Abstract
Relapse is more frequent after autologous than allogeneic bone marrow transplantation (BMT), due in part to lack of T-lymphocyte mediated allogeneic graft-versus-leukemia (GVL) effects. Infusions of leukemia-reactive T cells to patients after autologous BMT may be a means for providing a GVL effect. Costimulation of T cells by binding of the CD28 receptor on T cells with B7-counter receptors on antigen presenting cells amplifies antigen-specific T-cell responses. To enhance generation of leukemia reactive cytotoxic T lymphocytes (CTL), the murine B7-1– and B7-2–costimulatory molecule cDNAs were introduced into the MHC class I+, class II−, murine meyloid leukemia cell line C1498. B7-1 expression greatly enhanced the ability of the leukemia cells to generate and expand leukemia reactive CTL in vitro. A highly cytolytic and C1498 specific CD8+ CTL line was generated by B7-1 costimulation. This CTL line proliferated autonomously and produced interleukin-2 when provided B7-1 or B7-2 costimulation by C1498 leukemia cells. To test the in vivo antileukemia properties of this CTL line, irradiated syngeneic BMT recipients were given graded doses of leukemia cells on day 0, followed by CTL infusions beginning on day 1 post-BMT. Recipients of 107 CTL had a 3 log reduction in leukemia burden such that 100% of mice were protected from a supralethal leukemic cell dose. Sustained immune responses were detectable up to 3 months postinfusion of the CTL line. B7-1 or B7-2 costimulation in vivo did not augment antileukemia effects of infused CTL post BMT. These results suggest that B7 costimulation of leukemia reactive CTL may be important for their ex vivo generation and expansion for use in human adoptive immunotherapy of leukemia.
BONE MARROW transplantation (BMT) has become a mainstay of treatment for patients with acute myeloid leukemia (AML). Owing to a relative lack of matched donors, many patients undergo autologous BMT. However, relapse following autologous BMT remains a significant barrier to success. The risk of relapse varies between 30% to 70% depending on remission status and the preparative regimen used.1,2 Although gene marking studies show that leukemia cells contaminating the autologous marrow product can contribute to relapse,3 there is no clearly demonstrated role for purging of autologous marrow with regard to relapse.2 This suggests that failure to eradicate the total body burden of leukemia with chemotherapy and/or irradiation may be the primary cause for failure post autologous BMT.
Allogeneic BMT for AML on the other hand, is thought to offer an in vivo graft-versus-leukemia (GVL) benefit, which can serve to eliminate residual leukemia cells that survive the chemotherapy/irradiation of the preparative regimen. Relapse rates following matched related or unrelated donor BMT for AML with preparative regimens similar or identical to autologous BMT are 15% to 30% depending on patient remission status and the degree to which donors are matched with the recipients.4,5 Although the lower incidence of relapse with allogeneic versus autologous BMT could be attributed to lack of contamination with leukemia cells, there are several lines of evidence that suggest that this may not be the major explanation. First, it was shown that relapse was higher after syngeneic compared to allogeneic BMT, indicating that alloreactivity of the donor cells were able to eliminate leukemia.6 Furthermore, patients that experience graft-versus-host disease (GVHD) have a lower incidence of AML relapse.6 Finally, it has now been shown that AML patients that relapse after allogeneic BMT can undergo remission reinduction or prolongation with infusion of donor leukocytes, often with concommitant induction of GVHD.7
Alloreactive T cells that can cause GVHD are also the likely effector cells in GVL. Indeed, cytotoxic T lymphocytes (CTL) with relative specificity for host leukemia cells have been cloned from the peripheral blood lymphocytes of matched sibling donors pretransplant.8 Therefore, we reasoned that if CTLs with reactivity for leukemia could be generated from syngeneic (autologous) donors and adoptively transferred to BMT recipients, we could duplicate the GVL effects of allogeneic BMT. Therefore, we have pursued this hypothesis in a murine model of AML.
We have previously reported our investigations of CD8+ CTL immune responses to the murine cell line C1498, a MHC class I+, class II− myelomonocytic leukemia.9 We found that leukemia reactive CTLs could be generated in syngeneic mice by subcutaneous (SC) immunization, but that intravenous (IV) injection was ineffective in expanding leukemia reactive CTL precursors. However, when the leukemia cells were genetically engineered to produce interleukin-2 (IL-2), CTL precursors were expanded and a systemic CD8+ T-cell immune response was generated. These results implied to us that the leukemia cells were able to directly signal T cells through the T-cell receptor (TCR), but the overall signal generated was ineffective for expansion of precursor CTLs. Therefore, we hypothesized that if we could enhance the T-cell stimulatory capacity of the leukemia cells, we might enhance their ability to generate leukemia reactive CTLs.
The clonotypic TCR complex on T cells confers antigen specificity to a particular T cell and stimulation through this receptor is required for the initial activation of an individual naive cell. Stimulation through the TCR results in upregulation of high-affinity IL-2 receptor complexes and in production of IL-2, leading to autocrine growth stimulation. However, recent reports suggest that stimulation through pathways other than the TCR, referred to as costimulation, play a critical role in activation of T cells.10 In particular, stimulation of CD28 on T cells by B7-1 and B7-2 on antigen presenting cells has been shown to be essential under some circumstances to activate naive T cells in vitro to proliferate and to develop cytotoxic function.11
Because C1498 leukemia cells were found to lack expression of B7-1 (or B7-2), to test this hypothesis, we have genetically engineered the leukemia cells to express murine B7-1 (and B7-2), and have studied the ability of these cells to stimulate CD8+ T cells. We could generate leukemia reactive CD8+ CTLs from mice that were immunized with B7-1–expressing leukemia cells, and in fact use of B7-1–expressing leukemia cells as stimulators in vitro greatly facilitated generation and expansion of leukemia reactive CTL lines. One such line was studied in vivo in an autologous BMT model and found to mediate a 3 log reduction in leukemia burden resulting in prolonged survival of mice. Furthermore, sustained immunity was apparent up to 3 months post infusion of the CTL line. These results show that CD8+ CTLs generated by costimulation can mediate significant GVL in vivo even in a setting of autologous BMT.
MATERIALS AND METHODS
Cell lines.C1498 was originally derived from a female C57Bl/6J (H-2b) mouse and subsequently adapted to tissue culture and was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and grown in RPMI (GIBCO, Grand Island, NY) with 10% heat inactivated fetal bovine serum (Hyclone, Ogden, UT), 2 mmol/L L-glutamine, MEM amino acids, 1 mmol/L sodium pyruvate, 50 mmol/L 2-mercaptoethanol, and penicillin/streptomycin (referred to as CM) and was used for all tissue culture including T-cell cultures. EL4 is a murine T-cell lymphoma (ATCC) and FBL is a murine erythroleukemia (kind gift from Martin Cheever, Seattle, WA), both also derived originally from a female C57Bl/6 mouse. Frozen stocks that had been screened for presence of mycoplasma were used for experiments.
Mice and BMT/leukemia model.Female C57Bl/6 (B6, CD45.1) or congenic B6.SJL (B6 Ly5.2, CD45.2) mice aged 6 to 12 weeks were obtained from the National Institutes of Health (Bethesda, MD) and used for in vivo experiments. Mice were housed in microisolator cages and fed ad libitum according to University of Minnesota Research Animal Resources guidelines (Minneapolis). Leukemia induction in mice was always accomplished by IV injection into the lateral tail vein. Mice undergoing BMT were conditioned with 800 cGy irradiation from a x-ray source on day −1, with IV infusion of 5 × 106 syngeneic bone marrow (BM) on day 0 and were housed under specific pathogen free conditions. Leukemia was induced by injection of the appropriate dose of C1498 leukemia cells in a volume of 0.2 cc phosphate-buffered saline (PBS). In the BMT model, leukemia was injected on day 0, admixed with BM. In mice that also received CTLs, injections were started on day +1 post BMT and also injected IV into the lateral tail vein in PBS.
B7-gene transfer.The murine B7-1 cDNA containing retroviral vector construct and transduction procedure has been previously described.12 Briefly, the muB7-1 cDNA was inserted into the LXSN retroviral vector by subcloning into the BamHI site of LXSN. The LB7-1SN retrovirus was introduced into the amphotropic retroviral packaging cell line PA-317 and clones screened for high-viral titer. High-viral titer supernatant was then used to transduce C1498 leukemia cells by three separate 24-hour incubations with fresh supernatant at a 4:1 viral particle to cell ratio in the presence of 8 μg/mL polybrene. The transduced bulk leukemia cell population was then grown in CM with 0.8 mg/mL G418 to select transduced cells. Seven days following culture in selective media, cells were tested for B7-1 expression and found to be uniformly B7-1+. A frozen stock for subsequent experiments was established at that time. Clones established from the initial C1498B7-1 stock all displayed identical levels of B7-1 expression implying a relatively homogeneous population of B7-1 expressing cells were selected. Control transduced C1498 leukemia cells (C1498-LN) have been previously described.9 C1498B7-1 (and C1498-LN) cells were always cultured with G418 to insure maintenance of B7-1 expression.
Murine B7-2 gene transfer was accomplished by transfection rather than transduction. The murine B7-2 cDNA13 was inserted into the pREP9 expression vector according to manufacturer′s instructions (Invitrogen, San Diego, CA ) and transfected into C1498 cells by electroporation (Bio-Rad, Hercules,CA) with capacitance = 25 μF and voltage = 2,250 V. Cells were grown for 48 hours following electroporation and then placed into limiting dilution cultures in the presence of G418 (0.8 mg/mL). Positive wells were expanded in G418 and screened for expression by staining with CTLA-4Ig and anti-B7-2 monoclonal antibody (MoAb). Frozen stocks of the highest expressing line were established for further experiments.
Flow cytometry.B7 molecule expression was assessed by staining cells with CTLA-4Ig, a fusion protein of CTLA-4 and human Ig that binds all B7-family members.14 Cells for flow cytometric analysis were washed in PBS containing 1% fetal bovine serum and 0.1% sodium azide and a total of 106 cells stained with saturating concentration of protein for 30 minutes on ice, followed by goat antihuman Ig biotin plus SA-PE. Control staining was accomplished in the absence of CTLA4Ig. Fluorescein isothyiocyanate-conjugated MoAbs specific for murine B7-1 (clone 16-10A1) and B7-2 (clone GL1) and isotype matched control MoAbs were used to confirm specific B7-1 or B7-2 expression. (all MoAbs obtained from Pharmingen, San Diego, CA) Stained cells were washed three times and 10,000 events analyzed by a Becton Dickinson (San Jose, CA) FACScan and Consort 30 software.
Generation of leukemia reactive CTL lines.B6 Ly5.2 mice were primed to leukemia cells in vivo by injection of 106 live leukemia cells SC, either control transduced C1498-LN or C1498B7-1, and draining lymph node cells procured after 3 weeks. The lymph node cells were restimulated in vitro with cells procured from the C1498-LN immunized group restimulated by irradiated C1498-LN and the C1498B7-1 group restimulated by irradiated C1498B7-1 cells. Stimulators were irradiated to 10,000 rad and restimulation was in CM with a responder to stimulator ratio of 10:1. On day 5 of culture cells were expanded in CM with IL-2 at 20 IU/mL. The cells from the C1498-LN group became overrun with viable leukemia cells. Limiting dilution cultures were then performed with the remaining group of C1498B7-1 restimulated lymph node cells. Lymph node cells were placed into flat-bottom 96-well plates at 1 and 10 cells/well along with 104 irradiated (10,000 rads) C1498-LN or C1498B7-1 and IL-2 at 5 IU/mL in CM. Cells were refed at 4 to 5-day intervals with fresh CM plus IL-2 10 IU/mL by replacement of 50% of the media. After 14 days of culture, all wells were expanded into 24-well plates with 105 irradiated C1498-LN or C1498B7-1 leukemia cells/well and IL-2 20 IU/mL in CM. These cultures were refed at 5- to 7-day intervals by 50% replacement of media with CM plus IL-2 20 IU/mL. Individual wells with evidence of proliferation were expanded into 10-mL flasks with irradiated C1498-LN or C1498B7-1 and IL-2 20 IU/mL in CM.
Expansion of T15 CTL line.The cytolytic T-cell line that was used in vivo (T15) was expanded by restimulation every 2 weeks with irradiated C1498B7-1 (or C1498B7-2) and IL-2. T15 CTLs were stimulated with C1498B7-1 (irradiated 10,000 rads) at a responder:stimulator ratio of 20:1 with IL-2 10 IU/mL in 10-mL flasks. After 2 days cells were expanded into 50-mL flasks with 100 IU IL-2/mL. Thereafter, cells were split as neccessary to maintain cell density less than 2 to 3 × 105/mL.
In vitro costimulation assay.Leukemia cells were plated at various densities in triplicate either with or without an optimal concentration of T cells in a final volume of 150 μL and incubated for 48 hours. All stimulating cells were fixed in 0.15% paraformaldehyde for 10 minutes. Tritiated thymidine was added the last 16 hours of incubation and incorporated thymidine was assessed by scintillation counting. In some experiments, the fusion protein CTLA-4Ig that binds all B7 family molecules was added to wells at 10 μg/mL to block B7-1/B7-2 mediated costimulation.14
Statistical analysis.The Kaplan-Meier product-limit method was used to assess the survival of mice. The log-rank statistic was used to test differences between groups.
RESULTS
B7-1 expression by myeloid leukemia enhances the in vitro generation and expansion of CD8+ CTL lines.We had previously cloned CD8+ cytotoxic T cells from B6 mice immunized with irradiated C1498 cells by limiting dilution in the presence of splenic accessory cells and IL-2 (data not published). However, after successive rounds of stimulation, the CD8+ cytotoxic T cells lost almost all cytotoxicity. Subsequently we generated a B7-1 expressing C1498 leukemia line (Fig 1) and tried stimulation of the cytotoxic T cells to restore the lost cytotoxicity but without success. Therefore, studies were initiated to determine if provision of B7-1 costimulation might enhance generation of long-term CTL lines. Mice were injected SC with either 106 live C1498-LN (control transduced) or C1498B7-1 and draining lymph nodes cells procured at 3 weeks. The C1498-LN group had demonstrable SC masses at the time of lymph node harvest whereas the C1498B7-1 did not. The harvested lymph node cells were initially cultured in vitro with the C1498-LN group restimulated by irradiated C1498-LN and the C1498B7-1 group restimulated with irradiated C1498B7-1. Both cultures had clusters of cells at 5 days suggesting proliferating T cells, and cells were further expanded in IL-2 (20 IU/mL) in bulk cultures. However, the C1498-LN cultures became overgrown with leukemia cells that had metastasized to the lymph node from the original SC injection. Limiting dilution cultures were performed with T cells from the C1498B7-1 bulk cultures, using either irradiated C1498-LN versus C1498B7-1 as stimulators. The presence of B7-1 on the stimulating leukemia cells increased cloning efficiency approximately threefold, and allowed establishment and expansion of long-term CTL lines. No T-cell lines could be established by stimulation with C1498-LN. Of the three established T-cell lines generated by B7-1 costimulation, 2 were cytolytic although all three were CD8+, CD4−, TCR αβ+.
B7 expression by C1498 leukemia. C1498 leukemia cells or lines with either murine B7-1 or B7-2 cDNA introduction were stained with CTLA-4Ig and goat antihuman IgG biotin plus SA-PE and fluorescence intensity measured by flow cytometry. Control staining (lefthand figures) was performed in the absence of CTLA-4Ig. (A) C1498, (B) C1498B7-1, and (C) C1498B7-2.
B7 expression by C1498 leukemia. C1498 leukemia cells or lines with either murine B7-1 or B7-2 cDNA introduction were stained with CTLA-4Ig and goat antihuman IgG biotin plus SA-PE and fluorescence intensity measured by flow cytometry. Control staining (lefthand figures) was performed in the absence of CTLA-4Ig. (A) C1498, (B) C1498B7-1, and (C) C1498B7-2.
Antileukemia CD8+ CTL line proliferates autonomously and optimally with B7-1 and B7-2 costimulation.The most cytolytic line was chosen for further study and was also positive for CD28. When this T-cell line (designated T15) was restimulated with either irradiated C1498LN or C1498B7-1, expansion was 7 to 25 times greater at the end of 7 days of culture when restimulation took place with the B7-1–expressing leukemia cells, even in the presence of optimal concentrations of IL-2. To better study the costimulatory requirements of this T-cell line, we generated a B7-2–expressing C1498 line to compare to C1498B7-1. Both the C1498B7-1 and C1498B7-2 lines were able to costimulate significant autonomous proliferation of T15 in the absence of exogenous IL-2 (Fig 2A). The T15 was shown to produce IL-2, but not IL-4 (by bioassay with CTLL-2 and CT.4S indicator lines) in response to B7-1 or B7-2 costimulation provided by C1498, but not by parental C1498. Furthermore, addition of CTLA-4Ig or anti-LFA-1 MoAbs completely inhibited autonomous proliferation of T15 in response to B7-1 or B7-2 costimulation by C1498 (data not shown). Optimal proliferation of T15 was dependent on B7-mediated costimulation even in the presence of exogenous IL-2 (Fig 2B). Together these data showed the presence of a functional B7 counter-receptor on the T15 T-cell line and a “helper-independent” CD8+ cytolytic phenotype.15
Autonomous proliferation and expansion of CTL line T15 with B7 mediated costimulation by leukemia cells. (A) The anti-C1498 CTL line T15 was placed into microwells at 2.5 × 104 cells/well and the indicated paraformaldehyde fixed stimulators were added at the indicated concentration (in triplicate). Thymidine incorporation was assessed the last 16 hours of a 48-hour incubation and expressed as CPM. Values shown are after subtraction of CPM in wells without T15 CTL added. (B) T15 CTL cells were stimulated with irradiated (10,000 rads) leukemia cells as indicated at a 20:1 responder stimulator ratio with either 5 U or 50 U of IL-2 added on day 2 of stimulation. Viable T cells were counted by trypan exclusion.
Autonomous proliferation and expansion of CTL line T15 with B7 mediated costimulation by leukemia cells. (A) The anti-C1498 CTL line T15 was placed into microwells at 2.5 × 104 cells/well and the indicated paraformaldehyde fixed stimulators were added at the indicated concentration (in triplicate). Thymidine incorporation was assessed the last 16 hours of a 48-hour incubation and expressed as CPM. Values shown are after subtraction of CPM in wells without T15 CTL added. (B) T15 CTL cells were stimulated with irradiated (10,000 rads) leukemia cells as indicated at a 20:1 responder stimulator ratio with either 5 U or 50 U of IL-2 added on day 2 of stimulation. Viable T cells were counted by trypan exclusion.
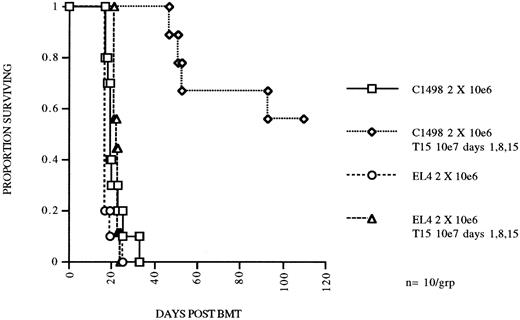
A highly cytolytic CD8+ CTL line mediates specific antileukemia killing both in vitro and in vivo.The T15 cytolytic line was further studied for specificity of target recognition both in vitro and in vivo. Specificity of target recognition in vitro was determined with chromium release cytotoxicity assays. T15 killing of C1498 leukemia cells was specific with regard to other related (EL4 and FBL derived from the same mouse strain) leukemias and syngeneic or congenic conA stimulated blasts (Table 1). Blocking by anti-CD8 or anti-LFA-1 MoAbs was performed, both of which nearly completely blocked cytotoxicity by T15. Both the specificity and blocking results correlated very well with our previously reported data obtained with bulk CTLs generated against C1498.9 The in vivo properties of this CTL line were investigated by adoptive transfer of expanded T15 cells to syngeneic BMT mice. We transferred 107 T15 cells/mouse 1 day after challenge with 2 × 106 C1498 cells, and repeated the T15 infusions two more times at 1-week intervals. Mice receiving T15 CTLs had a significant survival advantage (P = .00025) as compared to the no treatment group (Fig 3). Furthermore, the in vivo effect was highly specific because there was no prolongment of survival of mice receiving the related leukemia EL4 (Fig 2) or FBL (data not shown). Specificity was also examined by infusion of the T-OVA specific CD8+ CTL clone (GX-1) on the same dosage schedule or infusion of 30 to 50 × 106 splenocytes and no effect on survival of mice challenged with 2 × 105 C1498 was found (data not shown).
Comparison of Cytotoxicity of Targets by the Anti-C1498 Autologous CTL Line T15 and Allogeneic CTLs In Vitro
| Targets . | T15 . | DBA . | |
|---|---|---|---|
| . | 1:1* . | 10:1 . | Anti-B6 . |
| . | . | . | 100:1 . |
| C1498 | 65.2 | 91.0 | 34.0 |
| (anti-CD8)† | 3.2 | 30.9 | |
| (anti-LFA-1) | 3.9 | 5.5 | |
| C1498 B7-1 | 65.0 | 82.8 | |
| EL4 | 8.3 | 8.8 | |
| FBL | 0 | 0 | |
| Ly5.1 blasts | 0 (15) | 9.1 (5.0) | 28.2 (7.5) |
| Ly5.2 blasts | 0 (5.1) | 8.1 (8.2) | 37.9 (7.5) |
| Targets . | T15 . | DBA . | |
|---|---|---|---|
| . | 1:1* . | 10:1 . | Anti-B6 . |
| . | . | . | 100:1 . |
| C1498 | 65.2 | 91.0 | 34.0 |
| (anti-CD8)† | 3.2 | 30.9 | |
| (anti-LFA-1) | 3.9 | 5.5 | |
| C1498 B7-1 | 65.0 | 82.8 | |
| EL4 | 8.3 | 8.8 | |
| FBL | 0 | 0 | |
| Ly5.1 blasts | 0 (15) | 9.1 (5.0) | 28.2 (7.5) |
| Ly5.2 blasts | 0 (5.1) | 8.1 (8.2) | 37.9 (7.5) |
Values represent mean % lysis as measured by 4-hour chromium release; standard deviation was <3% unless noted in parentheses.
Indicates effector to target ratio.
C1498 Lysis in the presence of 10 μg/mL of the indicated monoclonal antibody.
CTL line T15 mediates specific eradication of myeloid leukemia in an autologous BMT model. C57Bl/6 mice were irradiated with 800 cGy total body irradiation and transplanted with 5 × 106 syngeneic BM and received 2 × 106 of C1498 or EL4 (lymphoid) leukemia cells IV on day 0. Two groups received T15 CD8+ CTL cells 107 IV on days 1, 8, and 15.
CTL line T15 mediates specific eradication of myeloid leukemia in an autologous BMT model. C57Bl/6 mice were irradiated with 800 cGy total body irradiation and transplanted with 5 × 106 syngeneic BM and received 2 × 106 of C1498 or EL4 (lymphoid) leukemia cells IV on day 0. Two groups received T15 CD8+ CTL cells 107 IV on days 1, 8, and 15.
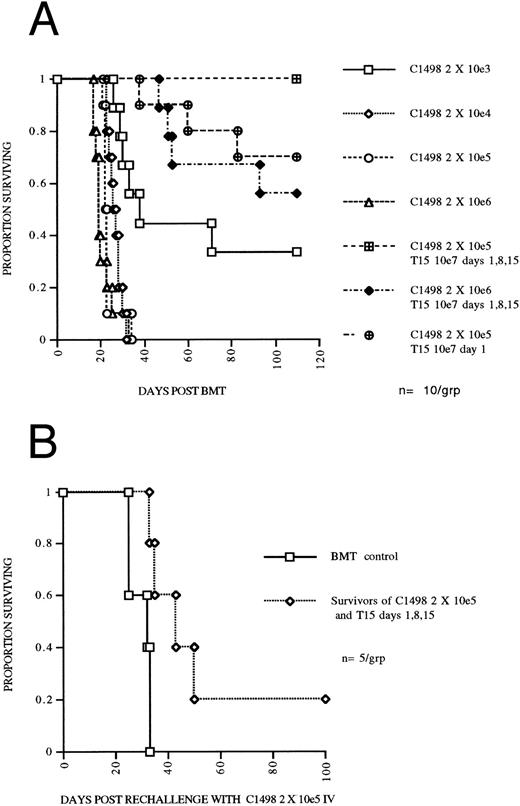
A highly cytolytic CTL line mediates an apparent three log reduction in leukemia burden in syngeneic BMT mice and results in a persistent antileukemia effect.To determine the degree to which T15 CTLs could eliminate C1498 leukemia cells in vivo, mice undergoing syngeneic BMT were given leukemia doses ranging from 2 × 103 to 2 × 106 in log increments on day 0 and compared to mice receiving 107 T15 cells on days 1, 8, 15, and either 2 × 105 or 2 × 106 leukemia cells on day 0. Mice challenged with 2 × 106 leukemia and receiving 3 doses of T15 had survival at least as good as mice challenged with 2 × 103 leukemia (Fig 4A), consistent with a three log reduction in leukemia burden by the infused T15 CTLs. To assess the necessity for multiple infusions of CTLs, one group that was given 2 × 105 leukemia received only one T15 infusion on day 1, resulting in 70% survival of mice and suggesting a two log reduction in leukemia. To assess the persistence of the antileukemia effect of infused T15 CTLs, 5 mice initially challenged with 2 × 105 C1498 and treated with 3 infusions of T15 were rechallenged at day 70 post BMT (day 55 following the last T15 infusion) with 2 × 105 C1498 IV. Mice undergoing syngeneic BMT and given T15 CTLs had a significantly prolonged survival (P = .017) compared to syngeneic BMT control mice challenged on day 70 post BMT with 2 × 105 C1498 leukemia cells (Fig 4B). These results suggest persistence of T15 CTLs for at least 8 weeks after infusion.
(A) CTL line T15 mediates an apparent three log reduction of leukemia in an autologous BMT model. C57Bl/6 mice were irradiated with 800 cGy TBI and transplanted with 5 × 106 syngeneic BM and received graded doses of C1498 leukemia cells IV on day 0. Two groups received T15 CD8+ CTL cells 107 IV on days 1, 8, and 15. (B) Persistence of adoptively transferred T15 CTLs. Mice surviving until day 70 following 2 × 105 C1498 leukemia and receiving T15 107 cells on days 1, 8, and 15 were rechallenged with 2 × 105 C1498 leukemia. Control mice had received syngeneic BMT 70 days before challenge with 2 × 105 C1498 leukemia.
(A) CTL line T15 mediates an apparent three log reduction of leukemia in an autologous BMT model. C57Bl/6 mice were irradiated with 800 cGy TBI and transplanted with 5 × 106 syngeneic BM and received graded doses of C1498 leukemia cells IV on day 0. Two groups received T15 CD8+ CTL cells 107 IV on days 1, 8, and 15. (B) Persistence of adoptively transferred T15 CTLs. Mice surviving until day 70 following 2 × 105 C1498 leukemia and receiving T15 107 cells on days 1, 8, and 15 were rechallenged with 2 × 105 C1498 leukemia. Control mice had received syngeneic BMT 70 days before challenge with 2 × 105 C1498 leukemia.
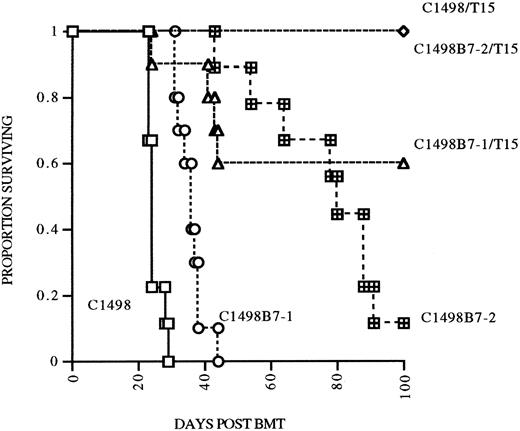
B7 costimulation in vivo does not result in augmented persistence of infused T15 CTLs.Because B7-1 or B7-2 costimulation was necessary for autonomous proliferation of T15 in vitro, we hypothesized that provision of B7-mediated costimulation in vivo would also augment expansion and perhaps persistence of anti-C1498 CTLs in the postsyngeneic BMT model. To address this hypothesis, in a separate experiment, mice undergoing syngeneic BMT were given either C1498, C1498B7-1, or C1498B7-2 leukemia cells at 2 × 105 IV/mouse on day 0, with mice in each group also receiving 107 T15 on days 1, 8, and 15. Mice were followed until day 105 (90 days after last T15 infusion) and survivors rechallenged with 2 × 105 C1498 IV as a measure of persistence of T15 CTLs. Suprisingly, mice receiving C1498B7-1 and T15 had a lower survival compared to the C1498 and T15 group (P = .016), implying that B7-1 costimulation may have impaired the ability of T15 CTLs to eliminate C1498 cells in vivo (Fig 5). When the three surviving groups of mice were rechallenged at day 105, all had significant prolongation of survival compared to controls (Fig 6). However, there were no differences between the three groups, signifying that B7-1 or B7-2 costimulation in vivo did not have a demonstrable effect on expansion and/or persistence of T15 CTLs.
Effect of B7-1 or B7-2 expression on eradication of leukemia postsyngeneic BMT. Syngeneic BMT mice received either C1498, C1498B7-1, or C1498B7-2, 2 × 105 IV on day 0, with treatment groups receiving T15 107 IV on days 1, 8, and 15 (n = 10/grp).
Effect of B7-1 or B7-2 expression on eradication of leukemia postsyngeneic BMT. Syngeneic BMT mice received either C1498, C1498B7-1, or C1498B7-2, 2 × 105 IV on day 0, with treatment groups receiving T15 107 IV on days 1, 8, and 15 (n = 10/grp).
Persistence of T15 GVL effect postsyngeneic BMT is not dependent on or enhanced by in vivo B7 costimulation. Mice surviving until 105 days post BMT having received either C1498, C1498B7-1, or C1498B7-2 leukemia and T15 CTLs were rechallenged with 2 ×105 C1498 leukemia cells. Control mice received syngeneic BMT alone 105 days before. P values shown are compared to control BMT group.
Persistence of T15 GVL effect postsyngeneic BMT is not dependent on or enhanced by in vivo B7 costimulation. Mice surviving until 105 days post BMT having received either C1498, C1498B7-1, or C1498B7-2 leukemia and T15 CTLs were rechallenged with 2 ×105 C1498 leukemia cells. Control mice received syngeneic BMT alone 105 days before. P values shown are compared to control BMT group.
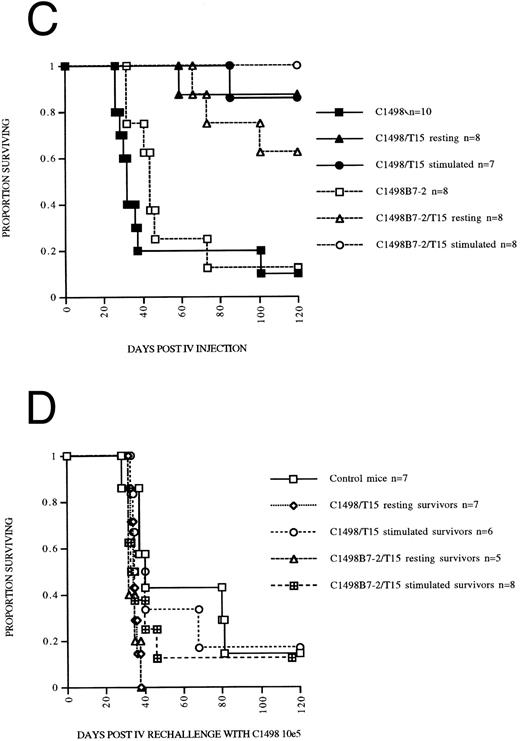
We had previously noted that T15 CTL that had been “rested” in low dose IL-2 (5 U IL-2) had a higher capacity for B7-mediated autonomous proliferation compared to T15 maintained in high-dose IL-2 (50 to 100 U IL-2). Therefore, an experiment was performed to address differences in the in vivo costimulation and expansion/persistence between “rested” and “stimulated” T15 CTL. C1498B7-2 was studied as the costimulator because it consistently stimulated a higher degree of proliferation in T15, presumably caused by a higher level of expression of B7-costimulatory molecules compared to C1498B7-1 (as assessed by CTLA-4Ig staining, Fig 1). Only “resting” T15 CTL that were provided B7 costimulation proliferated autonomously in vitro (Fig 7A). However, both “resting” and “stimulated” T15 resulted in a similar degree of in vitro cytolysis of C1498 (Fig 7B). The “resting” and “stimulated” T15 CTL populations were infused in vivo to mice on the same day as the in vitro analyses to study differences in in vivo costimulatory signaling. Both “resting” and “stimulated” T15 CTL populations were able to significantly prolong survival of mice receiving C1498 or C1498B7-2, (Fig 7C) although “stimulated” T15 CTL were better able to eliminate C1498B7-2 than “resting” T15 (P = .033). At day 120 postleukemia injection surviving mice were rechallenged with C1498 105 IV to test for persistence or expansion of T15 CTL. None of the rechallenge groups had prolonged survival compared to control mice, (Fig 7D) indicating that in vivo B7 mediated costimulation of the adoptively transferred T15 CTL did not lead to enhancement of a long-term memory state.
Optimally “rested” T15 CTL cells undergo significant in vitro autonomous expansion in response to B7 costimulation by leukemia, but do not have an apparent equivalent response to costimulation in vivo. CTL line T15 was stimulated 2 weeks previously with irradiated C1498B7-2 and expanded the first week in IL-2 100 U/mL. In the second week, “resting” T15 CTL were maintained in 5 U/mL IL-2 while “stimulated” T15 CTL continued to expand in 100 U/mL of IL-2. These 2 populations were studied in vitro and in vivo on the same day. (A) “resting” versus “stimulated” T15 CTL were stimulated with paraformaldehyde fixed C1498 or C1498B7-2 in vitro in the absence of exogenous IL-2 for 48 hours, and thymidine incorporation measured the last 16 hours. As a positive control incorporation of thymidine without leukemia stimulation in the presence of IL-2 100 U/mL was measured and found to be 153,967 for “resting” T15 CTL and 185,437 for “stimulated” T15 CTL. (B) Cytotoxicity of chromium labeled C1498 by “resting” versus “stimulated” T15 CTL measured in a 4-hour chromium release assay. (C) Female B6 mice were irradiated to 500 cGy on day −1, and received C1498 or C1498B7-2 105 IV on day 0. On day +1 mice received either 5 × 106 “rested” or “stimulated” T15 CTL or no treatment. (D) Survivors from the above experiment were rechallenged on day 120 with C1498 105 IV with aged matched female B6 mice serving as controls.
Optimally “rested” T15 CTL cells undergo significant in vitro autonomous expansion in response to B7 costimulation by leukemia, but do not have an apparent equivalent response to costimulation in vivo. CTL line T15 was stimulated 2 weeks previously with irradiated C1498B7-2 and expanded the first week in IL-2 100 U/mL. In the second week, “resting” T15 CTL were maintained in 5 U/mL IL-2 while “stimulated” T15 CTL continued to expand in 100 U/mL of IL-2. These 2 populations were studied in vitro and in vivo on the same day. (A) “resting” versus “stimulated” T15 CTL were stimulated with paraformaldehyde fixed C1498 or C1498B7-2 in vitro in the absence of exogenous IL-2 for 48 hours, and thymidine incorporation measured the last 16 hours. As a positive control incorporation of thymidine without leukemia stimulation in the presence of IL-2 100 U/mL was measured and found to be 153,967 for “resting” T15 CTL and 185,437 for “stimulated” T15 CTL. (B) Cytotoxicity of chromium labeled C1498 by “resting” versus “stimulated” T15 CTL measured in a 4-hour chromium release assay. (C) Female B6 mice were irradiated to 500 cGy on day −1, and received C1498 or C1498B7-2 105 IV on day 0. On day +1 mice received either 5 × 106 “rested” or “stimulated” T15 CTL or no treatment. (D) Survivors from the above experiment were rechallenged on day 120 with C1498 105 IV with aged matched female B6 mice serving as controls.
DISCUSSION
Our studies show that B7-mediated costimulation can play an important role in the generation of leukemia reactive CTLs. Expression of B7-1 enhanced the ability of the leukemia cells to stimulate leukemia specific CTLs in vitro. In fact, only with the provision of B7-1 costimulation were we able to generate and expand leukemia specific CTL lines. One of these CTL lines was able to mediate potent and specific GVL effects in vivo when adoptively transferred to syngeneic BMT mice. Interestingly, although we could show marked autonomous proliferation of the CTL line in response to B7 costimulation provided by leukemia cells in vitro, we could not show a significant role for B7 costimulation in vivo.
When investigating the generation of antileukemia CTLs in vitro, we found a significant advantage to the B7-1 expressing leukemia cells when used as stimulators. This is in agreement with the results reported by one of the first groups to describe enhanced in vivo immunity to B7-1 expressing murine tumor cells.16 We had previously generated a CD8+ CTL line with the untransduced C1498 cells in the presence of IL-2 and irradiated splenocytes as accessory cells. However, we were unable to maintain expansion and cytotoxicity over time. In contrast, the T15 cell line generated by stimulation with C1498B7-1 was maintained for more than 10 months without loss of cytotoxicity or proliferative capacity. Furthermore, the T15 line was found to secrete IL-2 and proliferate autonomously (Fig 2A) when stimulated by C1498B7-1 (or C1498B7-2) but not C1498, unlike the CTL line derived previously without B7-1 costimulation (data unpublished). This implies that biologically different CTLs may be generated with provision of costimulation. Not only did we find that B7-1 expression served to augment initial generation of antileukemia lines, but during expansion for in vivo adoptive transfer, provision of B7 costimulation was critical for optimum expansion (Fig 2B). It is now recognized that costimulation of T cells through the B7/CD28 pathway can result in multiple effects including upregulation of Bcl-xL ,17 an effect that may help promote survival of in vitro maintained T cells.
Largely because of the ability to generate large numbers of the antileukemia CTL line was it possible to study the in vivo effects following adoptive transfer. Even a single dose of 107 T15 CTLs was able to mediate a 2 log reduction in leukemia burden. The in vivo effect was specific because EL4 leukemia (Fig 3) or FBL leukemia (data not shown) was not eliminated. When 3 doses of T15 CTLs were infused 1 week apart, an apparent 3 log reduction in leukemia could be discerned. It is important to note that the CTLs were infused 24 hours after leukemia cells, to allow leukemia cells time to migrate to peripheral tissues. These data suggest a direct correlation between number of infused effector cells and the number of leukemia cells that can be eradicated. A 50-fold excess of CTLs per infused leukemia cell was necessary to effect a 2 log reduction in leukemia in vivo. This may reflect a low survival of infused CTLs or it may represent the ineffeciency of homing of CTLs to sites of leukemia migration. Other investigators have shown that lack of the ability to home specifically to sites of leukemia can account for inefficiency of infused CTLs.18 However, since the leukemia cells grow in vivo, the exact ratio of CTL to leukemia at the time the CTL home to sites of leukemia burden is unknown.
We do not know what proportion of infused CTLs survive after initial injection in our model. We did find that when surviving postsyngeneic BMT mice were rechallenged up to 90 days following the last T15 infusion there was still evidence of enhanced resistance to leukemia (Fig 6). This implied that infused CTLs were surviving up to 3 months following injection although other mechanisms of enhanced immunity might also be responsible. Others have shown that exogenous cytokines are necessary for optimal function and expansion of antileukemia CTLs in mice.19 In lieu of exogenous cytokines, others have demonstrated that CD4+ helper T-cell immunity may be necessary for optimal expansion and persistence of infused antileukemia CTLs in mice20 or anti-CMV CTLs in humans.21 Our hypothesis was that costimulation by B7 might be capable of overcoming any dependence on exogenous cytokines or CD4+ helper immunity by stimulation of autocrine production of cytokines such as IL-2.
Therefore, we predicted that if we could provide both antigen stimulation and costimulation via B7-1 or B7-2 in vivo, we might detect a significant expansion and/or persistence of infused T15 CTLs. However, in the syngeneic BMT model, there was no difference in survival of rechallenged mice if they received B7-1 or B7-2 costimulation in vivo compared to no B7 costimulation (Fig 6). To further address the issue of in vivo costimulation of T15 CTL, an experiment was performed in a non-BMT setting in which “resting” versus “stimulated” T15 CTL were infused to mice that had received C1498 or C1498B7-2 1 day before. The two T15 CTL populations were tested for autonomous proliferation and cytotoxicity on the same day as infusion and were very similar in cytotoxic potential (Fig 7B), although only the “resting” T15 CTL could be stimulated to proliferate autonomously with B7-2 costimulation (Fig 7A). Despite the significant ability of the “resting” T15 CTL to proliferate in response to B7-2 costimulation in vitro, mice receiving T15 and C1498B7-2 in vivo had no survival advantage when rechallenged with parental C1498 compared to other groups or even control mice (Fig 7D). These results argue strongly against any role for B7-mediated costimulation of T15 CTL in vivo with regard to expansion and/or persistence and establishment of a memory state of this adoptively transferred CTL line.
Our data also show that B7-2 could provide costimulation of the T15 CTL similarly to B7-1 as reported by others.13 In fact, the C1498B7-2 leukemia line consistently resulted in higher autonomous proliferation of T15 in vitro and also higher survival of post BMT mice not receiving T15 (Fig 5). Others have reported that B7-1 but not B7-2 was able to generate T-cell immunity to murine myeloid leukemia in a different model system.22 In our experiments, a greater costimulatory ability for C1498B7-2 could be explained by a higher level of expression as assessed by CTLA-4Ig (Fig 1). In other model systems differences between the survival advantages conferred by B7-1 as compared to B7-2 expressing tumor cells could be dependent on the tumor cell type, factors that are mouse strain specific, or whether B7 expressing tumor cells are able to provide sufficient tumor antigens for directly stimulating T-cell responses.
Interestingly, in the post syngeneic BMT model with infused T15 CTLs, we found a statistically lower survival rate for the B7-1 group as compared to the non-B7-1 leukemia groups. This implied that provision of B7-1 costimulation downregulated the efficacy of infused T15 CTLs. The explanation for this may be based on the recent discovery of a negative signaling role for CTLA-4, a counter receptor for B7-1 (and B7-2) on activated T cells.23 In a recent report it was found that a B7-1 transfected tumor line was partially rejected by immunocompetent mice, but was fully rejected by mice receiving anti-CTLA-4 monoclonal antibody.24 These results suggest that some of the positive signaling of in situ antitumor T cells by B7-1 through CD28 may be offset by negative signaling through CTLA-4. Our data suggest a similar effect may occur with infused CTLs. However, this effect was not seen with the C1498B7-2 leukemia group and further experiments would be necessary to confirm this hypothesis.
There are previous attempts to generate autologous CTLs reactive to human AML documented in the literature. The earliest attempts documented that autologous remission lymphocytes could in some cases be stimulated by acute leukemia cells25 and generate cytotoxic cells26 and clones.27 However, in these early attempts there is no data concerning the phenotype of the cells generated because many of the reagents (cytokines and MoAbs) were not yet available. In more recent attempts major histocompatibility complex (MHC)-nonrestricted cytotoxic cells have been generated from remission lymphocytes stimulated in the presence of irradiated autologous acute leukemia blasts, irradiated pooled allogeneic cells, and 30 U/mL of IL-2.28 Interestingly, the cytotoxic cells were all CD8+ and CD28−. The authors speculated that since the leukemia stimulators were found to lack B7-1, CD28+ cells were not stimulated and may have accounted for the inability to generate MHC-restricted CTLs.
In summary, we have shown that B7-1 costimulation can augment generation of leukemia reactive CD8+ CTLs in vitro. B7-1 or B7-2 costimulation by leukemia cells resulted in autonomous proliferation and greatly augmented in vitro expansion of the CTLs even in the presence of optimal concentrations of exogenous IL-2. These in vitro generated CTLs were potent mediators of GVL effects when infused after syngeneic BMT. B7-1 or B7-2 costimulation in vivo did not, however, augment persistence of infused CTLs. Other strategies utilizing in vivo IL-2, post infusion immunization, or coadministration of CD4+ T-cell lines may be necessary to generate better CTL persistence and memory cell responses. However, our data suggest that high dose, repeat infusions of highly cytolytic CD8+ CTL may in fact be sufficient to eliminate a lethal leukemia burden despite lack of a potent memory response. We conclude that B7-mediated costimulation of antileukemia CTLs may be useful in the ex vivo generation of potent GVL effectors, which could be adoptively transferred to patients undergoing BMT for high-risk leukemia.
Supported in part by Grants from the National Institutes of Health (PO1-CA-21737) (Bethesda, MD) and the Children's Cancer Research Fund (Minneapolis, MN). B.R.B. is a scholar of the Edward Mallinckrodt Jr Foundation.
Address reprint requests to Michael W. Boyer, MD, Emory University Department of Pediatrics, 2040 Ridgewood Dr, Atlanta, GA 30322.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal