose (FMC Biologicals, Rockland, ME) system in triplicate. GM-CFC assays were performed using optimal doses of r-metHuSCF (100 ng/culture), recombinant human IL-3 (rhIL-3) (100 ng/culture), rhIL-6 (100 ng/culture), rhGM-CSF (100 ng/culture). Cultures were incubated for 14 days after which GM-CFC were scored using a dissecting microscope, counting colonies containing 50 or more cells. All growth factors were prepared and provided by Amgen Inc and were purified to greater than 99% purity.
BFU-E cultures were performed using the same doses of r-metHuSCF, rhIL-3, and rhIL-6 as were used for GM-CFC cultures with the addition of rhEpo (4 U/culture) and cultures were incubated for 14 days. BFU-E were scored using a dissecting microscope counting all hemoglobinized colonies containing 50 or more cells.
CD34+ Cell Analysis
Samples were directly labeled by incubation with a phycoerythrin-conjugated antibody to CD34 (HPCA-2; Becton Dickinson, Mountain View, CA) for 30 minutes. Unbound antibody was removed by washing; the cells were then resuspended in 2 mL of phosphate-buffered saline (PBS; GIBCO, Grand Island) plus 2% BSA and treated with lysing solution (FACS lysing solution; Becton Dickinson). The fluorescence was analyzed using a FACSCAN analyzer (Becton Dickinson). A blast window was established on forward- versus side-scatter analysis and positive fluorescence determined by comparison of the side-scatter versus fluorescence for cells in this blast window. A nonspecific antibody control was run for all samples and the nonspecific labeling was subtracted from the test sample. For each sample, 50,000 events in the blast window were collected and represented a range of 100,000 to 750,000 total events accumulated. The percent CD34+ cells in the sample were calculated as follows: (% of CD34+ events in blast window) × (% of total cells in the blast window) − (nonspecific AB control). The calculations for CD34+ cells/kg were: (% CD34 in sample × WBC of leukapheresis × volume of leukapheresis)/kg weight of patient.
Statistics
A modified intent-to-treat analysis was done and included all patients who received hematopoietic growth factors and who were leukapheresed on days 5, 6, and 7 (N = 35). These data were also analyzed by the extent of previous chemotherapy received, due to the importance of this covariable in this patient population. Previous treatment was retrospectively classified as extensive if the patient had received one of the following stem cell toxic chemotherapy drugs: procarbazine, nitrogen mustard, melphalan, nitrosoureas (two or more cycles of any of these agents), or high-dose cytosine arabinoside (≥7.5 g).
Efficacy was assessed separately during the collection phase and the treatment phase. The collection phase included mobilization and apheresis. Mobilization variables during the collection phase included GM-CFC, CD34+ cells, BFU-E, and MNC. The treatment phase included PBPC transplant, treatment with Filgrastim, and engraftment. Progenitor cell mobilization and subsequent recovery variables were analyzed by treatment cohort (A through E), by hematopoietic growth factors used (Filgrastim alone v Filgrastim and r-metHuSCF), and amount of prior chemotherapy. Total harvests, for all 3 days of leukapheresis, are summarized for each mobilization variable. During the treatment phase, variables included time to neutrophil recovery (≥0.5 × 109/L and ≥1.0 × 109/L) and time to platelet recovery (≥20.0 × 109/L and ≥50.0 × 109/L) without transfusion for 48 hours. Analysis of variance (ANOVA) was used to assess prior chemotherapy (extensive, not extensive) along with treatment (Filgrastim alone, all Filgrastim and r-metHuSCF combinations), and their interaction with respect to mobilization variables (CD34+ cells, GM-CFC, BFU-E, MNC). The CD34+ cells, GM-CFC, and BFU-E variables analyzed were log transformed to adhere to parametric distribution assumptions in this model.
The Generalized Wilcoxon Test25 was used to compare prior chemotherapy across all dose cohorts as this test was used to compare Filgrastim alone to all r-metHuSCF and Filgrastim combinations within each level of prior chemotherapy for time to ANC and platelet recovery.
All statistical analyses were conducted at the .05 level of significance and were two-sided. All calculations were performed using SAS version 6.09 (SAS Institute Inc, Cary, NC) using SUN OS release 4.13 (SUN Microsystems Inc, Mountain View, CA).
RESULTS
Patient Profile
Thirty-eight patients diagnosed with intermediate-grade or immunoblastic high-grade NHL in second remission or who had chemosensitive disease in first relapse were enrolled on this study at one of four study sites. Patient characteristics are outlined in Table 1.
Patient Demographics
| Treatment Cohort . | A . | B . | C . | D . | E . | Total . |
|---|---|---|---|---|---|---|
| r-metHuSCF ( μg/kg/d) | 0 | 5 | 10 | 15 | 20 | |
| Filgrastim ( μg/kg/d) | 10 | 10 | 10 | 10 | 10 | |
| Age (yr) | ||||||
| N | 11 | 6 | 6 | 7 | 8 | 38 |
| Median | 51.0 | 51.0 | 45.0 | 55.0 | 48.5 | 49.0 |
| Range | 21.0-61.0 | 26.0-63.0 | 23.0-58.0 | 41.0-59.0 | 29.0-61.0 | 21.0-63.0 |
| Sex | ||||||
| Men | 6 | 6 | 5 | 5 | 6 | 28 |
| Women | 5 | 0 | 1 | 2 | 2 | 10 |
| Karnofsky performance | 90-100 | 80-100 | 80-100 | 80-100 | 90-100 | 80-100 |
| Status range (median) | (100) | (100) | (85) | (90) | (90) | (90) |
| Disease stage at enrollment | ||||||
| II | 1 | 0 | 3 | 1 | 1 | 6 |
| IIE | 0 | 0 | 0 | 0 | 1 | 1 |
| III | 4 | 2 | 0 | 1 | 0 | 7 |
| IIIE | 1 | 0 | 0 | 0 | 2 | 3 |
| IV | 5 | 3 | 3 | 5 | 3 | 19 |
| Chemosensitive disease in first relapse | 5 | 4 | 5 | 4 | 4 | 22 |
| Second remission | 5 | 2 | 1 | 3 | 3 | 14 |
| Treatment Cohort . | A . | B . | C . | D . | E . | Total . |
|---|---|---|---|---|---|---|
| r-metHuSCF ( μg/kg/d) | 0 | 5 | 10 | 15 | 20 | |
| Filgrastim ( μg/kg/d) | 10 | 10 | 10 | 10 | 10 | |
| Age (yr) | ||||||
| N | 11 | 6 | 6 | 7 | 8 | 38 |
| Median | 51.0 | 51.0 | 45.0 | 55.0 | 48.5 | 49.0 |
| Range | 21.0-61.0 | 26.0-63.0 | 23.0-58.0 | 41.0-59.0 | 29.0-61.0 | 21.0-63.0 |
| Sex | ||||||
| Men | 6 | 6 | 5 | 5 | 6 | 28 |
| Women | 5 | 0 | 1 | 2 | 2 | 10 |
| Karnofsky performance | 90-100 | 80-100 | 80-100 | 80-100 | 90-100 | 80-100 |
| Status range (median) | (100) | (100) | (85) | (90) | (90) | (90) |
| Disease stage at enrollment | ||||||
| II | 1 | 0 | 3 | 1 | 1 | 6 |
| IIE | 0 | 0 | 0 | 0 | 1 | 1 |
| III | 4 | 2 | 0 | 1 | 0 | 7 |
| IIIE | 1 | 0 | 0 | 0 | 2 | 3 |
| IV | 5 | 3 | 3 | 5 | 3 | 19 |
| Chemosensitive disease in first relapse | 5 | 4 | 5 | 4 | 4 | 22 |
| Second remission | 5 | 2 | 1 | 3 | 3 | 14 |
Thirty-six of 38 patients received cytokine(s) and were included in the safety analysis. The other two patients had evidence of tumor progression before beginning hematopoietic growth factor injections. One patient was not included in the efficacy analysis because he received only two doses of hematopoietic growth factors before going off study due to a moderate multi-system allergic-like reaction; no leukaphereses were performed on this patient during the time he was on study (see Results, Toxicity data). Two patients were not included in the evaluation for engraftment in the treatment phase; one patient in cohort A did not meet the minimum collection of MNC required to continue on to transplant and one patient was not leukapheresed on the correct days specified in the protocol (days 6, 7, and 8 rather than 5, 6, and 7).
Collection Phase
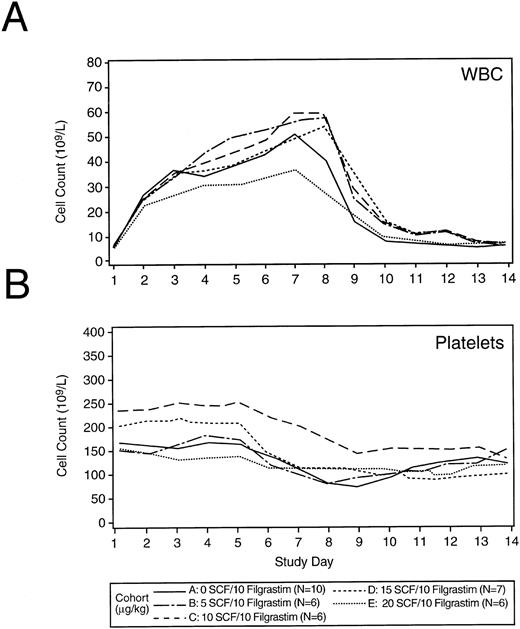
Blood cell counts.The peripheral WBC count peaked on days 7 through 8 post initiation of hematopoietic growth factors (17 to 116.5 × 109/L) and returned to baseline by about day 10 (Fig 2A). No dose reductions in Filgrastim were required. The administration of r-metHuSCF did not result in a dose-dependent augmentation of WBC and ANC count when combined with 10 μg/kg/d of Filgrastim. Hemoglobin concentration was unaffected during the collection phase (data not shown). Platelet counts were stable until the initiation of leukapheresis (day 5) when the expected decrease in platelet counts was observed (Fig 2B).
Median WBC count and platelet values by treatment cohort alone during the collection phase. Panels (A) and (B) show the peripheral WBC count and platelet counts during the collection phase of the study for each treatment cohort. Cytokines (Filgrastim or various doses r-metHuSCF in combination with Filgrastim) were administered on days 1 through 7 for all patients. The dose of Filgrastim was 10 μg/kg/d for all patients. The dose of r-metHuSCF (0 to 20 μg/kg/d) is identified by cohort in the legend. The number of patients in each group are also listed in the legend.
Median WBC count and platelet values by treatment cohort alone during the collection phase. Panels (A) and (B) show the peripheral WBC count and platelet counts during the collection phase of the study for each treatment cohort. Cytokines (Filgrastim or various doses r-metHuSCF in combination with Filgrastim) were administered on days 1 through 7 for all patients. The dose of Filgrastim was 10 μg/kg/d for all patients. The dose of r-metHuSCF (0 to 20 μg/kg/d) is identified by cohort in the legend. The number of patients in each group are also listed in the legend.
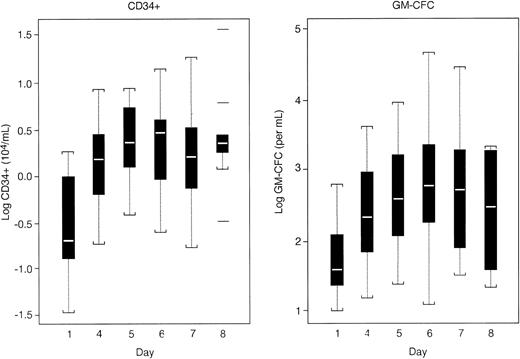
Progenitor cell quantitation.Mobilization of PBPCs as measured by the number of circulating CD34+ cells, MNC, GM-CFC, and BFU-E occurred in all patients. CD34+ cells and GM-CFC in the PB peaked on days 5 through 7 of the collection phase in all cohorts (Fig 3). No difference was observed in the median levels of CD34+ cells and GM-CFC in patients mobilized by Filgrastim compared to patients mobilized with the combination of r-metHuSCF and Filgrastim. Based on these data, it appears that the best collection could be obtained by leukapheresing patients on days 5, 6, and 7. This appeared to be true for all cohorts evaluated in this study. BFU-E values in the PB peaked on days 4 through 6 except for patients receiving 15 μg/kg of r-metHuSCF in combination with Filgrastim (data not shown). In these patients, BFU-E continued to increase through day 7 (last day of hematopoietic growth factor administration). There was no significant difference in the total number of PBPCs, either in the PB or in the apheresis product, using Filgrastim alone at 10 μg/kg/d (cohort A) versus Filgrastim at 10 μg/kg/d in combination with doses of 5 to 15 μg/kg/d of r-metHuSCF (cohorts B-D) as measured by the numbers of CD34+, GM-CFC, BFU-E, and MNC (Table 2). There was a nonstatistically significant decrease in the median collection of PBPCs seen in cohort E, (r-metHuSCF 20 μg/kg/d in combination with Filgrastim) for all measures of PBPC collection (CD34+ cells, GM-CFC, and BFU-E), which may be attributed to the fact that all patients in this cohort were considered to be extensively pretreated (see section titled “Effect of Prior Therapy” for definition of extensively pretreated patients).
Boxplot of PB CD34+ and GM-CFC levels during the collection phase (all patients). PB levels of CD34+ cells and GM-CFC were evaluated for all patients on days 1, 4, 5, 6, 7, and 8 during the mobilization phase of the study. Patients received cytokines (Filgrastim or r-metHuSCF and Filgrastim) on days 1 through 7. All patients were leukapheresed on days 5 through 7.
Boxplot of PB CD34+ and GM-CFC levels during the collection phase (all patients). PB levels of CD34+ cells and GM-CFC were evaluated for all patients on days 1, 4, 5, 6, 7, and 8 during the mobilization phase of the study. Patients received cytokines (Filgrastim or r-metHuSCF and Filgrastim) on days 1 through 7. All patients were leukapheresed on days 5 through 7.
Total Progenitor Collection by Treatment Cohort
| Treatment Cohort . | A . | B . | C . | D . | E . |
|---|---|---|---|---|---|
| r-metHuSCF ( μg/kg/d) | 0 | 5 | 10 | 15 | 20 |
| Filgrastim ( μg/kg/d) | 10 | 10 | 10 | 10 | 10 |
| (N = 10) | (N = 6) | (N = 6) | (N = 7) | (N = 6) | |
| CD34+ cells (×106/kg) | |||||
| Median | 3.02 | 3.92 | 2.65 | 3.32 | 0.37 |
| Range | (0.03-20.68) | (0.09-5.84) | (1.03-6.84) | (1.40-12.04) | (0.10-5.90) |
| GM-CFC (×104/kg) | |||||
| Median | 19.21 | 32.37 | 32.47 | 65.89 | 4.11 |
| Range | (0.10-123.07) | (6.34-95.68) | (7.97-121.0) | (26.73-223.76) | (0.13-16.47) |
| BFU-E (×104/kg) | |||||
| Median | 16.89 | 54.19 | 75.60 | 122.71 | 6.93 |
| Range | (0.36-131.55) | (4.79-171.95) | (27.14-161.31) | (23.07-601.46) | (0.00-46.86) |
| MNC (×109/L) | |||||
| Median | 7.55 | 9.31 | 6.95 | 7.41 | 6.50 |
| Range | (2.94-22.42) | (2.21-13.12) | (3.37-10.99) | (5.45-11.58) | (4.81-11.24) |
| Treatment Cohort . | A . | B . | C . | D . | E . |
|---|---|---|---|---|---|
| r-metHuSCF ( μg/kg/d) | 0 | 5 | 10 | 15 | 20 |
| Filgrastim ( μg/kg/d) | 10 | 10 | 10 | 10 | 10 |
| (N = 10) | (N = 6) | (N = 6) | (N = 7) | (N = 6) | |
| CD34+ cells (×106/kg) | |||||
| Median | 3.02 | 3.92 | 2.65 | 3.32 | 0.37 |
| Range | (0.03-20.68) | (0.09-5.84) | (1.03-6.84) | (1.40-12.04) | (0.10-5.90) |
| GM-CFC (×104/kg) | |||||
| Median | 19.21 | 32.37 | 32.47 | 65.89 | 4.11 |
| Range | (0.10-123.07) | (6.34-95.68) | (7.97-121.0) | (26.73-223.76) | (0.13-16.47) |
| BFU-E (×104/kg) | |||||
| Median | 16.89 | 54.19 | 75.60 | 122.71 | 6.93 |
| Range | (0.36-131.55) | (4.79-171.95) | (27.14-161.31) | (23.07-601.46) | (0.00-46.86) |
| MNC (×109/L) | |||||
| Median | 7.55 | 9.31 | 6.95 | 7.41 | 6.50 |
| Range | (2.94-22.42) | (2.21-13.12) | (3.37-10.99) | (5.45-11.58) | (4.81-11.24) |
Toxicity data.Mild to moderate cutaneous skin reactions, which occurred predominantly at the injection site, were reported in 88% of the patients who received r-metHuSCF. These injection site reactions occurred within 24 to 48 hours after r-metHuSCF administration and spontaneously resolved within 24 to 48 hours of first observation. The severity of the reactions was not dose related and frequently improved during leukapheresis. Two patients (8%) developed more generalized cutaneous toxicity, including erythema in one patient and urticaria with pruritus in another.
Musculoskeletal symptoms believed to be related to hematopoietic growth factor therapy were seen in 23% (6 of 26) of the patients treated with r-metHuSCF plus Filgrastim and in 20% (2 of 10) of the patients treated with Filgrastim alone. Musculoskeletal symptoms included bone pain, back pain, and arthralgia. Other types of pain believed to be related to hematopoietic growth factor administration included chest pain reported in 12% (3 of 26); and generalized pain and headache, which were each reported in 8% (2 of 26) of patients treated with r-metHuSCF plus Filgrastim.
Respiratory symptoms believed to be related to hematopoietic growth factors were reported in 12% (3 of 26) of patients treated with r-metHuSCF plus Filgrastim. Growth factors were discontinued in only one of these patients. One of the three patients had a cough that was reported to be mild on day 3 of hematopoietic growth factor injections; it resolved spontaneously within 24 hours without discontinuing r-metHuSCF. The second patient reported a mild change in voice (dysphonia) after the second administration of r-metHuSCF; laryngoscopy showed mild laryngeal edema. r-metHuSCF was continued and the dysphonia resolved spontaneously in 7 days. The third patient who reported respiratory symptoms developed localized urticaria distant from the injection site 15 minutes after the second dose of r-metHuSCF. This patient also had a history of anxiety requiring anxiolytic medications. After the urticaria developed, he complained of chest tightness, nasal congestion, and pruritus. He received an additional dose of Benadryl (50 mg orally) (LNK International Inc, Hauppauge, NY) and the respiratory symptoms resolved within 3 hours. r-metHuSCF was discontinued and the patient was removed from study. All other side effects were similar in all treatment groups (data not shown).
Transplant Phase
Hematologic recovery.The rate of hematopoietic reconstitution was comparable in all treatment groups (Filgrastim 10 μg/kg/d alone v doses of 5, 10, 15, or 20 μg/kg/d r-metHuSCF in combination with Filgrastim 10 μg/kg/d) (Table 3). The median times to reach an ANC of ≥0.5 × 109/L and 1.0 × 109/L ranged from 9 to 11.5 days and 10 to 12 days, respectively. Median times to platelet recovery to ≥20 × 109/L and ≥50 × 109/L (unsupported by transfusion for a minimum of 48 hours) ranged from 10 to 13.5 days and 14 to 21.5 days, respectively, for each treatment group (Table 3).
Time to ANC and Platelet Engraftment of 0.5 × 109/L and 1.0 × 109/L by Treatment Cohort
| Treatment Cohort . | A . | B . | C . | D . | E . | |
|---|---|---|---|---|---|---|
| r-metHuSCF ( μg/kg/d) | 0 | 5 | 10 | 15 | 20 | |
| Filgrastim ( μg/kg/d) | 10 | 10 | 10 | 10 | 10 | |
| (N = 10) | (N = 6) | (N = 6) | (N = 7) | (N = 6) | Total | |
| (N = 35) | ||||||
| ANC engraftment* to: | ||||||
| 0.5 × 109/L (d) | ||||||
| Median | 9.5 | 10 | 10 | 9 | 11.5 | 10 |
| Range | (9-14) | (8-17) | (10-11) | (8-11) | (9-13) | (8-17) |
| 1.0 × 109/L (d) | ||||||
| Median | 10 | 10 | 11 | 10 | 12 | 10 |
| Range | (9-15) | (8-20) | (10-12) | (8-12) | (9-14) | (8-20) |
| Platelet engraftment† to: | ||||||
| 20 × 109/L (d) | ||||||
| Median | 12.5 | 13 | 12 | 10 | 13.5 | 12 |
| Range | (10-42) | (11-26) | (9-15) | (8-15) | (9-37) | (8-42) |
| 50 × 109/L (d) | ||||||
| Median | 16 | 16 | 15 | 14 | 21.5 | 15 |
| Range | (12-193) | (13-30) | (12-22) | (10-17) | (10-66) | (10-193) |
| Treatment Cohort . | A . | B . | C . | D . | E . | |
|---|---|---|---|---|---|---|
| r-metHuSCF ( μg/kg/d) | 0 | 5 | 10 | 15 | 20 | |
| Filgrastim ( μg/kg/d) | 10 | 10 | 10 | 10 | 10 | |
| (N = 10) | (N = 6) | (N = 6) | (N = 7) | (N = 6) | Total | |
| (N = 35) | ||||||
| ANC engraftment* to: | ||||||
| 0.5 × 109/L (d) | ||||||
| Median | 9.5 | 10 | 10 | 9 | 11.5 | 10 |
| Range | (9-14) | (8-17) | (10-11) | (8-11) | (9-13) | (8-17) |
| 1.0 × 109/L (d) | ||||||
| Median | 10 | 10 | 11 | 10 | 12 | 10 |
| Range | (9-15) | (8-20) | (10-12) | (8-12) | (9-14) | (8-20) |
| Platelet engraftment† to: | ||||||
| 20 × 109/L (d) | ||||||
| Median | 12.5 | 13 | 12 | 10 | 13.5 | 12 |
| Range | (10-42) | (11-26) | (9-15) | (8-15) | (9-37) | (8-42) |
| 50 × 109/L (d) | ||||||
| Median | 16 | 16 | 15 | 14 | 21.5 | 15 |
| Range | (12-193) | (13-30) | (12-22) | (10-17) | (10-66) | (10-193) |
Time to ANC engraftment: The number of days between PBPC infusion and the first of 2 consecutive days of ANC ≥0.5 × 109/L and ≥1.0 × 109/L (after the ANC nadir ≤0.5 × 109/L).
† Time to platelet engraftment: The number of days between PBPC infusion and the first of 2 consecutive days of platelets ≥20 × 109/L and ≥50 × 109/L (after platelet nadir ≤20 × 109/L) after PBPC infusion and at least 48 hours after any platelet transfusion support.
Toxicity data.Thirty-five patients were evaluable for safety during the transplant phase of the treatment program. There were no differences in the incidence of expected toxicities after myeloablative therapy between patients receiving PBPCs mobilized with Filgrastim versus r-metHuSCF and Filgrastim. Nausea, vomiting, diarrhea, mucositis, and fever were the most common events and were seen in ≥77% of the patients. Petechiae, ecchymosis, or microscopic hematuria were seen in 14 patients (44%) and were generally mild to moderate in severity. Gastrointestinal bleeding was observed in 4 patients. CNS bleeding was not noted. The incidence of infectious episodes during the treatment phase was approximately 23% and included central venous catheter infections, cellulitis, fungal dermatitis, pneumonia, and urinary tract infection. Four potentially serious or life-threatening events occurred. There was one case of each of the following: septic shock, portal vein thrombosis, bilateral pneumonia, and congestive heart failure. None of these was believed to be related to hematopoietic growth factor therapy. There was no in-hospital mortality.
At a median follow-up of approximately 1 year in this small group of patients, there were no differences in survival in patients mobilized with Filgrastim as compared to patients mobilized with r-metHuSCF in combination with Filgrastim. Median survival has not been reached. Patient follow-up is continuing.
Effect of Prior Therapy
Extensive prior therapy definition.This study was not initially designed to take into account the extent of prior exposure to specific chemotherapeutic agents known to impair progenitor and/or stem cell development4,26-30 and mobilization. During the course of this study, it became apparent that the treatment arms were not balanced with regard to this important variable. Therefore, at the completion of the study patients were assessed in a blinded fashion (blinded to mobilization and engraftment information), with regard to the type of prior treatment received. Based on previous reports in the literature,4,26-30 study patients were considered “extensively pretreated” if they had been exposed to specific stem cell toxic chemotherapeutic agents (≥2 cycles of any one drug or ≥7.5 g of cytosine arabinoside). The specific drugs used in this definition included only the following: procarbazine, nitrogen mustard, nitrosoureas, melphalan, and high-dose cytosine arabinoside (defined as ≥7.5 g).26,27 31-34 A list of the specific chemotherapy regimens received by each patient before enrollment is provided in Table 4.
Prior Treatment Characteristics
| Patient . | Cohort . | Initial Regimen . | Salvage Regimen 1 . | Salvage Regimen 2 . | Extensive Prior . |
|---|---|---|---|---|---|
| . | . | . | . | . | Chemotherapy4-151 . |
| 102 | A | CHOP × 8 | DHAP × 2 | Y | |
| 107 | A | PROMACE-CYTABOM × 4 | CHOP × 2 | N | |
| 110 | A | CHOP × 2 | DHAP × 2 | Y | |
| 203 | A | MACOP-B × 3 | CHOP × 3 | N | |
| 205 | A | CHOP × 4 | DHAP × 1 | N | |
| 208 | A | CHOP × 12 | ICE × 3 | N | |
| 301 | A | CHOP × 8 | DHAP × 2 | Y | |
| 307 | A | CHOP × 6 | Radiation4-150 | N | |
| 400 | A | PROMACE-CYTABOM × 6 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 407 | A | CHOP × 6 | DHAP × 2 | ICE × 3 | Y |
| 101 | B | PROMACE-CYTABAM × 4 | ICE × 2 | N | |
| 103 | B | CHOP × 4 | DHAP × 2 | Y | |
| 104 | B | CHOP × 4 | DHAP × 2 | Y | |
| 105 | B | CHOP × 4 | DHAP × 2 | Y | |
| 108 | B | CHOP × 8 | DHAP × 2 | Y | |
| 109 | B | DOX, VCR × 4 | ICE × 2 | N | |
| 201 | C | CHOP × 4 | DHAP × 3 | Y | |
| 202 | C | CHOP × 6 | ICE × 2 | N | |
| 204 | C | MACOP-B × 3 | ICE × 2 | N | |
| 206 | C | CHOPE × 2 | DHAP × 2 | Y | |
| 207 | C | ABVD × 5 | ICE × 2 | EPOCH × 1 | N |
| 209 | C | CHOP × 6 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 300 | D | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 302 | D | CHOP × 4 | ESHAP × 1 | N | |
| 303 | D | CHOP 2 | CHOP × 4 | N | |
| 304 | D | PROMACE-MOPP × 4 | ICE × 3 | Y | |
| 305 | D | CVP × 8 | COPBLAM × 4 | Y | |
| 306 | D | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 308 | D | CHOP × 6 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 401 | E | CHOP-BLEO × 3 | Dexa-BEAM × 2 | Y | |
| 402 | E | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 403 | E | CHOP × 6 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 404 | E | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 405 | E | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 406 | E | CHOP × 6 | CHOP × 4 | C-MOPP × 4 | Y |
| 407 | E | CHOP × 6 | DHAP × 2 | ICE × 3 | Y |
| 408 | E | CHOP × 6 | MOPP × 6 | DHAP × 1/HD CTX | Y |
| Patient . | Cohort . | Initial Regimen . | Salvage Regimen 1 . | Salvage Regimen 2 . | Extensive Prior . |
|---|---|---|---|---|---|
| . | . | . | . | . | Chemotherapy4-151 . |
| 102 | A | CHOP × 8 | DHAP × 2 | Y | |
| 107 | A | PROMACE-CYTABOM × 4 | CHOP × 2 | N | |
| 110 | A | CHOP × 2 | DHAP × 2 | Y | |
| 203 | A | MACOP-B × 3 | CHOP × 3 | N | |
| 205 | A | CHOP × 4 | DHAP × 1 | N | |
| 208 | A | CHOP × 12 | ICE × 3 | N | |
| 301 | A | CHOP × 8 | DHAP × 2 | Y | |
| 307 | A | CHOP × 6 | Radiation4-150 | N | |
| 400 | A | PROMACE-CYTABOM × 6 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 407 | A | CHOP × 6 | DHAP × 2 | ICE × 3 | Y |
| 101 | B | PROMACE-CYTABAM × 4 | ICE × 2 | N | |
| 103 | B | CHOP × 4 | DHAP × 2 | Y | |
| 104 | B | CHOP × 4 | DHAP × 2 | Y | |
| 105 | B | CHOP × 4 | DHAP × 2 | Y | |
| 108 | B | CHOP × 8 | DHAP × 2 | Y | |
| 109 | B | DOX, VCR × 4 | ICE × 2 | N | |
| 201 | C | CHOP × 4 | DHAP × 3 | Y | |
| 202 | C | CHOP × 6 | ICE × 2 | N | |
| 204 | C | MACOP-B × 3 | ICE × 2 | N | |
| 206 | C | CHOPE × 2 | DHAP × 2 | Y | |
| 207 | C | ABVD × 5 | ICE × 2 | EPOCH × 1 | N |
| 209 | C | CHOP × 6 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 300 | D | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 302 | D | CHOP × 4 | ESHAP × 1 | N | |
| 303 | D | CHOP 2 | CHOP × 4 | N | |
| 304 | D | PROMACE-MOPP × 4 | ICE × 3 | Y | |
| 305 | D | CVP × 8 | COPBLAM × 4 | Y | |
| 306 | D | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 308 | D | CHOP × 6 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 401 | E | CHOP-BLEO × 3 | Dexa-BEAM × 2 | Y | |
| 402 | E | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 403 | E | CHOP × 6 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 404 | E | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 405 | E | NHL-15 | ICE × 3 | MTX/HIDARA-C × 5 | Y |
| 406 | E | CHOP × 6 | CHOP × 4 | C-MOPP × 4 | Y |
| 407 | E | CHOP × 6 | DHAP × 2 | ICE × 3 | Y |
| 408 | E | CHOP × 6 | MOPP × 6 | DHAP × 1/HD CTX | Y |
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; PROMACE-CytaBOM, prednisone, cyclophosphamide, doxorubicin etoposide, ara-c, bleomycin, vincristine, methotrexate; MACOP-B, methotrexate, cyclophosphamide, doxorubicin, vincristine prednisone, bleomycin, COPBLAM, cytoxan, vincristine, procarbazine, bleomycin, leucovorin, doxorubicin, methotrexate; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; NHL-15, doxorubicin, vincristine, high-dose cyclophosphamide; CVP, cyclophosphamide, vincristine, prednisone; DHAP, decadron, high-dose ara-c, cisplatin; ICE, ifosfamide, carboplatin, etoposide; PROMACE-MOPP, prednisone, cyclophosphamide, doxorubicin etoposide, nitrogen mustard, vincristine, procarbazine; Dexa-BEAM, decadron, carmustine, etoposide ara-c, melphalan; EPOCH, infusional CHOP plus etoposide; MTX/HIDARA-C, methotrexate, high-dose Ara-C; ESHAP, etoposide, solumedrol, ARA-C, cisplatin.
The salvage regimen for patient 307 consisted of surgical resection and radiation therapy for a local recurrence in his neck.
Patients were defined as having received extensive prior chemotherapy if they had received 2 or more cycles of any of the following drugs: procarbazine, nitrogen mustard, nitrosoureas (including BCNU), melphalan, or high-dose cytosine arabinoside (defined as ≥7.5 g).
Using this definition, the original patient cohorts could be divided with respect to previous extensive chemotherapy as follows: cohort A, 50% extensively pretreated; cohort B, 67% extensively pretreated; cohort C, 50% extensively pretreated; cohort D, 71% extensively pretreated; cohort E, 100% extensively pretreated (see Table 4). Thus, most patients in this study (64%) were extensively pretreated, but were unbalanced between treatment cohorts. No other retrospective statistical analyses were done.
Effect of prior chemotherapy on PB counts.Peripheral WBC count at baseline for the mobilization period was similar for patients when they were grouped by the extent of their prior chemotherapy and by the mobilization regimen used. However, the peak WBC count achieved was higher for the nonextensively treated patients than the extensively pretreated patients, although no patient had a WBC count ≥100 × 109/L (data not shown). Patients treated with the combination of r-metHuSCF and Filgrastim had higher WBC count than the patients mobilized with Filgrastim. Of interest, patients who were considered to be non–extensively pretreated had a median baseline platelet count of 250 × 109/L as compared to 150 × 109/L in the extensively pretreated patients. The median platelet count continued to be higher in the non–extensively pretreated patients through the completion of cytokine dosing (day 7), although all groups experienced the expected decrease in median platelet counts during the 3 days of leukapheresis (data not shown).
Effect of prior chemotherapy on mobilization.The assessment of the effect of prior chemotherapy on mobilization and engraftment was done due to observed differences in the amount and type of prior stem cell toxic chemotherapy patients had received and the knowledge that the treatment arms were not balanced with respect to this variable. Because of small sample sizes for the individual dose cohorts and because of the lack of an observed dose response in the combination cytokine cohorts, the patients receiving r-metHuSCF and Filgrastim were combined into one group and compared to the group of patients mobilized with Filgrastim alone to evaluate the effect of prior chemotherapy on mobilization.
In this analysis, the amount of prior chemotherapy had a statistically significant impact (P ≤ .01) for all mobilization parameters (Table 5). When comparing the effect of prior chemotherapy in conjunction with the mobilization regimen, extensively pretreated patients who received r-metHuSCF in combination with Filgrastim had a sixfold increase in median number of CD34+ cells/kg collected and a fourfold increase in the median GM-CFC/kg compared with the extensively pretreated cohort receiving Filgrastim alone (Table 5). These respective increases were not found to be statistically significant, perhaps because of small sample size. In contrast, a fourfold, statistically significant (P = .034) increase in median BFU-E was observed in patients receiving the combination of r-metHuSCF and Filgrastim compared with Filgrastim alone.
Previous Chemotherapy, Impact on Mobilization Total Yields
| . | Previous Chemotherapy/Treatment5-150 . | |||||
|---|---|---|---|---|---|---|
| . | Extensive . | Nonextensive . | . | . | ||
| . | Filgrastim Alone . | All Combinations . | Filgrastim Alone . | All Combinations . | . | . |
| CD34+ (106/kg) | ||||||
| N | 5 | 18 | 5 | 7 | ||
| Median | 0.28 | 1.76 | 3.68 | 5.27 | ||
| Range | (0.03-3.39) | (0.09-12.04) | (2.95-20.68) | (1.79-8.94) | ||
| GM-CFC (104/kg) | ||||||
| N | 5 | 18 | 5 | 7 | ||
| Median | 5.01 | 20.43 | 40.21 | 47.93 | ||
| Range | (0.10-49.64) | (0.13-223.76) | (18.7-123.07) | (9.11-121.00) | ||
| BFU-E (104/kg) | ||||||
| N | 5 | 18 | 5 | 7 | ||
| Median | 8.90 | 36.87 | 64.92 | 122.71 | ||
| Range | (0.36-21.28) | (0.00-601.46) | (12.50-131.55) | (35.82-283.00) | ||
| . | Previous Chemotherapy/Treatment5-150 . | |||||
|---|---|---|---|---|---|---|
| . | Extensive . | Nonextensive . | . | . | ||
| . | Filgrastim Alone . | All Combinations . | Filgrastim Alone . | All Combinations . | . | . |
| CD34+ (106/kg) | ||||||
| N | 5 | 18 | 5 | 7 | ||
| Median | 0.28 | 1.76 | 3.68 | 5.27 | ||
| Range | (0.03-3.39) | (0.09-12.04) | (2.95-20.68) | (1.79-8.94) | ||
| GM-CFC (104/kg) | ||||||
| N | 5 | 18 | 5 | 7 | ||
| Median | 5.01 | 20.43 | 40.21 | 47.93 | ||
| Range | (0.10-49.64) | (0.13-223.76) | (18.7-123.07) | (9.11-121.00) | ||
| BFU-E (104/kg) | ||||||
| N | 5 | 18 | 5 | 7 | ||
| Median | 8.90 | 36.87 | 64.92 | 122.71 | ||
| Range | (0.36-21.28) | (0.00-601.46) | (12.50-131.55) | (35.82-283.00) | ||
Results from analysis of variance showed a statistically significant difference (P ≤ .01) between extensive and non–extensively treated patients for all mobilization parameters.
CD34+, GM-CFC, and BFU-E were log transformed.
Time to ANC and Platelet Engraftment Evaluated by Previous Chemotherapy and Mobilization Regimen
| Chemotherapy . | Extensive Prior Chemotherapy . | Not Extensive Prior Chemotherapy . | ||
|---|---|---|---|---|
| . | Filgrastim Alone . | All Combinations . | Filgrastim Alone . | All Combinations . |
| . | (N = 5) . | (N = 18) . | (N = 5) . | (N = 7) . |
| ANC engraftment* to: | ||||
| 0.5 × 109/L (d) | ||||
| Median | 14.0 | 11.0 | 9.0 | 10.0 |
| Range | (9-14) | (8-17) | (9-10) | (8-10) |
| 1.0 × 109/L (d) | ||||
| Median | 14.0 | 11.0 | 10.0 | 10.0 |
| Range | (9-15) | (8-20) | (9-10) | (8-11) |
| Platelet engraftment† to: | ||||
| 20 × 109/L (d) | ||||
| Median | 23.0 | 12.5 | 12.0 | 10.0 |
| Range | (10-42) | (8-37) | (10-24) | (9-18) |
| 50 × 109/L (d) | ||||
| Median | 23.0 | 15.5 | 14.0 | 15.0 |
| Range | (12-193) | (10-66) | (12-33) | (12-18) |
| Chemotherapy . | Extensive Prior Chemotherapy . | Not Extensive Prior Chemotherapy . | ||
|---|---|---|---|---|
| . | Filgrastim Alone . | All Combinations . | Filgrastim Alone . | All Combinations . |
| . | (N = 5) . | (N = 18) . | (N = 5) . | (N = 7) . |
| ANC engraftment* to: | ||||
| 0.5 × 109/L (d) | ||||
| Median | 14.0 | 11.0 | 9.0 | 10.0 |
| Range | (9-14) | (8-17) | (9-10) | (8-10) |
| 1.0 × 109/L (d) | ||||
| Median | 14.0 | 11.0 | 10.0 | 10.0 |
| Range | (9-15) | (8-20) | (9-10) | (8-11) |
| Platelet engraftment† to: | ||||
| 20 × 109/L (d) | ||||
| Median | 23.0 | 12.5 | 12.0 | 10.0 |
| Range | (10-42) | (8-37) | (10-24) | (9-18) |
| 50 × 109/L (d) | ||||
| Median | 23.0 | 15.5 | 14.0 | 15.0 |
| Range | (12-193) | (10-66) | (12-33) | (12-18) |
Effect of prior chemotherapy on engraftment.When the amount and type of prior chemotherapy was considered without regard to treatment group, a statistically significant delay in engraftment of ANC was seen in extensively pretreated patients (P < .01) (Table 6). Recovery of platelets to ≥20 × 109/L or ≥50 × 109/L was not significant in the extensively pretreated compared with the non–extensively pretreated groups (Table 6). However, there was a trend toward more rapid platelet recovery in patients who were not extensively pretreated.
When the effect of prior therapy was considered in conjunction with the cytokine regimen used for mobilization, more rapid recovery of platelets was seen in the extensively pretreated patients who received Filgrastim and r-metHuSCF compared with those who received Filgrastim alone. The median number of days to platelet engraftment to 20 × 109/L was 23 days in the extensively pretreated patients mobilized with Filgrastim alone versus 12.5 days in the patients extensively pretreated and mobilized with Filgrastim in combination with r-metHuSCF (Table 6). However, perhaps because of the small numbers of patients per group, no statistical significance was observed.
DISCUSSION
In this phase I/II study, we observed that r-metHuSCF used in combination with Filgrastim for hematopoietic progenitor cell mobilization could be administered in a safe and efficacious manner, r-metHuSCF and Filgrastim mobilized PBPCs effectively promote hematopoietic reconstitution and rapid platelet engraftment after myeloablative therapy, the type and extent of prior chemotherapy is an important predictor of mobilization, and r-metHuSCF in combination with Filgrastim appears to be better than Filgrastim alone in mobilizing PBPCs in patients who have received extensive prior therapy. In the phase I studies, one concern regarding the use of r-metHuSCF was the observation of adverse events that appeared to be mast cell–related. Patients receiving r-metHuSCF for 14 consecutive days were found to have small, but significantly increased, numbers of cutaneous mast cells as well as elevated levels of mast cell tryptase.35 r-metHuSCF can stimulate histamine release by cultured mast cells in vitro and enhance the IgE-dependent release of histamine from cultured tissue mast cells and PB basophils in vitro.36 In addition, cutaneous mast cell degranulation is observed histologically at r-metHuSCF injection sites.35 Because of these concerns, we proactively used a premedication regimen that included H1 and H2 histamine receptor antagonists, in conjunction with an inhaled β2 agonist and found that this premedication regimen eliminates most mast cell–mediated side effects of r-metHuSCF, with the exception of mild to moderate local injection site reactions. It appears that the use of premedications has contributed to the decrease in the type of mast cell–mediated side effects seen and has permitted the clinical trials using r-metHuSCF to be conducted safely.
Although the use of PBPC transplantation has been shown to be safe and effective in patients diagnosed with NHL, the optimum method for mobilization of progenitor cells in these patients continues to be investigated. Initial clinical investigations with Filgrastim and GM-CSF showed the ability of these respective hematopoietic growth factors to mobilize PBPCs. In one study by Sheridan et al,5 patients receiving PBPCs mobilized with Filgrastim in addition to BM exhibited significantly faster platelet recovery compared with patients receiving BM alone. There have been a number of studies addressing the use of Filgrastim-mobilized PBPCs as the only source of progenitor cell rescue post myeloablative therapy.1,4,5,8 37-39 In general, provided that there is a high yield of PBPCs, as measured by CD34+ cells/kg or CFU-GM/kg, there is rapid neutrophil and platelet engraftment.
Other studies have shown that chemotherapy can induce the mobilization of PBPCs during the recovery phase after BM hypoplasia.2 3 Chemotherapy plus hematopoietic growth factors such as Filgrastim or GM-CSF have been shown to markedly increase the number of PBPC collected; however, when these cells are used as the autograft, more rapid engraftment is not observed when compared with PBPC mobilized by hematopoietic growth factor alone. The use of chemotherapy plus hematopoietic growth factors carries a significant risk of hospitalization for neutropenic fever and/or sepsis and can create difficulty in scheduling the apheresis procedures due to variable rates of recovery. For these reasons, and because chemotherapy mobilization may not be appropriate for all patients, we examined the use of r-metHuSCF and Filgrastim in this setting.
In this study, we evaluated the use of an early acting hematopoietic growth factor, r-metHuSCF, used in combination with Filgrastim in an attempt to improve autologous PBPC mobilization and collection in these patients. r-metHuSCF acts on hematopoietic cells that are not lineage-committed and are more primitive than targets for Filgrastim or rGM-CSF. A major effect is expansion of primitive CD34+ progenitor cells in the BM and PB.17 In our study we showed that PBPCs mobilized with r-metHuSCF and Filgrastim can effectively rescue patients with relapsed intermediate- or immunoblastic high-grade NHL after myeloablative therapy. There were no patients mobilized with r-metHuSCF in combination with Filgrastim who required infusion of their backup BM stem cells for delayed engraftment. One patient of 10 mobilized with Filgrastim alone did not collect the minimum number of MNC required to continue on the transplant. However, when all patients were analyzed, there was no difference in the amount of CD34+ cells/kg mobilized in any of the treatment cohorts (Filgrastim at 10 μg/kg/d v Filgrastim at 10 μg/kg/d in combination with 5, 10, 15, or 20 μg/kg/d of r-metHuSCF) (Table 2).
Recent investigations have highlighted the importance of prior exposure to specific stem cell toxic chemotherapy4,26,27 29 and its impact on optimal mobilization. In this trial, the extent of prior therapy was not assessed prospectively; however, during the course of the conduct of this study it became clear that the treatment arms were not balanced with regard to this important variable. Therefore, upon completion of the study, blinded to individual patient's mobilization and engraftment outcome, we re-analyzed our data to assess the impact of prior (extensive v nonextensive) therapy on these endpoints. Based on previously published reports of the effect of specific drugs on the mobilization of PBPC, patients were defined as being extensively pretreated if they had received a specified amount of one of the following specific chemotherapeutic agents: nitrogen mustard, procarbazine, nitrosoureas, melphalan, or greater than 7.5 g of high-dose cytosine arabinoside.
Other factors have been described as being associated with poor PBPC mobilization including the effect of tumor involvement of the BM32 and prior radiation.31-33 These factors could not be assessed in this study because BM that was histologically positive for lymphoma or radiation to greater than 25% of the BM excluded patients from participating in the study. The number of cycles of previous chemotherapy has also been reported to impact mobilization26,27,31,33; however, we did not analyze our data in this manner.
After grouping patients based on the extent of their prior exposure to stem cell toxic chemotherapy, the data were re-analyzed to assess the ability of r-metHuSCF in combination with Filgrastim or Filgrastim alone to mobilize PBPC. The subset of patients who were extensively pretreated based on this definition had increases in progenitor cell yields when mobilized with the combination of r-metHuSCF and as compared to Filgrastim alone. Patients mobilized with the combination of r-metHuSCF and Filgrastim collected a median of 1.76 × 106/kg CD34+ cells in three aphereses versus 0.28 × 106/kg collected with Filgrastim alone. The increase in number of CD34+ cells collected translated into a 10-day improvement in the time to reach a transfusion-independent platelet count of 20 × 109/L. Although the study was not powered to detect statistical significance, these differences are clinically important. Similar results were seen in the comparison of these mobilizing regimens for GM-CFC (20.5 v 5.01 × 104/kg) and BFU-E (36.9 v 8.9/kg).
The duration of hospitalization for autologous transplant patients is directly correlated to the time to reach an ANC of 0.5 × 109/L. The need for continuing medical observation is influenced by the time to achieve a platelet count of 20 × 109/L. The ability of the combination of r-metHuSCF and Filgrastim to mobilize greater numbers of PBPCs may, therefore, decrease the cost of a PBPC transplant in extensively pretreated patients. If 2.0 to 5.0 × 106 CD34+ cells/kg can be collected, one can reliably predict that engraftment will occur.27 31-34 The number of patients in whom 2.0 or 5.0 × 106/kg CD34+ cells were collected was also assessed. In the extensively pretreated patient group, twice as many patients who were mobilized with the combination of r-metHuSCF and Filgrastim (44%) collected ≥2.0 106/kg CD34+ cells compared to patients mobilized with Filgrastim alone (20%). None of the extensively pretreated patients mobilized with Filgrastim alone achieved a collection of ≥5.0 × 106/kg CD34+ cells during 3 days of collection whereas 11% of the patients receiving r-metHuSCF and Filgrastim collected ≥5.0 × 106/kg CD34+ cells using the same leukapheresis schedule. These encouraging data suggest that use of r-metHuSCF with Filgrastim may allow more patients who have received extensive prior therapy to receive a PBPC transplant and may decrease the morbidity of PBPC transplantation in patients receiving myeloablative chemotherapy.
As treatment programs become more dose intense, the number of patients who will be potentially poor mobilizers is likely to increase. In some patients, insufficient numbers of CD34+ cells/kg are obtained in PBPC harvests making these patients ineligible for PBPC transplantation. Mobilization techniques that improve the quality or quantity of PBPCs collected may allow more patients to receive a potentially curative high-dose chemotherapy with PBPC transplant, including those who would not otherwise be able to mobilize the minimum number of PBPC required to be eligible for transplant. In addition, by collecting an optimal number of PBPC, a more rapid and consistent engraftment can be obtained, decreasing the incidence of morbidity and mortality as compared with an autologous BM transplant.5 34
Future protocols using the combination of r-metHuSCF and Filgrastim with an effective premedication regimen will prospectively evaluate the effectiveness of this mobilization regimen in extensively pretreated patients with NHL, Hodgkin's disease, or multiple myeloma.
ACKNOWLEDGMENT
We thank the following people for contributing to the conduct of the clinical trial and preparation of this manuscript: Linda A. Battiato, Neal Birkett, Marilyn Bishop, Robert Briddell, Mark Davis, Alison Gencarelli, Sheila T. Hoff, Bill Parker, Nancy Rad, Maria Toia, and Brenda Van Arsdall.
Address reprint requests to Craig H. Moskowitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 350, New York, NY 10021.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal